Introduction
Brazil has emerged as a leading hub for first-in-human studies, attracting attention for its unique combination of a diverse population and a rapidly evolving regulatory landscape. The country’s vast array of clinical trials, which saw a significant increase in activity recently, underscores its growing prominence in the global clinical research arena. With a wealth of medical conditions represented among its populace, Brazil offers an unparalleled opportunity for robust clinical findings.
Coupled with the expertise of organizations like bioaccess®, which provide comprehensive clinical trial management services, the environment is ripe for innovation in medical technology. As Brazil streamlines its regulatory processes and enhances its healthcare infrastructure, it positions itself as an ideal location for pioneering research that not only advances medical science but also transforms patient outcomes.
Why Brazil is an Ideal Location for First-in-Human Studies
Brazil emerges as a prime destination for first-in-human studies, largely due to its expansive and diverse population. This diversity offers a substantial pool of participants representing various medical conditions, enhancing the robustness of research findings. According to recent statistics, Brazil carried out more than 1,500 research studies in 2024, indicating a 15% rise from the prior year, highlighting its increasing significance in the research field.
Moreover, bioaccess® provides extensive research management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
This ensures a streamlined process for sponsors. With more than 20 years of expertise in Medtech, bioaccess® is well-prepared to manage the intricacies of research studies. Furthermore, Brazil’s regulatory environment has experienced significant changes, streamlining procedures and speeding up approvals, which greatly aids research studies.
Dr. Maria Silva, a prominent authority in clinical studies, states, 'Brazil's evolving regulatory environment is making it easier for sponsors to begin studies, ensuring quicker access to innovative treatments.' The cost of conducting trials in Brazil remains competitive compared to other nations, making it an appealing choice for sponsors seeking efficient and effective study environments. The nation is also home to numerous academic institutions and advanced hospitals, equipped with cutting-edge medical technology.
Recent news highlights a successful first-in-human trial conducted at Hospital das Clinicas, which demonstrated promising results in a novel treatment for diabetes. This combination of regulatory advancements, competitive costs, and medical infrastructure positions Brazil as a leader in innovative medical exploration, facilitating successful health studies and transforming lives through advanced Medtech.
Step-by-Step Process for Conducting First-in-Human Studies in Brazil
- Research Design: Start by creating a detailed research protocol that clearly defines the objectives, methodology, and endpoints of the clinical examination. This document serves as the foundation for your study, guiding every phase of the investigation. It is important to note that researchers propose approximately 30 interviews to reach theoretical saturation, emphasizing the need for thorough data collection and monitoring.
- Regulatory Approval: Once your protocol is in place, submit it to the Brazilian Health Regulatory Agency (ANVISA) for approval. This submission must include essential documentation such as informed consent forms and investigator brochures, ensuring that all regulatory requirements are met. Partnering with bioaccess®, a vetted CRO and consulting partner, can streamline this process with their extensive experience in navigating complex regulatory landscapes.
- Ethics Committee Review: It is crucial to obtain approval from a local ethics committee, known as the Comite de Etica em Pesquisa. This review is designed to safeguard participant safety and uphold ethical standards throughout the trial. As Esther Dyson aptly stated, "Things will be both better and worse. Numerous individuals will be deceased and many others will suffer lasting harm, physically, mentally, or financially," highlighting the ethical considerations in medical studies.
- Site Selection: Identify and choose clinical sites that are equipped with the necessary facilities and have access to the patient populations needed for your research. Proper site selection is vital for successful recruitment and data collection. bioaccess® specializes in feasibility assessments and site selection, ensuring that your research is conducted in the most suitable environments.
- Recruitment: Implement a robust recruitment strategy that adheres to defined inclusion and exclusion criteria. This strategy should concentrate on enrolling participants who fulfill the project's requirements, ensuring that the research is carried out with a representative sample.
- Data Collection and Monitoring: Carry out the research while rigorously ensuring data integrity and compliance with Good Clinical Practice (GCP). Ongoing monitoring assists in recognizing any issues that may occur during the experiment, which is essential for maintaining the quality of the data gathered. The case analysis on mental health and technology illustrates the real-world implications of research design and regulatory processes, especially regarding marginalized groups and the impact of technology on health outcomes. bioaccess® provides extensive project management services to assist with continuous monitoring and data management throughout the study.
- Reporting: Following the completion of the study, submit the results to ANVISA and other relevant stakeholders. This includes detailed reports on any adverse events encountered during the study, which are crucial for regulatory oversight and public safety. By entrusting your reporting processes to bioaccess®, you can ensure that all documentation meets regulatory expectations, thereby enhancing the credibility and impact of your study.
- Economic Impact: Acknowledging the wider consequences of research studies, collaborating with bioaccess® not only enables successful experiments but also aids local economies by generating employment, enhancing healthcare advancements, and encouraging global cooperation. Their expertise in managing Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH) positions bioaccess® as a leader in advancing Medtech innovation in Latin America.
Navigating Challenges in Brazilian Clinical Trials
Carrying out medical studies in Brazil poses a distinct array of obstacles that can greatly influence the success of research endeavors. Regulatory delays are a considerable hurdle, as navigating the complex legal landscape requires an in-depth understanding of local regulations and compliance requirements. Almost 50% of medical studies in Brazil face delays because of these obstacles, prolonging schedules and raising expenses. Additionally, cultural differences play a pivotal role in patient engagement; understanding these nuances is essential for effective communication and recruitment strategies. As Secretary of State Antony J. Blinken noted, 'the way we acted – decisively, strategically, with humility and confidence' is crucial in addressing these cultural challenges. Logistical issues, especially concerning transportation and site management, further complicate execution, necessitating proactive planning and resource allocation.
To effectively navigate these challenges, it is essential to leverage comprehensive clinical management services like those offered by bioaccess®, which include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
These services are specifically designed to address regulatory delays by ensuring compliance with local laws and facilitating faster approvals. Fostering robust relationships with local stakeholders, including investigators and ethics committees, is crucial. Such collaborations facilitate smoother regulatory processes and enhance the project's credibility within the community. For instance, the Anti-Xenophobia Campaigns implemented by UNHCR and partners demonstrate the importance of community engagement in fostering understanding and support for marginalized populations.
Employing bilingual staff can bridge communication gaps, thereby improving participant recruitment and retention rates. Moreover, understanding regional differences in healthcare practices and patient expectations is crucial; customizing engagement strategies to correspond with these elements can greatly improve the quality of patient interactions and overall success. Katherine Ruiz, an expert in Regulatory Affairs, emphasizes that her specialized knowledge in navigating these complex environments is instrumental in overcoming regulatory challenges and expediting processes. This expertise not only ensures compliance but also contributes to economic growth and healthcare improvement in the regions involved. For instance, medical research has been demonstrated to generate jobs and invigorate local economies, with metrics showing a rise in healthcare-related employment prospects by up to 30% in regions hosting medical experiments.
The Role of CROs in First-in-Human Studies in Brazil
Contract Research Organizations (CROs) are crucial in advancing first-in-human trials in Brazil, primarily by providing specialized expertise in regulatory compliance, site management, and patient recruitment. Organizations like bioaccess® leverage over 20 years of Medtech expertise to navigate Brazil's intricate regulatory framework, ensuring adherence to local laws and guidelines. Their extensive management services for research experiments include:
- Feasibility assessments
- Selection of research locations and lead investigators
- Compliance evaluations
- Setup, which are essential for preserving the integrity of research projects
Importantly, bioaccess® also manages Pilot Studies and Post-Market Clinical Follow-Up Studies (PMCF), further showcasing their extensive capabilities. With services extending to import permits and project management, bioaccess® is well-equipped to support sponsors in their research endeavors. Additionally, they offer comprehensive reporting on study status and adverse events, enhancing transparency and trust in the research process. By integrating these capabilities, sponsors can significantly enhance the efficiency and effectiveness of their clinical studies, ultimately driving successful outcomes in medical device research. Their profound comprehension of Brazil's regulatory landscape translates into tangible value for clients, ensuring that processes not only adhere to regulations but also progress smoothly towards successful completion.
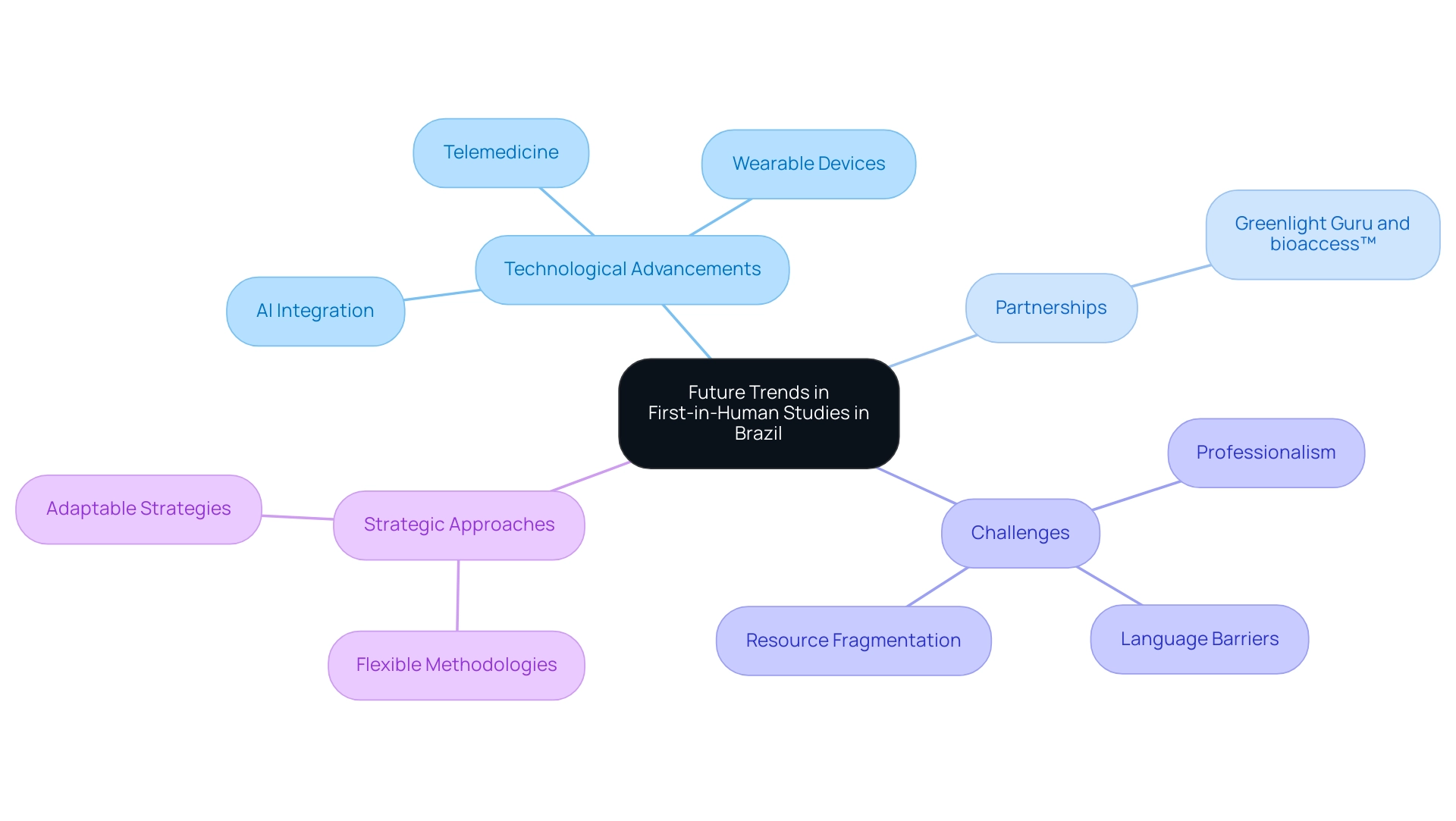
Future Trends in First-in-Human Studies in Brazil
The future of first-in-human studies in Brazil appears exceptionally promising, largely due to rapid advancements in technology and a growing emphasis on personalized medicine. Innovations such as telemedicine and digital health tools, including wearable devices and mobile health applications, are poised to transform patient engagement and enhance data collection methods, ensuring more efficient and accurate studies.
As pointed out by Paulo Fernandes, a prominent consultant and board member of the Brazilian Clinical Research Alliance, 'As these changes take effect, we can anticipate significant positive impacts on the research landscape in Brazil, making it an even more compelling location for conducting cutting-edge medical research.'
Additionally, the partnership between Greenlight Guru and bioaccess™ seeks to improve research capabilities, enabling early-stage studies and ensuring that Medtech innovations can enter the market more quickly and with reduced risk.
However, it is essential to acknowledge the challenges faced by US Medtech companies in Latin America, including professionalism, language barriers, and fragmentation of resources, which can impede effective communication and collaboration with local hospitals. The Brazilian government is actively enhancing its regulatory structure, establishing a more appealing setting for international research studies.
This evolving environment requires that investigators and sponsors adopt a flexible and adaptable strategy, embracing new methodologies and technologies to sustain competitiveness in the global trial arena. Looking ahead to 2024, trends indicate an increasing integration of artificial intelligence and machine learning in study designs, enhancing patient recruitment and data analysis processes.
With a notable 79% increase in clinical studies reported in Argentina from 2017 to 2021, Brazil stands poised to leverage similar growth trajectories in the coming years, particularly as it integrates advanced technological solutions into its clinical research practices.
Case studies from recent trials, including PAVmed's first-in-human study in Colombia, demonstrate the successful application of these technologies, showcasing their potential to streamline operations and improve outcomes. Addressing the urgent need for a solution-driven approach is critical to bridging the gap between innovation and execution in the region.
Conclusion
Brazil's emergence as a premier destination for first-in-human studies is underscored by its diverse population, evolving regulatory landscape, and the expertise of organizations like bioaccess®. The significant increase in clinical trials conducted within the country highlights its growing prominence in the global research arena. The combination of a rich variety of medical conditions, advanced healthcare infrastructure, and a commitment to regulatory efficiency positions Brazil as an ideal location for innovative medical research.
Navigating the complexities of conducting clinical trials in Brazil involves a meticulous process that encompasses study design, regulatory approval, ethics committee review, and robust site selection. It is essential for sponsors to understand and address the unique challenges posed by regulatory delays, cultural differences, and logistical issues. Partnering with experienced CROs like bioaccess® not only streamlines the trial management process but also enhances the overall success of the research initiatives through comprehensive support services.
Looking ahead, the future of first-in-human studies in Brazil is bright, with advancements in technology and a shift towards personalized medicine paving the way for more efficient trials. The incorporation of digital health tools and an emphasis on agile methodologies will further strengthen Brazil's position in the clinical research landscape. As the country continues to refine its regulatory framework and embrace innovative practices, it is poised to attract even more international collaboration, ultimately transforming patient outcomes and advancing the field of medical technology.
Frequently Asked Questions
Why is Brazil considered a prime destination for first-in-human studies?
Brazil's expansive and diverse population provides a substantial pool of participants representing various medical conditions, enhancing the robustness of research findings. In 2024, Brazil conducted over 1,500 research studies, indicating a 15% rise from the previous year.
What services does bioaccess® offer for research management?
bioaccess® provides extensive research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, ensuring a streamlined process for sponsors.
How has Brazil's regulatory environment changed to benefit research studies?
Brazil's regulatory environment has undergone significant changes, streamlining procedures and speeding up approvals, which aids research studies and allows for quicker access to innovative treatments.
What are the cost implications of conducting trials in Brazil?
The cost of conducting trials in Brazil remains competitive compared to other countries, making it an appealing choice for sponsors seeking efficient and effective study environments.
What recent success has Brazil seen in first-in-human trials?
A successful first-in-human trial at Hospital das Clinicas demonstrated promising results for a novel treatment for diabetes, showcasing Brazil's capabilities in innovative medical exploration.
What challenges do researchers face when conducting studies in Brazil?
Researchers face challenges such as regulatory delays, cultural differences affecting patient engagement, and logistical issues related to transportation and site management.
How can bioaccess® help overcome challenges in conducting research in Brazil?
bioaccess® offers comprehensive clinical management services, including feasibility assessments, site selection, compliance reviews, and project management, which help address regulatory delays and enhance project credibility.
What role do Contract Research Organizations (CROs) play in first-in-human trials in Brazil?
CROs like bioaccess® provide specialized expertise in regulatory compliance, site management, and patient recruitment, ensuring adherence to local laws and enhancing the efficiency of clinical studies.
What is the future outlook for first-in-human studies in Brazil?
The future of first-in-human studies in Brazil is promising due to advancements in technology and personalized medicine, with expectations for significant positive impacts on the research landscape.
What technological advancements are expected to influence clinical research in Brazil?
Innovations such as telemedicine, digital health tools, artificial intelligence, and machine learning are anticipated to enhance patient engagement and improve data collection methods in clinical research.




