Overview
Conducting medical device research in Brazil involves navigating a complex regulatory framework primarily governed by ANVISA, which sets forth detailed guidelines, classifications, and approval processes for healthcare instruments. The article emphasizes the importance of understanding these regulations, including compliance with Good Manufacturing Practices and the necessity of strategic collaborations, to successfully secure authorization and effectively manage research initiatives in the evolving medical device landscape.
Introduction
As the Brazilian medical device market continues to evolve, understanding the intricate regulatory framework becomes paramount for industry professionals.
Governed primarily by the National Health Surveillance Agency (ANVISA), the landscape is shaped by a series of guidelines and legislation that dictate the approval process for medical devices.
This article delves into the essential components of navigating Brazil's regulatory environment, from the classification of medical devices to the meticulous requirements for securing approval.
With insights from industry experts and a focus on emerging trends, it highlights the importance of strategic collaborations and staying informed about regulatory changes, particularly as Brazil positions itself as a leader in medical technology innovation.
Through careful navigation of these complexities, stakeholders can enhance their chances of success in this dynamic market.
Navigating Brazil's Medical Device Regulatory Framework
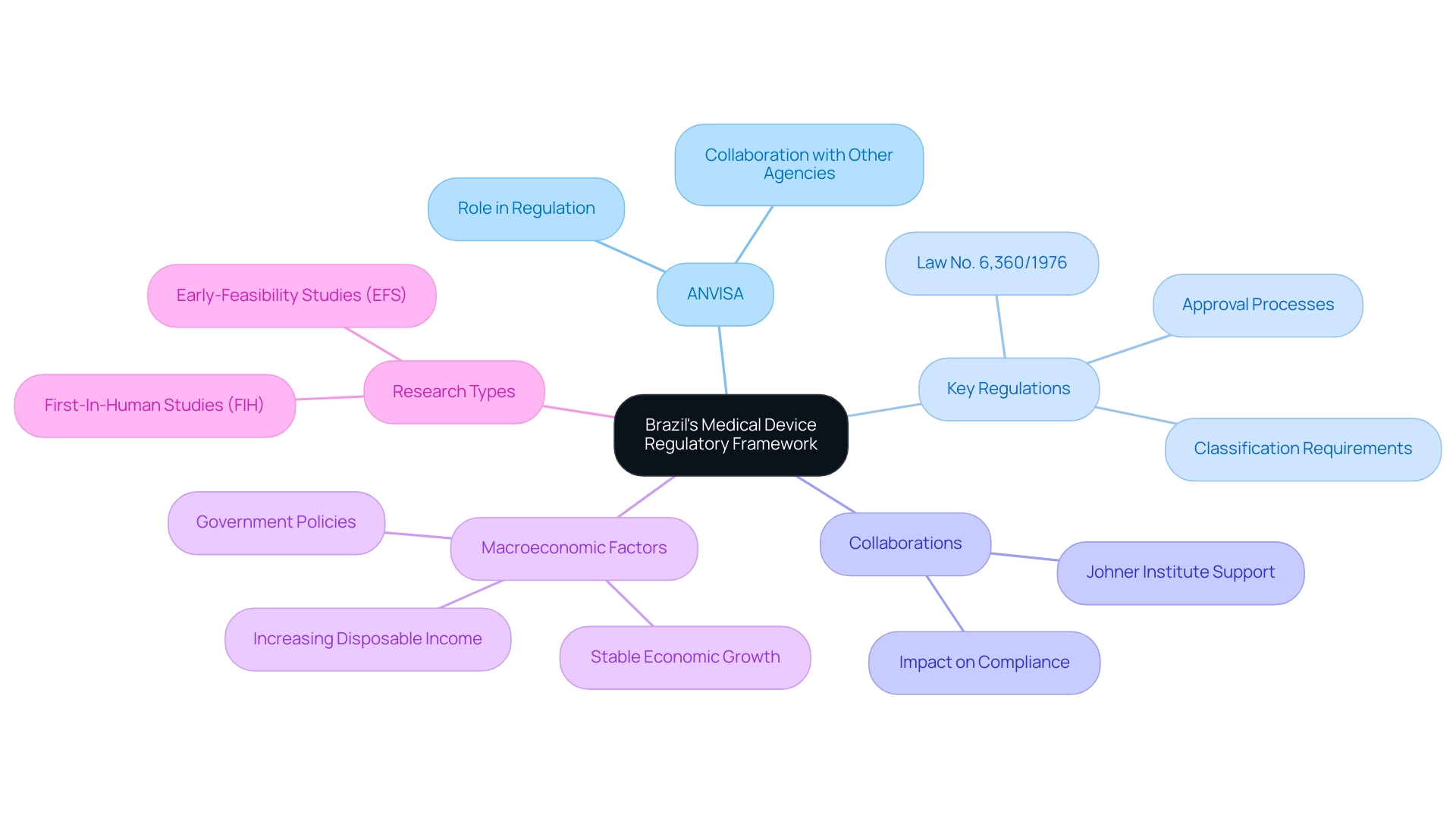
The Brazilian regulatory environment for healthcare instruments is primarily overseen by the National Health Surveillance Agency (ANVISA), making it essential for professionals in Medical Device Research Brazil to comprehend the key regulations that govern this sector. Understanding ANVISA's guidelines is crucial for Medical Device Research Brazil, as they outline the thorough steps required for conducting research on health-related instruments. Key legislation, particularly Law No. 6,360/1976, serves as the foundation for regulating the registration and control of health products, providing detailed classifications and approval processes for healthcare instruments. ANVISA's collaboration with other agencies to assess compliance breaches further underscores the importance of adhering to these regulations. Furthermore, macroeconomic elements, such as the stable economic growth of the country and increasing disposable income, are affecting the healthcare equipment market, influencing regulatory considerations as well.
Staying informed about these nuances is vital for navigating the research environment effectively. As emphasized by Margret Seidenfaden from the Johner Institute, collaborations can have a crucial impact:
'The Johner Institute team can assist you with the authorization of your healthcare instruments in South America.' Such collaborations are invaluable in ensuring compliance with ANVISA's evolving guidelines in Medical Device Research Brazil, particularly as updates for 2024, including recent changes in classification requirements and approval timelines, emerge.
bioaccess® specializes in managing a range of studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), backed by a team with over 20 years of expertise in Medtech. Their extensive experience equips them to navigate the complexities of the regulatory landscape effectively.
Essential Requirements for Medical Device Approval in Brazil
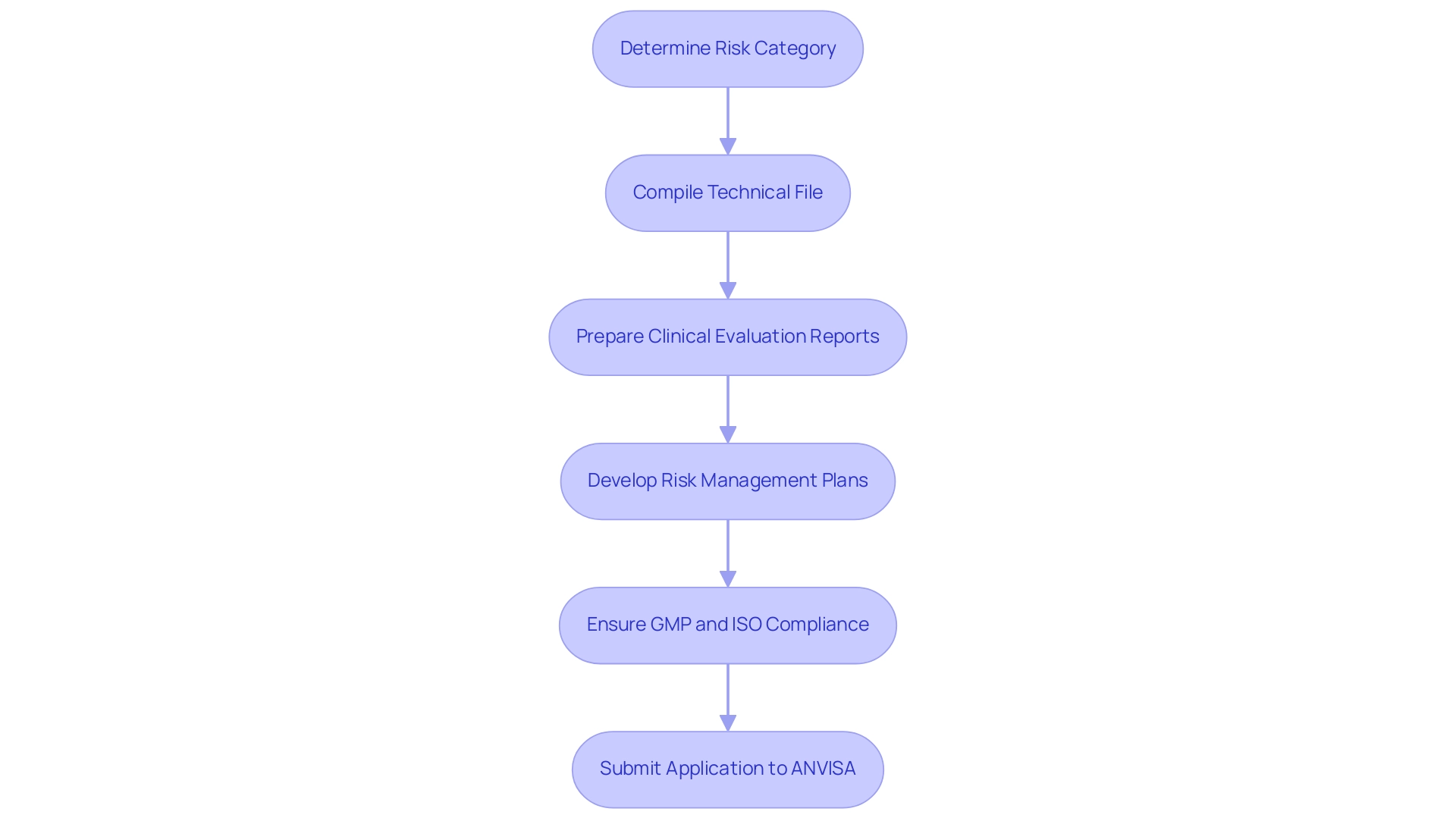
Obtaining authorization for healthcare equipment research in the country requires a careful strategy for the ANVISA application procedure, especially for First-In-Human and Early-Feasibility studies. Researchers must compile a detailed application that includes essential documentation such as the technical file, clinical evaluation reports, and comprehensive risk management plans. Margret Seidenfaden, a specialist in the field, stresses, 'Producers looking to promote a healthcare instrument in South America must first ascertain the risk category of the item,' underscoring the importance of grasping ANVISA's classification framework, which organizes healthcare instruments into categories I (low risk) to IV (high risk).
Additionally, ensuring compliance with Good Manufacturing Practices (GMP) and ISO 13485 is crucial for a successful submission. Familiarity with ANVISA's specific forms and templates is vital to streamline the application process and mitigate potential delays. As of 2024, compliance with these requirements will be essential as ANVISA continues to refine its approval criteria for healthcare products, particularly considering new regulations introduced in RDC 751/2022 that specifically address advanced technologies and their classifications.
The recent insights from the INMETRO certification process, as discussed in the LNE/G-MED North America webinar, can be instrumental for Medical Device Research Brazil in navigating the compliance landscape. By preparing comprehensively and grasping the compliance landscape, researchers can improve their likelihood of securing prompt authorization for their health products. This process is further supported by the comprehensive clinical trial management services provided by bioaccess®, which include expertise in:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
With over 20 years of experience in Medtech, bioaccess® provides a tailored approach to navigating the compliance landscape, ensuring that all aspects of feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting are expertly managed.
Understanding Medical Device Classification in Brazil
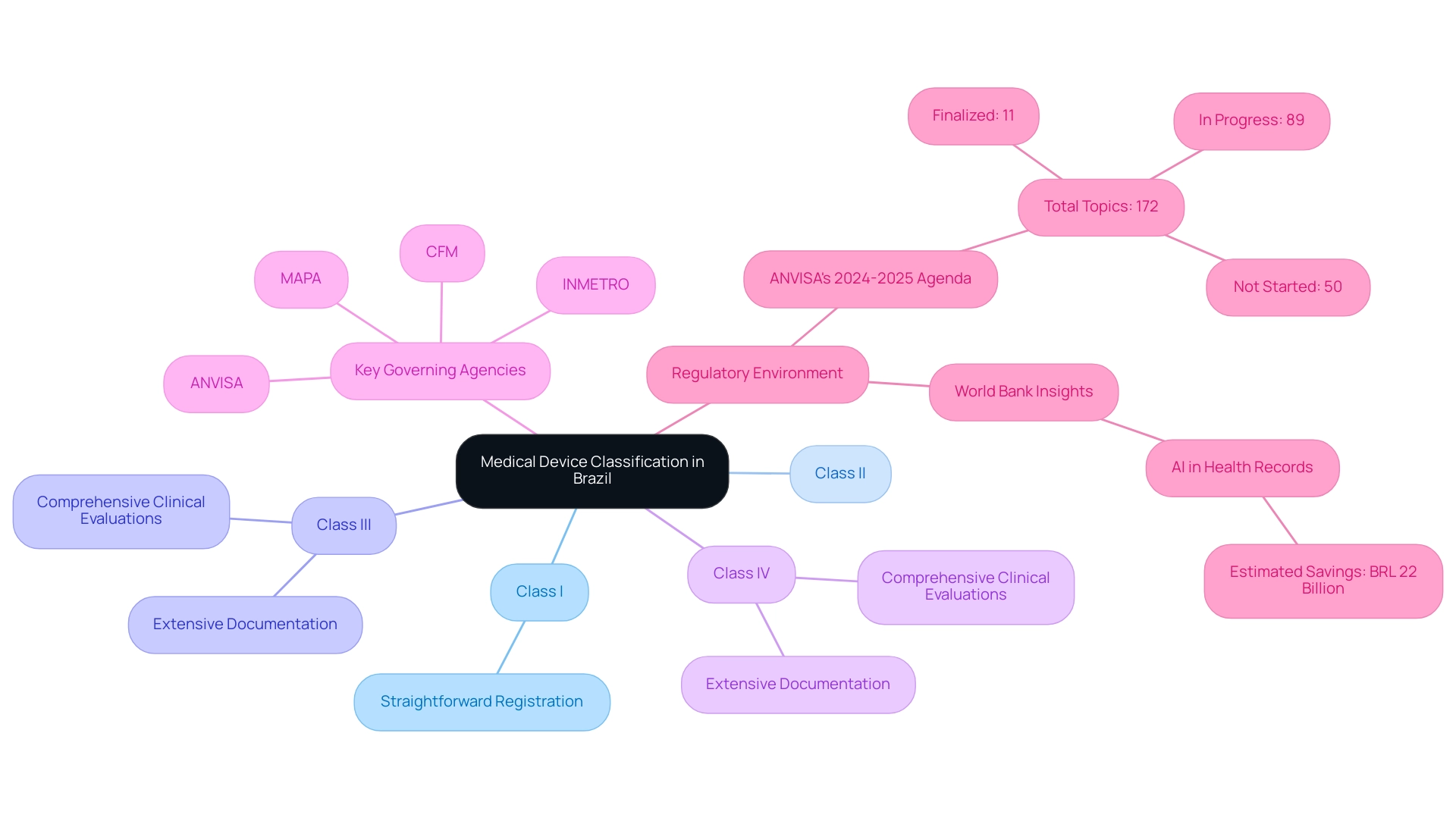
Medical Device Research Brazil categorizes medical instruments into four distinct classes—Class I, II, III, and IV—based on their associated risk levels, with Class I instruments presenting the lowest risk and Class IV instruments, which pose the highest risk, requiring the most stringent oversight. Each classification is subject to specific oversight requirements:
- Class I items often undergo a straightforward registration process.
- Class III and IV items demand comprehensive clinical evaluations and extensive documentation to meet compliance standards.
It is crucial to familiarize yourself with the classification criteria established by ANVISA, as accurate categorization is vital for a successful approval process.
Misclassification can lead to delays and complications, impacting not only product launch timelines but also potential market access. According to ANVISA's 2024-2025 agenda, there are 172 topics under consideration, with:
- 11 finalized
- 89 in progress
- 50 not started
This highlights the dynamic nature of the environment. Moreover, key governing agencies such as ANVISA, MAPA, CFM, and INMETRO play crucial roles in the supervision of Medical Device Research Brazil, and their enforcement practices can differ depending on case specifics, including the offender's intent and public health impact.
A recent World Bank study highlights the significance of effective oversight processes, suggesting that the use of artificial intelligence in examining electronic health records could save the country an estimated BRL 22 billion by reducing unnecessary tests and treatments. Insights from compliance experts like Ana Criado, Director of Affairs and CEO of Mahu Pharma, who holds degrees in chemical pharmacology and health economics, along with Katherine Ruiz, a specialist in Affairs for Medical Devices and In Vitro Diagnostics in Colombia, can further enhance understanding and navigation of these complexities in the framework. Their collective knowledge is essential in tackling the regulatory obstacles encountered by healthcare product firms in South America and throughout Latin America.
Fostering Collaboration and Local Production in Medical Device Research
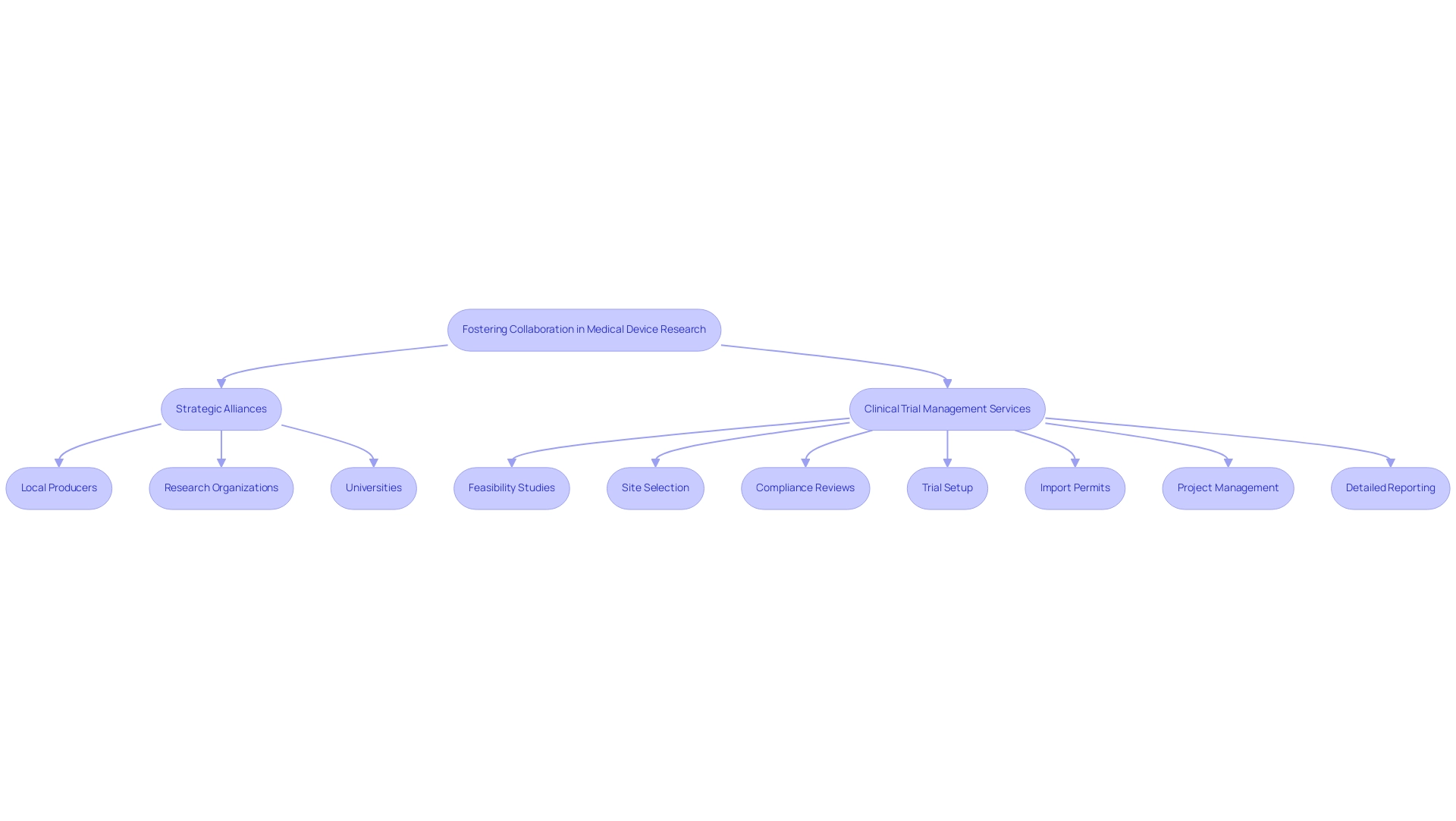
To enhance Medical Device Research Brazil, forming strategic alliances with local producers, research organizations, and universities is essential. Collaborating with regional entities in the context of Medical Device Research Brazil not only provides access to invaluable insights and resources but also enhances the overall effectiveness of research initiatives. Our comprehensive clinical trial management services at bioaccess® include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Detailed reporting on study status and adverse events—ensuring a streamlined process.
Notably, the revenue for Brazil's healthcare business collaboration tools is projected to reach US$ 988.9 million in 2024, underscoring the financial potential of these partnerships and their impact on local economies through job creation and healthcare improvement. Furthermore, fostering local production capabilities can substantially lower costs and increase the availability of medical devices in the market, which is essential for advancing Medical Device Research Brazil. However, it is essential to navigate the compliance environment, as borderline products like dermocosmetics and hyaluronic acid face classification challenges and may require registration with ANVISA.
Engaging with regulatory authorities such as ANVISA and MAPA is crucial for understanding compliance requirements and the enforcement of health regulations. Moreover, citing case studies on teamwork in Medical Device Research Brazil and healthcare equipment research in South America, like successful alliances that have resulted in innovative product developments, can offer valuable insights into the potential advantages and challenges of such collaborations. Engaging with industry associations and participating in local conferences is essential for building relationships that facilitate collaboration.
As Jennifer Mendoza, a research specialist in health, pharma, and medtech, highlights,
Contact us now
to discover how our particular services at bioaccess® can open doors in enhancing research on healthcare technology within the country.
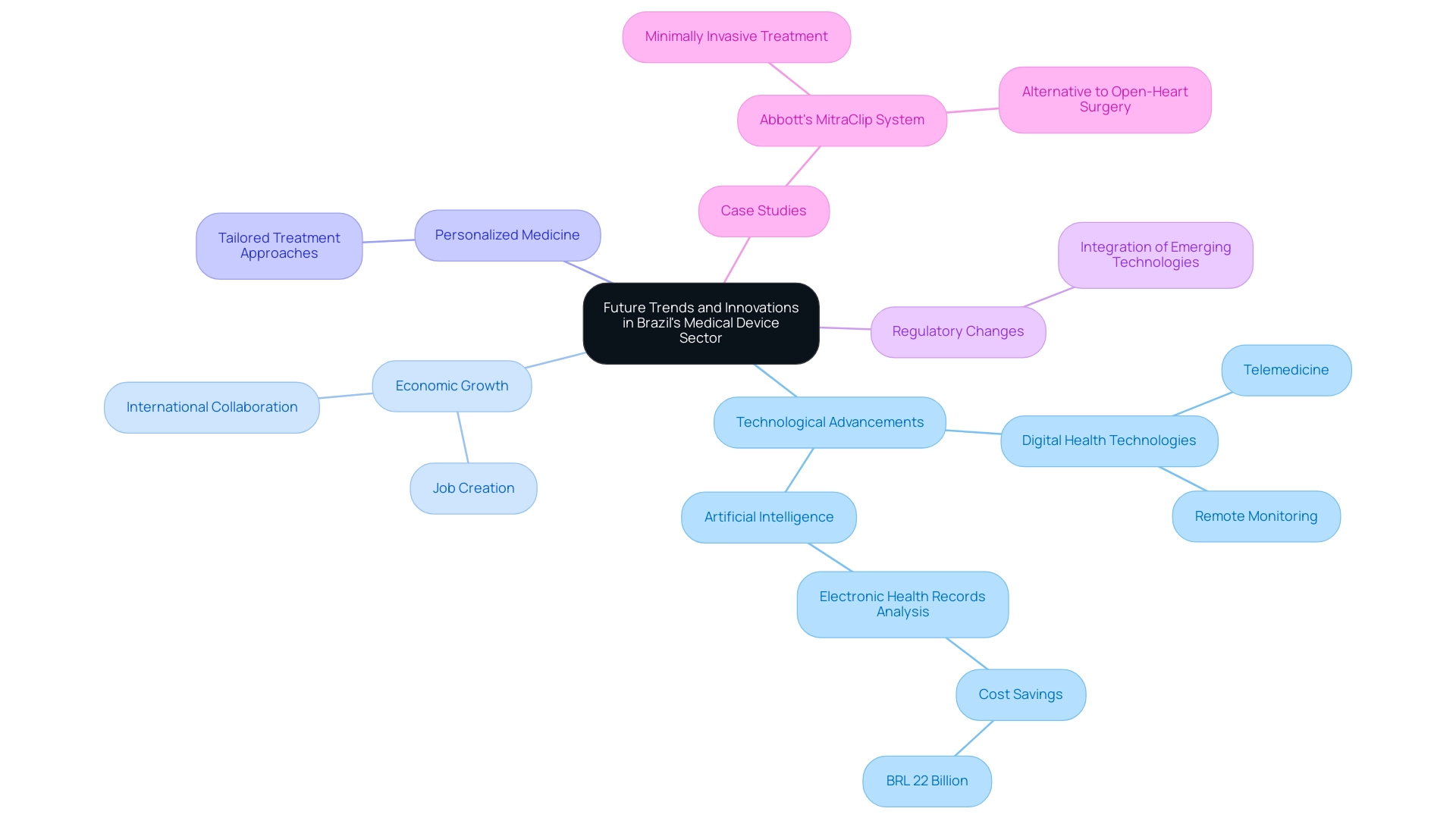
Future Trends and Innovations in Brazil's Medical Device Sector
The sector of Medical Device Research Brazil is on the brink of substantial growth, largely fueled by technological advancements and a rising demand for innovative healthcare solutions. Medical Device Research Brazil indicates that Medtech clinical studies play a pivotal role in this development, creating jobs and promoting economic growth while improving healthcare delivery through international collaboration. These studies not only enhance local economies but also contribute to the country's international recognition in the Medtech field, especially through Medical Device Research Brazil, fostering research and development that attracts global interest.
Significant trends involve the growing acceptance of digital health technologies, such as telemedicine and remote monitoring tools, which are transforming patient care and accessibility. Furthermore, there is a growing emphasis on personalized medicine, enabling tailored approaches to treatment. According to a World Bank study, the use of artificial intelligence in analyzing electronic health records could save the country BRL 22 billion by reducing unnecessary tests and treatments, showcasing the transformative potential of digital health technologies.
The case of Abbott's MitraClip System exemplifies this trend, offering a minimally invasive alternative for treating mitral valve regurgitation, thus reflecting the sector's shift towards innovative solutions. This system not only reduces patient recovery time but also minimizes the risks associated with traditional open-heart surgery. Furthermore, comprehending the demographic landscape, including the country's total population and life expectancy, is essential for researchers such as Dr. Sergio Alvarado, Clinical Trial Manager concentrated on innovative health research and artificial intelligence, especially in Medical Device Research Brazil, as these factors affect market dynamics and the demand for healthcare devices.
For clinical researchers, staying abreast of these developments and understanding regulatory changes will be crucial for integrating emerging technologies into their studies and maximizing new opportunities in this dynamic landscape. The interconnected advancements within Brazil's Medtech sector highlight the importance of Medical Device Research Brazil, which not only enhances local healthcare but also positions the country as a key player in international medical research and innovation.
Conclusion
The Brazilian medical device market presents a complex regulatory landscape that industry professionals must navigate with diligence and strategic foresight. Understanding the role of ANVISA and its regulatory guidelines is fundamental to successfully conducting clinical research and securing approvals for medical devices. Key legislation, particularly Law No. 6,360/1976, provides the framework for device classification and the approval process, emphasizing the importance of adhering to these regulations to avoid complications that could hinder market access.
The classification of medical devices into risk categories is another critical aspect that can significantly impact the approval process. By accurately determining the risk class, stakeholders can streamline their applications and enhance their chances of timely approval. The evolving regulatory environment, highlighted by recent updates and changes, necessitates ongoing education and collaboration among industry players. Engaging with local manufacturers and research institutions can further bolster research initiatives and foster innovation, ultimately contributing to the growth of the sector.
As Brazil's medical device sector continues to evolve, embracing emerging trends such as digital health technologies and personalized medicine will be vital. These advancements not only improve patient care but also position Brazil as a competitive player in the global Medtech arena. By staying informed about regulatory changes and leveraging strategic partnerships, stakeholders can navigate this dynamic market effectively, ensuring their contributions lead to enhanced healthcare delivery and innovative solutions that benefit both local and international communities.
Frequently Asked Questions
Who oversees the regulatory environment for healthcare instruments in Brazil?
The regulatory environment for healthcare instruments in Brazil is primarily overseen by the National Health Surveillance Agency (ANVISA).
Why is it important for professionals in Medical Device Research Brazil to understand ANVISA's guidelines?
Understanding ANVISA's guidelines is crucial as they outline the necessary steps for conducting research on health-related instruments and ensure compliance with regulatory requirements.
What is the foundation of the regulation for health products in Brazil?
The foundation of the regulation for health products in Brazil is Law No. 6,360/1976, which provides detailed classifications and approval processes for healthcare instruments.
How does ANVISA collaborate with other agencies?
ANVISA collaborates with other agencies to assess compliance breaches, emphasizing the importance of adhering to regulations in the healthcare sector.
What macroeconomic factors are influencing the healthcare equipment market in Brazil?
Stable economic growth and increasing disposable income in Brazil are macroeconomic factors influencing the healthcare equipment market and its regulatory considerations.
What role do collaborations play in the authorization of healthcare instruments in Brazil?
Collaborations, such as those with the Johner Institute, can significantly impact the authorization process by ensuring compliance with ANVISA's evolving guidelines.
What is required for obtaining authorization for healthcare equipment research in Brazil?
A careful strategy for the ANVISA application procedure is required, including a detailed application with essential documentation like technical files, clinical evaluation reports, and risk management plans.
How does ANVISA classify healthcare instruments?
ANVISA classifies healthcare instruments into categories I (low risk) to IV (high risk), which is crucial for producers to understand when promoting a healthcare instrument in South America.
What additional compliance standards must researchers adhere to for successful submissions?
Researchers must ensure compliance with Good Manufacturing Practices (GMP) and ISO 13485 for successful submissions to ANVISA.
What recent changes are affecting ANVISA's approval criteria for healthcare products?
As of 2024, compliance with new regulations introduced in RDC 751/2022, which specifically address advanced technologies and their classifications, will be essential.
What services does bioaccess® provide to assist in the regulatory process?
Bioaccess® provides comprehensive clinical trial management services, including expertise in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), and other pivotal studies.
How can researchers improve their chances of securing prompt authorization for health products?
By preparing comprehensively and understanding the compliance landscape, researchers can improve their likelihood of securing prompt authorization for their health products.

