Introduction
The initiation of pilot clinical studies in Argentina presents a unique set of challenges and opportunities for researchers and medical professionals alike. With the rapid evolution of medical technology and the increasing demand for innovative solutions, understanding the essential steps to successfully navigate this complex landscape is crucial.
From defining study objectives and assembling a qualified research team to engaging with stakeholders and adhering to regulatory requirements, each phase of the process plays a pivotal role in ensuring the study's success.
This article delves into the critical components necessary for launching pilot studies in Argentina, offering insights into:
- Effective recruitment strategies
- Data management practices
- The importance of compliance with local regulations
By following these guidelines, researchers can enhance their chances of conducting impactful studies that contribute to advancements in healthcare.
Essential Steps for Initiating Pilot Clinical Studies in Argentina
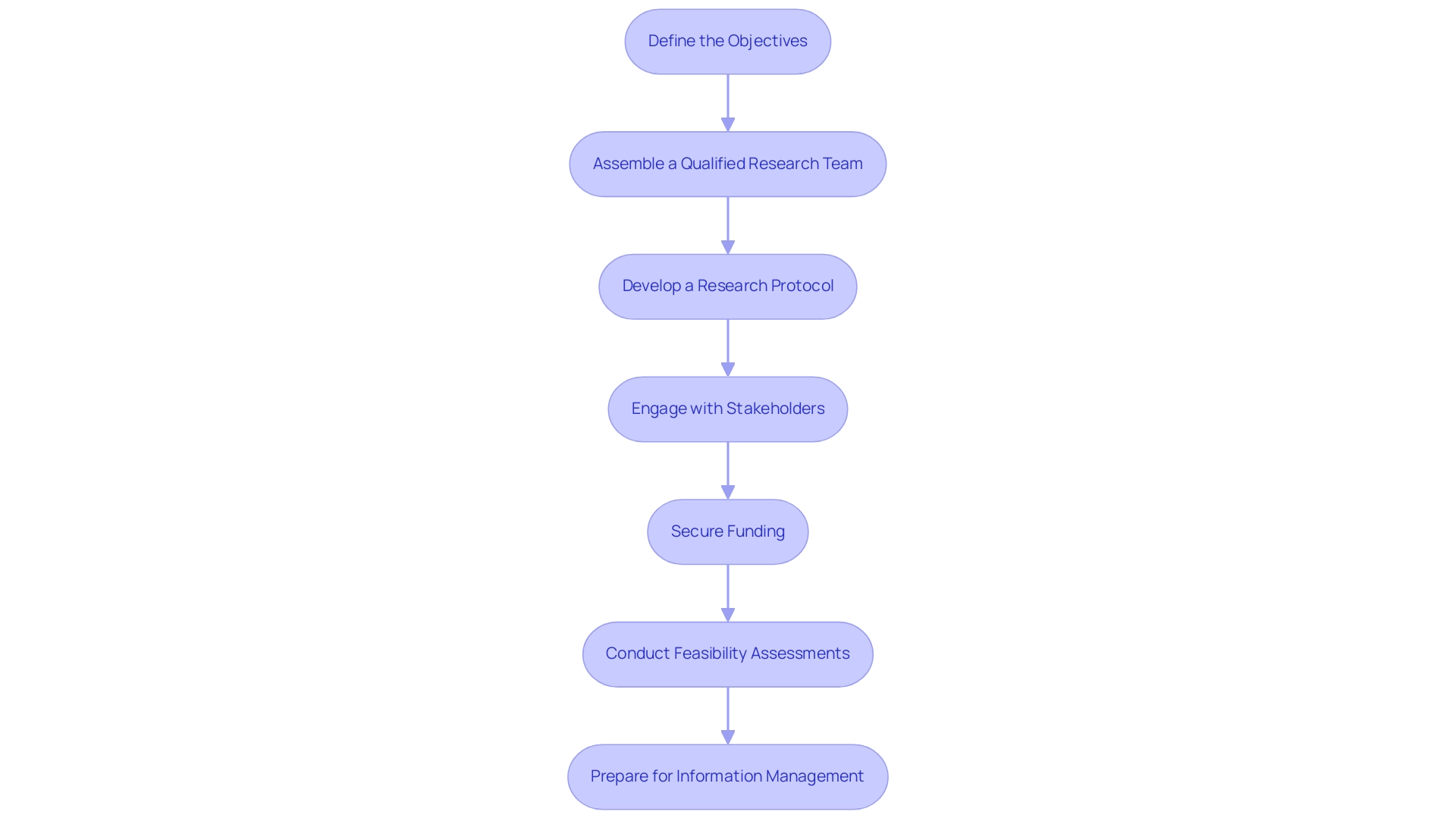
- Define the Objectives: The foundation of a successful pilot project lies in clearly articulating its goals. This encompasses identifying the specific medical device intended for testing, delineating the target population, and outlining the expected outcomes. Setting these parameters will not only direct the entire research process but also align it with regulatory expectations, particularly in the context of Pilot Clinical Studies for Medtech in Argentina, which are categorized by status and phase for 2023.
- Assemble a Qualified Research Team: The effectiveness of a pilot project significantly depends on the expertise of its research team. It is essential to recruit qualified professionals, including clinical investigators, research coordinators, and information managers, who possess experience in conducting clinical trials and a robust understanding of the medical device under investigation.
- Develop a Research Protocol: A comprehensive research protocol is critical for any pilot project. This document should outline the research design, methodology, participant eligibility criteria, and data collection procedures. A well-structured protocol ensures compliance with regulatory standards and serves as a roadmap for the research team.
- Engage with Stakeholders: Establishing early partnerships with key stakeholders—including healthcare providers, regulatory authorities, and ethics committees—can significantly enhance the approval process of the research. Proactive engagement fosters collaboration and can streamline the journey from conception to execution. Significantly, recent experiences emphasize that some research efforts have surpassed their completion date and remain unverified for over two years, underscoring the importance of these relationships.
- Secure Funding: Identifying and securing robust funding sources is vital for the sustainability of the research. Potential funding avenues include grants, partnerships with medical device companies, or institutional support. The cost of a professional account for teams of up to five people is $959 USD per month, billed annually, which is a crucial financial detail for managing resources effectively. Ensuring financial resources are adequately allocated will cover all necessary study-related expenses.
- Conduct Feasibility Assessments: A thorough feasibility assessment is crucial in evaluating the availability of participants, resources, and infrastructure to support the clinical trial. This proactive step is essential for anticipating challenges and developing strategies to mitigate them effectively. This includes the feasibility and selection of research sites and principal investigators, ensuring that the right environment and expertise are in place for the trial.
- Prepare for Information Management: Creating a thorough information management strategy is crucial for preserving the integrity of the research information. This plan should outline how information will be collected, stored, and analyzed while ensuring compliance with privacy regulations. Proper data management is fundamental to the validity and reliability of the results. Furthermore, establishing a strong reporting structure to monitor progress, inventory, and serious and non-serious adverse events is essential. By utilizing bioaccess®'s expertise in early-feasibility, first-in-human, and pilot clinical studies for Medtech in Argentina, research teams can navigate the complexities of medical device evaluations more efficiently.
Navigating Regulatory Requirements for Clinical Trials in Argentina
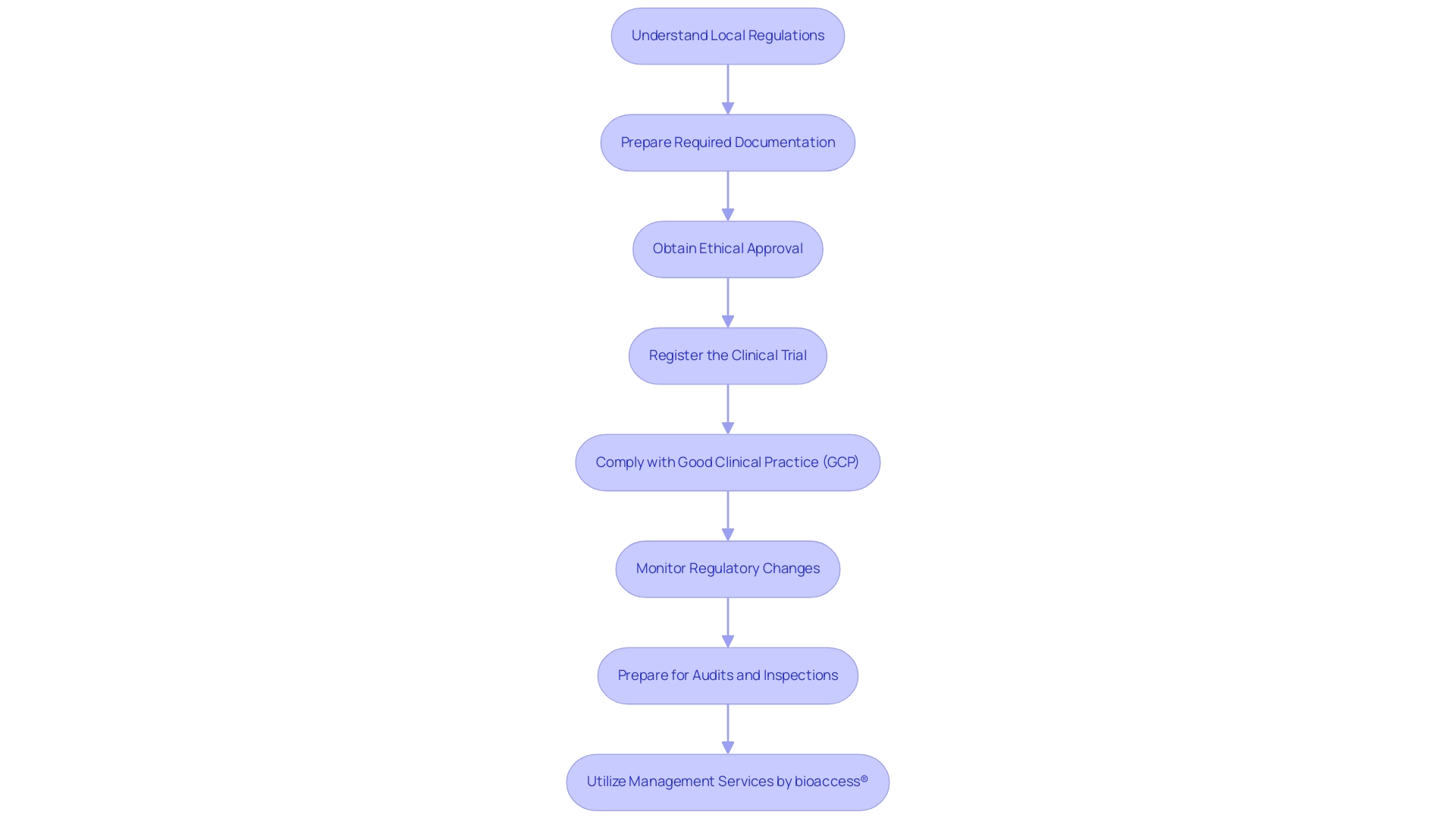
- Understand Local Regulations: It is essential to become acquainted with the local rules governing research studies in Argentina, especially those set by the National Administration of Drugs, Foods and Medical Technology (ANMAT). A thorough understanding of these regulations is essential for ensuring compliance and facilitating smooth operations. Notably, from 1994 to 2006, 64.8% of all protocols approved by ANMAT were foreign-sponsored or multinational trials, indicating the significant role of external sponsors in the Argentine clinical research landscape.
- Prepare Required Documentation: Compile all necessary documentation, including the research protocol, informed consent forms, and investigator qualifications. It is crucial to ensure that all documents are prepared in accordance with ANMAT guidelines to avoid potential delays or compliance issues.
- Obtain Ethical Approval: Submit your research protocol to an Institutional Review Board (IRB) or Ethics Committee for review and approval. This step is critical to ensure that the research adheres to ethical standards and adequately protects the rights and welfare of participants.
- Register the Clinical Trial: Register the clinical trial with ANMAT. The registration process typically involves submitting the research protocol along with other required documentation for comprehensive review.
- Comply with Good Clinical Practice (GCP): Adhere strictly to Good Clinical Practice (GCP) guidelines throughout the research. This compliance encompasses ensuring proper training for research staff, maintaining accurate records, and prioritizing participant safety.
- Monitor Regulatory Changes: Stay vigilant regarding any changes in local regulations that may impact the research. Regular consultations with regulatory experts are recommended to maintain ongoing compliance and adapt to any new requirements.
- Prepare for Audits and Inspections: Be prepared for potential audits or inspections by regulatory bodies. Keeping organized records and thorough documentation is vital in demonstrating compliance with all regulatory requirements.
Furthermore, utilizing the extensive management services provided by bioaccess®, which has more than 20 years of expertise in Medtech, including feasibility assessments, site selection, compliance evaluations, project setup, import permits, project oversight, and reporting, can greatly simplify the process of carrying out pilot research in Latin America. The results from a recent case analysis titled "Regulatory Framework of Clinical Registration in Argentina" emphasize that foreign-sponsored experiments frequently encounter difficulties because of a communication gap and restricted awareness about registration tools. This underscores the importance of implementing educational campaigns to bridge this gap and enhance regulatory compliance, particularly for sponsors and researchers navigating the complexities of clinical trial registration in Argentina.
Recruiting Participants for Pilot Clinical Studies
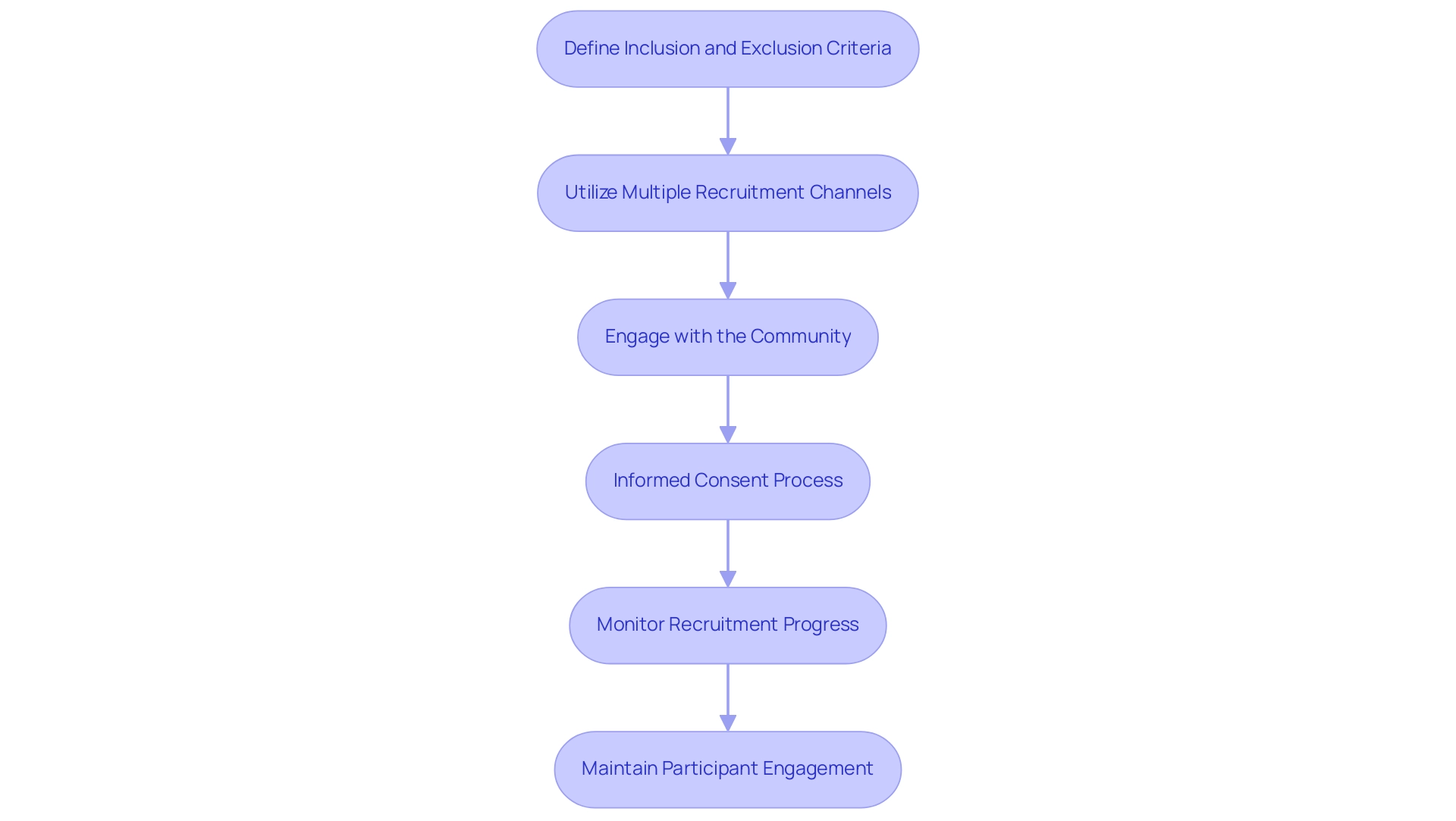
- Define Inclusion and Exclusion Criteria: Establishing clear inclusion and exclusion criteria is vital for ensuring that the study population aligns with the objectives of the pilot study. This precision aids in identifying suitable candidates, enhancing the relevance of the data collected.
- Utilize Multiple Recruitment Channels: Employ a diverse array of recruitment channels, such as social media platforms, healthcare providers, patient advocacy organizations, and clinical trial registries. A multi-channel approach not only broadens visibility but also improves outreach; this is essential given that 75% of patient recruitment originates from effective site selection. By leveraging various channels, researchers can significantly enhance their chances of reaching potential participants, as demonstrated by bioaccess™ and GlobalCare Clinical Trials, which achieved over a 50% reduction in recruitment time and over 95% retention rates.
- Engage with the Community: Host informational sessions or webinars to educate potential participants about the research, its potential benefits, and the particulars of participation. Building trust within the community is essential; as emphasized in recent cases, tackling communication issues can greatly improve recruitment efforts and lessen participant dropouts due to misunderstandings. For instance, the case analysis titled "Managing Participant Dropouts" illustrates how clarifying trial procedures and ensuring participant preferences are considered can improve retention rates.
- Informed Consent Process: A transparent and accessible informed consent process is paramount. Participants must fully comprehend the project's purpose, procedures, risks, and benefits before giving their consent to participate. This clarity fosters trust and aligns with the Pharmaceuticals and Medical Devices Agency (PMDA) recommendations, which advocate for alternatives to traditional methodologies during early development stages to enhance participant understanding.
- Monitor Recruitment Progress: Regularly evaluate recruitment metrics to ensure that targets are being achieved. If recruitment stalls, it may be necessary to adjust strategies or explore new outreach methods to attract participants effectively. This proactive approach is essential for maintaining momentum in clinical engagement.
- Maintain Participant Engagement: Keeping participants informed and engaged throughout the research is crucial. Consistent communication cultivates rapport and can greatly enhance retention rates, which are essential for the overall success of the research. Ensuring that participant preferences are considered can further enhance satisfaction and reduce dropout rates, as shown in the case study on managing participant dropouts. This method not only aids the success of individual studies but also promotes the wider aim of positioning Barranquilla as a premier location for medical research in Latin America, benefiting the local economy through job creation and enhanced healthcare. Furthermore, this initiative is backed by the support of Colombia's Minister of Health, reinforcing its legitimacy and importance in the region. The collaboration between bioaccess™ and GlobalCare Clinical Trials employed specific methodologies such as community outreach and tailored recruitment strategies, which have proven effective in achieving these impressive results.
Data Collection and Management in Pilot Clinical Studies
-
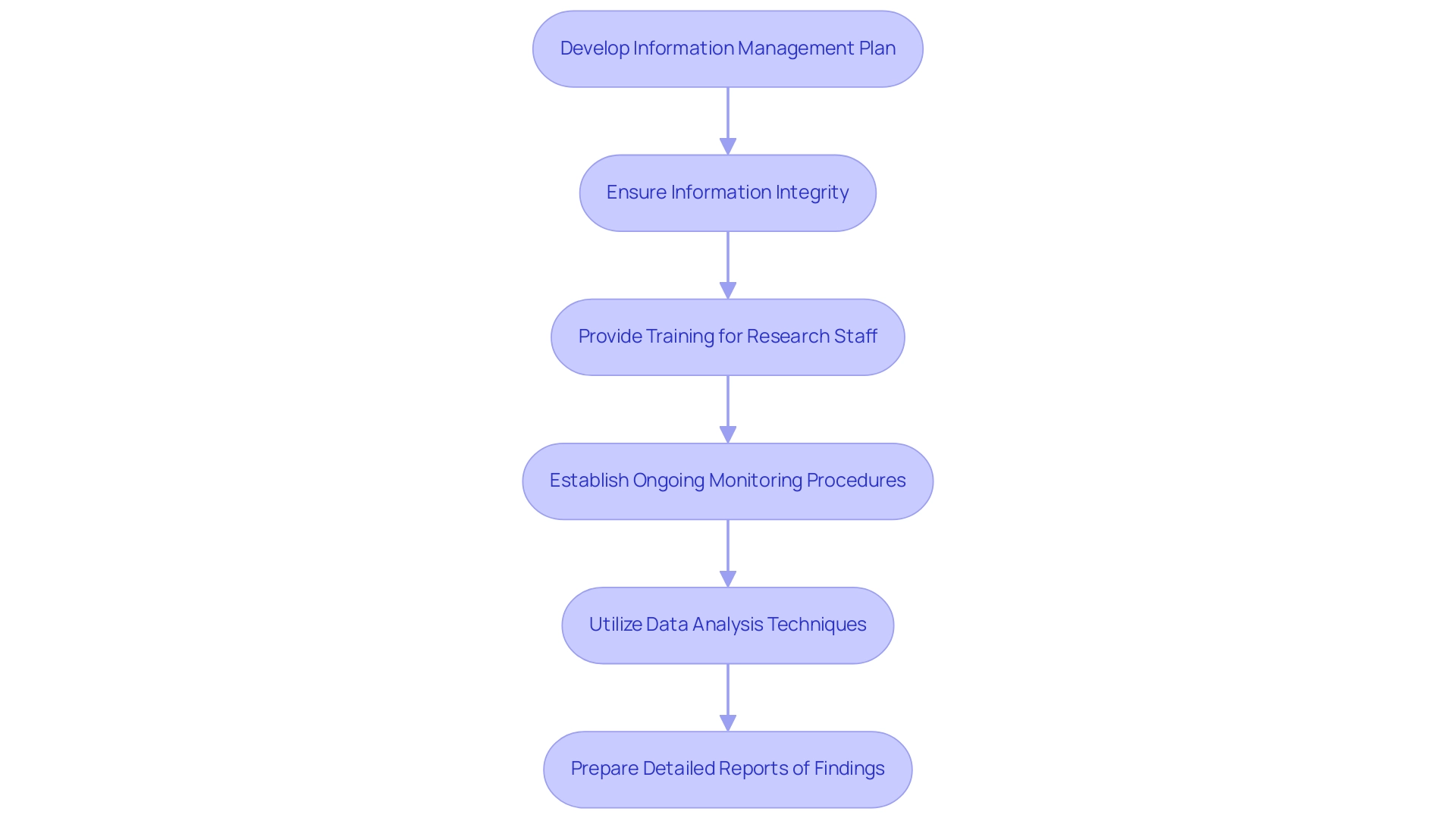
Develop a Comprehensive Information Management Plan: A robust information management plan is essential for outlining the methodologies for collection, storage, and analysis. This plan should specify the tools and software used in information entry and analysis, ensuring alignment with the latest best practices in trial management. Leveraging bioaccess®'s expertise and their network, including Datavant's ecosystem of over 500 real-world information partners, can significantly enhance the quality and breadth of the information collected, ensuring a more comprehensive approach to information management.
-
Implement Measures to Ensure Information Integrity: Preserving information integrity is essential for the success of research studies. Regular audits and validation checks should be instituted, alongside secure storage solutions. Such measures safeguard the accuracy and reliability of the information, a necessity underscored by the increasing demand for improved clinical information management standards to comply with regulatory requirements and expedite product commercialization. As Binny Krishnankutty from Dr. Reddy's Laboratories Ltd. states,
Clinical information management aims to streamline the drug development process, making it both faster and safer.
-
Provide Thorough Training for Research Staff: Training is crucial in reducing mistakes during information gathering and management. All research personnel should receive comprehensive training to fully understand the protocols and procedures governing information handling. This guarantees that every team member is prepared to maintain the integrity of the research, which is especially significant in the context of bioaccess®'s tailored approach to managing clinical trials.
-
Establish Ongoing Information Monitoring Procedures: Continuous observation of information throughout the study is vital. This includes monitoring participant compliance, evaluating entry precision, and recording any adverse events. Regular evaluations, as demonstrated by effective discrepancy management practices, assist in identifying and resolving discrepancies, which is essential for maintaining information integrity. For instance, implementing regular reviews in a pilot project can significantly enhance the ability to flag and address discrepancies in real time.
-
Utilize Effective Data Analysis Techniques: Employ appropriate statistical methods for data analysis that align with the project's objectives. The analysis should be conducted by qualified personnel to ensure that the findings are valid and reliable, ultimately enhancing the credibility of the results. This is particularly pertinent in the context of bioaccess®'s extensive management services for studies, which encompass setup and compliance evaluations to ensure regulatory adherence.
-
Prepare Detailed Reports of Findings: After examining the information, prepare a comprehensive report outlining the research’s findings, including analysis and interpretation. This report must adhere to regulatory requirements, ensuring that it is suitable for submission to relevant authorities and stakeholders. By incorporating structured reporting practices, researchers can effectively communicate their findings and insights, contributing to the advancement of medical technology and ensuring successful outcomes in clinical trials managed by bioaccess®.
Finalizing and Reporting Pilot Study Results
- Prepare a Comprehensive Final Report: Compile all research data, methodology, and findings into a comprehensive final report. This report should detail the study's objectives, methods, results, and conclusions, while adhering to INVIMA guidelines to ensure compliance with Colombian regulatory standards.
- Submit to Regulatory Authorities: Submit the final report to INVIMA (Colombia National Food and Drug Surveillance Institute) and any other relevant regulatory bodies such as ANMAT. Ensure that all documentation is complete and conforms to submission guidelines, given INVIMA's classification as a Level 4 health authority by PAHO/WHO.
- Obtain Ethics Committee Approval: Prior to initiating the trial, ensure that you have obtained the necessary approvals from the ethics committee. This step is crucial for compliance with ethical standards in clinical research.
- Publish Findings in Scientific Journals: Consider submitting the research findings to peer-reviewed scientific journals. This contributes to the scientific community and enhances the credibility of the research, building on your work as a leader in medical technology advancements.
- Present at Conferences: Share the research findings at relevant conferences to engage with other researchers and stakeholders. Presenting findings can foster collaboration and further research opportunities, highlighting the expertise of your team, including insights from Regulatory Affairs experts like Katherine Ruiz.
- Gather Feedback: Solicit input from peers and stakeholders on the findings and methodology. Constructive feedback can offer insights for future projects and enhance research practices, ensuring the alignment of your pilot program with industry standards.
- Plan for Future Research: Based on the findings of the pilot investigation, identify areas for further research or development. Use the insights gained to inform the design of Pilot Clinical Studies for Medtech in Argentina, utilizing the comprehensive clinical trial management services offered by bioaccess®, which include feasibility studies, site selection, and monitoring adverse events throughout the trial process.
Conclusion
The successful initiation of pilot clinical studies in Argentina hinges on a meticulous approach that encompasses:
- Defining objectives
- Assembling a qualified research team
- Navigating regulatory requirements
By establishing clear study goals and assembling a skilled team, researchers can lay a solid foundation for their trials. Furthermore, engaging stakeholders and securing funding are crucial steps that facilitate smoother processes and enhance study viability.
Recruiting participants effectively is another key component, emphasizing the importance of:
- Clear criteria
- Diverse recruitment strategies
By utilizing multiple channels and fostering community trust, researchers can improve participant engagement and retention, contributing to the overall success of the study. Moreover, a robust data management plan is essential for maintaining data integrity and ensuring compliance with regulatory standards, which is vital for the credibility of the findings.
Finally, the culmination of these efforts is reflected in the thorough reporting and dissemination of study results. Preparing comprehensive reports, submitting findings to regulatory bodies, and sharing insights at conferences not only contribute to the scientific community but also pave the way for future research opportunities. By following these essential steps, researchers can significantly enhance their chances of conducting impactful pilot studies that advance healthcare in Argentina. The collective efforts in navigating this complex landscape can ultimately lead to meaningful innovations in medical technology and improved patient outcomes.
Frequently Asked Questions
What is the first step in conducting a pilot project for medical devices?
The first step is to define the objectives, which includes articulating the goals, identifying the medical device for testing, delineating the target population, and outlining expected outcomes.
Why is assembling a qualified research team important?
A qualified research team is essential because the effectiveness of a pilot project relies on the expertise of professionals such as clinical investigators, research coordinators, and information managers who have experience in conducting clinical trials.
What should a research protocol include?
A research protocol should outline the research design, methodology, participant eligibility criteria, and data collection procedures to ensure compliance with regulatory standards.
How can engaging with stakeholders benefit a pilot project?
Engaging with stakeholders, including healthcare providers and regulatory authorities, can enhance the approval process and foster collaboration, which is crucial for the successful execution of the research.
What are the funding options for a pilot project?
Funding options include grants, partnerships with medical device companies, or institutional support, which are vital for covering study-related expenses.
What is the purpose of conducting feasibility assessments?
Feasibility assessments evaluate the availability of participants, resources, and infrastructure to support the clinical trial, helping to anticipate challenges and develop mitigation strategies.
Why is information management important in a pilot project?
A thorough information management strategy is crucial for preserving the integrity of research information, ensuring compliance with privacy regulations, and maintaining the validity and reliability of results.
What local regulations should researchers be aware of when conducting studies in Argentina?
Researchers should familiarize themselves with the regulations set by the National Administration of Drugs, Foods and Medical Technology (ANMAT) to ensure compliance and smooth operations.
What documentation is required for research studies?
Required documentation includes the research protocol, informed consent forms, and investigator qualifications, all prepared in accordance with ANMAT guidelines.
What steps are involved in obtaining ethical approval for a pilot project?
Researchers must submit their research protocol to an Institutional Review Board (IRB) or Ethics Committee for review and approval to ensure adherence to ethical standards.
What is Good Clinical Practice (GCP) and why is it important?
Good Clinical Practice (GCP) encompasses guidelines to ensure proper training for research staff, accurate record-keeping, and prioritization of participant safety throughout the research process.
How should researchers prepare for audits and inspections?
Researchers should keep organized records and thorough documentation to demonstrate compliance with regulatory requirements during potential audits or inspections.
What strategies can improve participant recruitment for clinical trials?
Utilizing multiple recruitment channels, engaging with the community, and maintaining transparent communication throughout the informed consent process can enhance participant recruitment.
Why is monitoring recruitment progress necessary?
Regularly evaluating recruitment metrics ensures that targets are being met and allows for adjustments in strategies if recruitment stalls.
What should be included in a final report after completing a pilot project?
A final report should compile all research data, methodology, findings, and conclusions, adhering to regulatory standards for submission to relevant authorities.
What are the benefits of publishing findings in scientific journals?
Publishing findings contributes to the scientific community and enhances the credibility of the research, establishing the researchers as leaders in medical technology advancements.
How can feedback from peers and stakeholders be utilized?
Gathering feedback can provide insights for future projects and improve research practices, ensuring alignment with industry standards.

