Introduction
In the realm of clinical research, Colombia has emerged as a promising landscape for pilot studies, offering a combination of regulatory efficiency and a commitment to high-quality healthcare. Navigating the intricate regulatory framework is essential for researchers aiming to initiate clinical trials within this vibrant market.
With the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) at the helm of oversight, understanding the necessary steps—from compliance with ethical standards to the meticulous preparation of documentation—becomes paramount.
This article delves into the critical aspects of conducting pilot clinical studies in Colombia, highlighting best practices for:
- Site selection
- Participant recruitment
- Protocol design
- Quality control measures
- Data analysis
By leveraging local expertise and resources, researchers can not only ensure regulatory compliance but also enhance the overall success of their clinical endeavors.
Navigating the Regulatory Framework for Clinical Studies in Colombia
To initiate pilot clinical studies for Medtech in Colombia, researchers must first familiarize themselves with the regulatory bodies involved, primarily the Institute Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA). This regulatory body plays a vital role in supervising research studies, ensuring adherence to health standards and protocols. The following steps outline the process:
- Understand Regulatory Requirements: Review the guidelines provided by INVIMA regarding clinical trials, including the necessary documentation and ethical approvals required before commencing any study.
- Prepare Essential Documents: Compile the required documents such as the Clinical Trial Protocol, Investigator’s Brochure, and informed consent forms. These documents must comply with Colombian regulations and international standards to facilitate a smooth approval process.
- Submit to INVIMA: Submit the compiled documentation to INVIMA for review. Be prepared for potential queries or requests for additional information from the regulatory body, as they ensure that all research meets the required safety and efficacy standards.
- Obtain Ethics Committee Approval: Concurrently, submit your research to a certified ethics committee for review. Their approval is crucial for ethical compliance and participant safety, ensuring that the trial adheres to ethical standards throughout its duration.
- Monitor Regulatory Changes: Stay updated on any modifications in regulations or guidelines that may impact your research. This can be accomplished by routinely reviewing INVIMA’s publications or industry news, along with engaging in forums that address research advancements in Colombia.
- Leverage Comprehensive Services: Utilize extensive trial management services such as feasibility assessments, site selection, project management, and compliance reviews provided by specialized organizations to streamline the process.
- Explore R&D Tax Incentives: Take advantage of Colombia's R&D tax incentives, which include a 100% tax deduction and other financial benefits for investments in science, technology, and innovation projects.
By following these steps and leveraging Colombia's competitive advantages—such as cost efficiency, regulatory speed, high-quality healthcare, and available R&D tax incentives—researchers can effectively conduct pilot clinical studies for Medtech in Colombia, ensuring their preliminary projects are compliant and ethically sound.
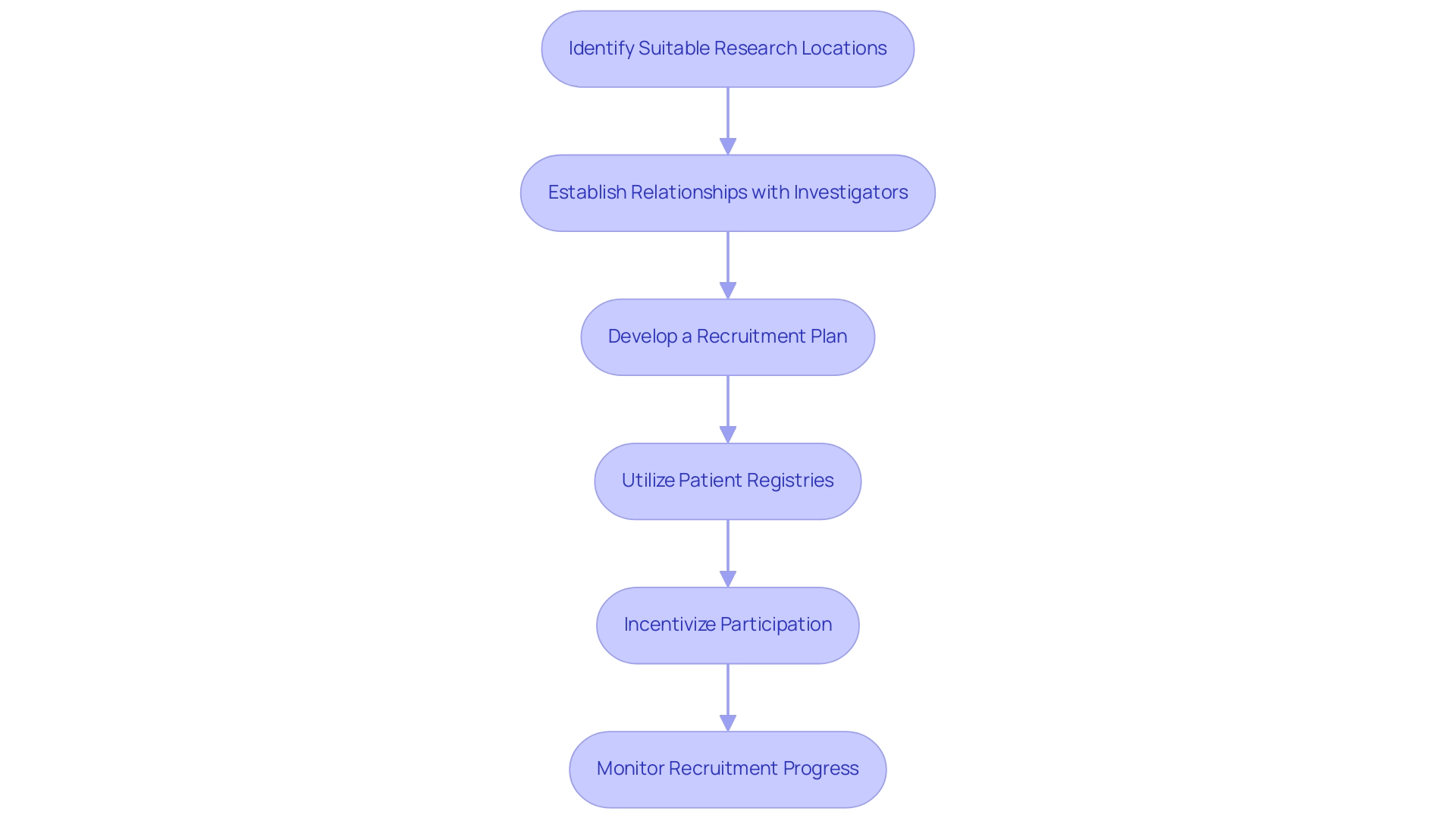
Best Practices for Conducting Pilot Clinical Studies: Site Selection and Recruitment Strategies
When carrying out pilot trials, adherence to best practices for site selection and recruitment is essential:
-
Identify Suitable Research Locations:
Assess potential sites based on their prior experience with studies, available facilities, and access to the target patient group. Consider locations that have effectively carried out similar research in the past, like those partnering with bioaccess®, which has over 20 years of experience in overseeing trials in Latin America.
-
Establish Relationships with Investigators:
Engage with experienced investigators who have a strong track record in clinical research. Their expertise, particularly in early-feasibility and pivotal research, can facilitate smoother execution and enhance recruitment efforts.
-
Develop a Recruitment Plan:
Create a comprehensive recruitment strategy that outlines how participants will be identified and approached.
Utilize various channels such as social media, local healthcare providers, and community outreach to maximize reach, leveraging bioaccess®'s extensive networks and successful collaborations, like those with Caribbean Health Group, to attract participants.
-
Utilize Patient Registries:
Leverage existing patient registries to identify potential participants who meet the research criteria. This can significantly reduce the time required for recruitment, as evidenced by GlobalCare Clinical Trials' partnership with bioaccess® which achieved over a 50% reduction in recruitment time.
-
Incentivize Participation:
Consider offering incentives for participation, such as compensation for travel expenses or providing health check-ups. This can motivate individuals to enroll in the study.
-
Monitor Recruitment Progress:
Regularly assess recruitment efforts and remain flexible in adapting strategies as needed. This may involve revisiting site selection or enhancing outreach efforts, ensuring adherence to local regulations and ethics requirements as managed by bioaccess®, which includes conducting feasibility assessments and compliance reviews.
By implementing these best practices, researchers can optimize site selection and enhance participant recruitment for pilot clinical studies for Medtech in Colombia, which will lead to more successful preliminary trials. With organizations like bioaccess® at the forefront of research management, including Early-Feasibility Studies, Pivotal Studies, and Post-Market Follow-Up Studies, the potential for significant research in Latin America is more promising than ever.
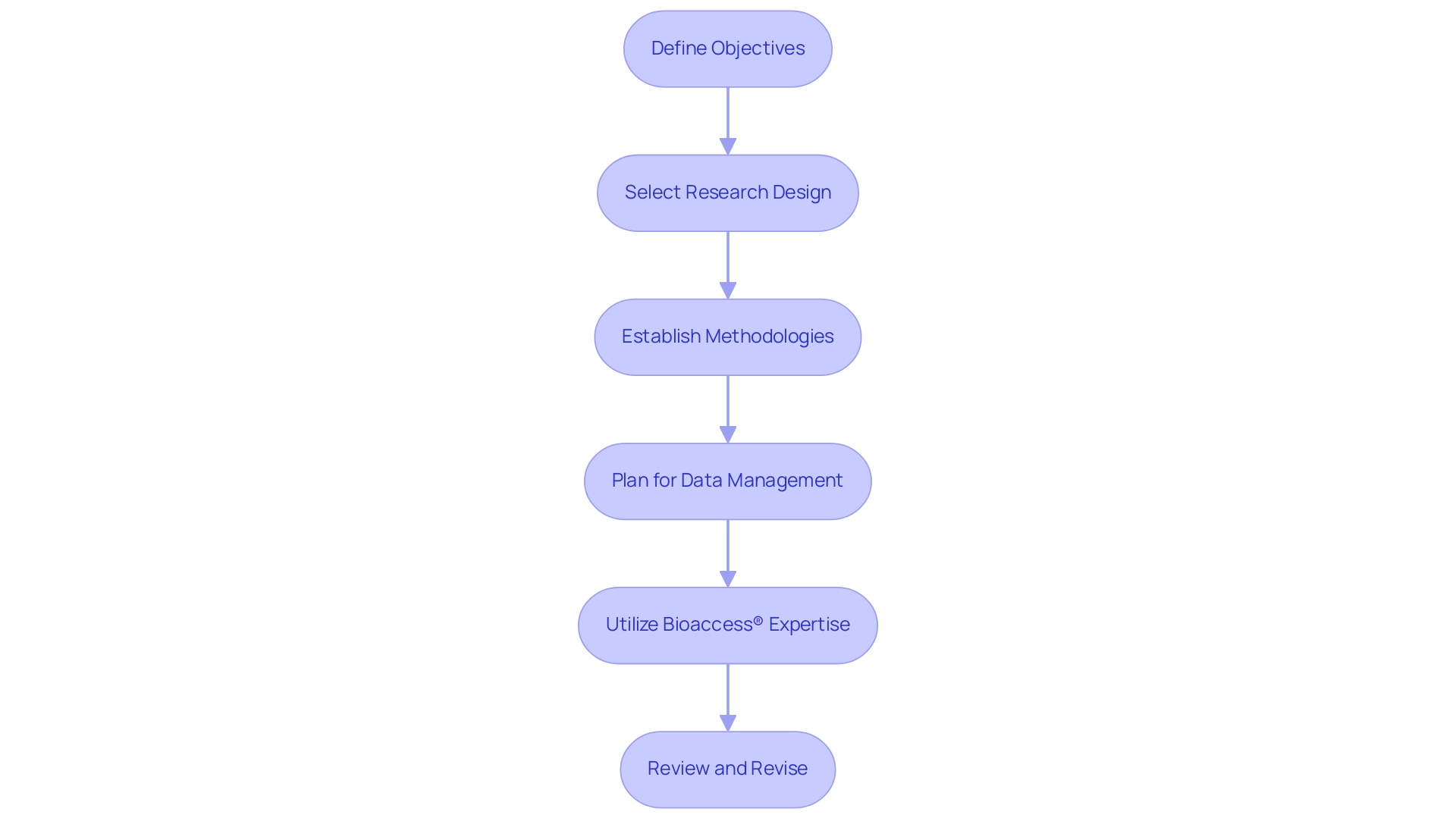
Designing the Pilot Study Protocol
Developing a preliminary research protocol includes various essential steps, particularly when utilizing the extensive clinical management services provided by bioaccess® in Latin America, supported by over 20 years of experience in Medtech:
-
Define Objectives: Clearly outline the primary goals of the trial. What specific questions are you aiming to answer? This will guide the entire research process.
-
Select Research Design: Choose a suitable research framework (e.g., randomized controlled experiment, observational analysis) that aligns with your objectives and the nature of the intervention.
- Determine Sample Size: Calculate the sample size needed to achieve statistically significant results, considering potential dropouts to ensure a representative sample of the target population.
-
Establish Methodologies: Detail the methodologies for data collection, intervention delivery, and outcome measurement. Ensure these methods are feasible and ethically sound, complying with regulations.
-
Plan for Data Management: Develop a data management plan outlining how data will be collected, stored, and analyzed. Ensure compliance with data protection regulations.
-
Utilize Bioaccess® Expertise: Bioaccess® offers customized services for feasibility and location selection, ensuring that you have the appropriate research site and principal investigator (PI) for your preliminary project. Their expertise also includes comprehensive compliance reviews and project management to facilitate smooth trial execution.
-
Review and Revise: Before finalizing the protocol, conduct a thorough review with stakeholders and revise as necessary to address any concerns or gaps. Bioaccess® can aid in examining research documents to ensure adherence to local regulations.
By following these steps and utilizing the expertise of bioaccess®, researchers can develop a strong preliminary research protocol that effectively directs their research endeavors and accelerates Pilot Clinical Studies for Medtech in Colombia.
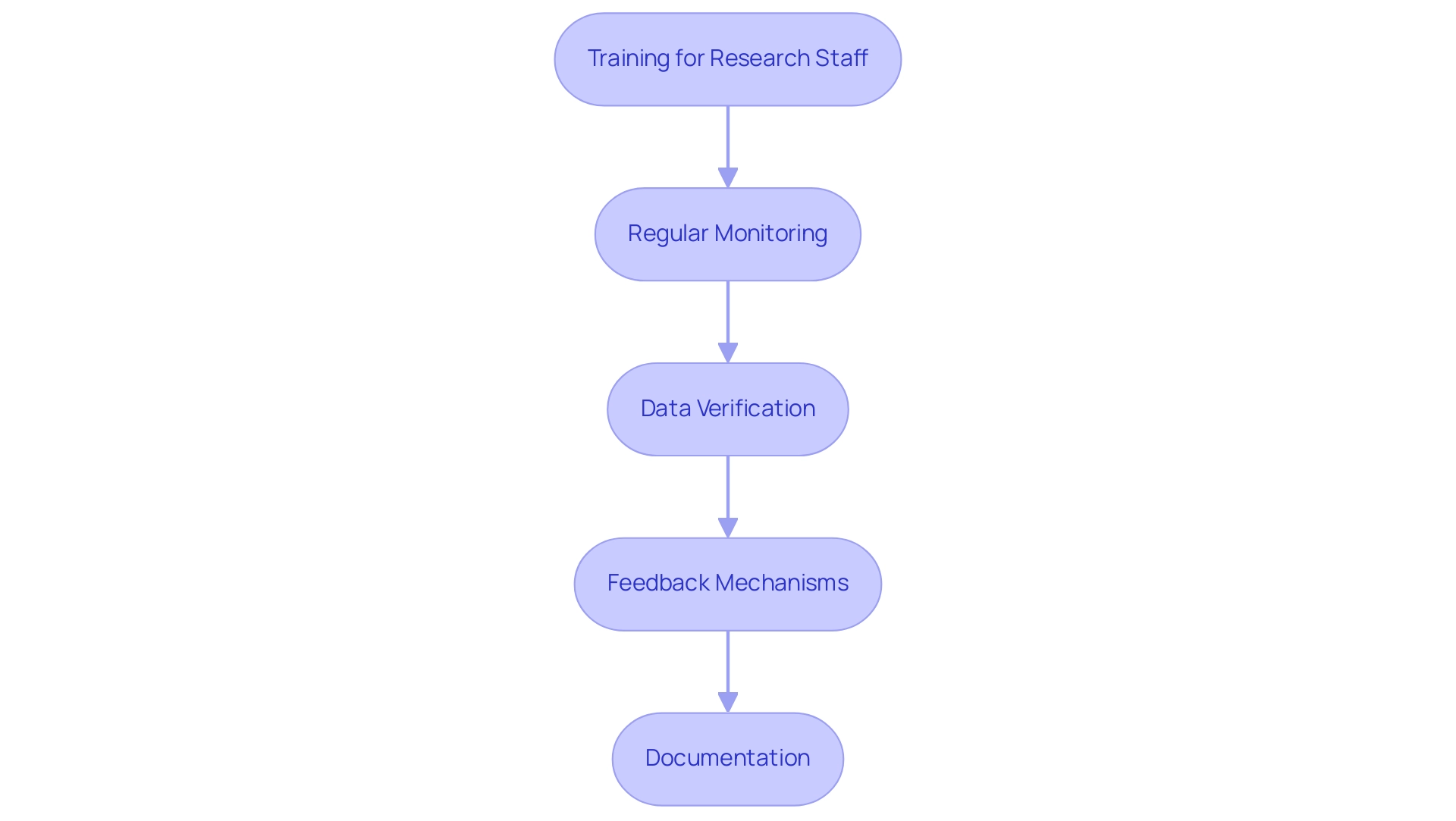
Implementing Quality Control Measures
To safeguard the integrity of your pilot clinical study, it is crucial to implement robust quality control measures that bioaccess® expertly manages, backed by over 20 years of experience in Medtech:
- Training for Research Staff: Comprehensive training for all personnel is vital to ensure a thorough understanding of research protocols, procedures, and ethical considerations. Recent advancements indicate that training for research assistants typically follows a structured three-stage process over a two- to four-week pre-training period. This method improves readiness and greatly minimizes measurement error in medical evaluations, as noted in the case analysis titled 'Recommendations for Future Training,' which stresses the necessity for thorough skill assessments and personalized re-certification schedules.
- Regular Monitoring: Conducting regular monitoring visits to research sites is essential for assessing compliance with the research protocol. These visits, facilitated by bioaccess®, allow for early identification of potential issues, enabling proactive risk mitigation before problems escalate.
- Data Verification: Implementing stringent data verification processes is necessary to guarantee the accuracy and completeness of collected information. This includes double-checking data entries and performing periodic audits to uphold data integrity, as emphasized in recent quality control measures adopted in clinical trials.
- Feedback Mechanisms: Establishing effective feedback mechanisms is crucial for enabling participants and research staff to report any concerns or challenges encountered during the research. Timely feedback can lead to prompt interventions, enhancing the overall learning experience and outcomes.
- Documentation: Maintaining meticulous documentation of all study-related activities is paramount. This includes recording any deviations from the protocol and the corrective actions taken, ensuring transparency and accountability throughout the research process.
By incorporating these quality control measures, along with bioaccess®'s expertise in feasibility studies, compliance reviews, and project management, researchers can significantly enhance the reliability of their pilot clinical studies for Medtech in Colombia, ensuring that the data collected meets the highest standards of quality. As Madhukar H. Trivedi, M.D., states, "thorough training and quality control are essential components of successful medical research," ultimately leading to more trustworthy outcomes. Trust in bioaccess® for a comprehensive management approach to your clinical studies in Latin America.
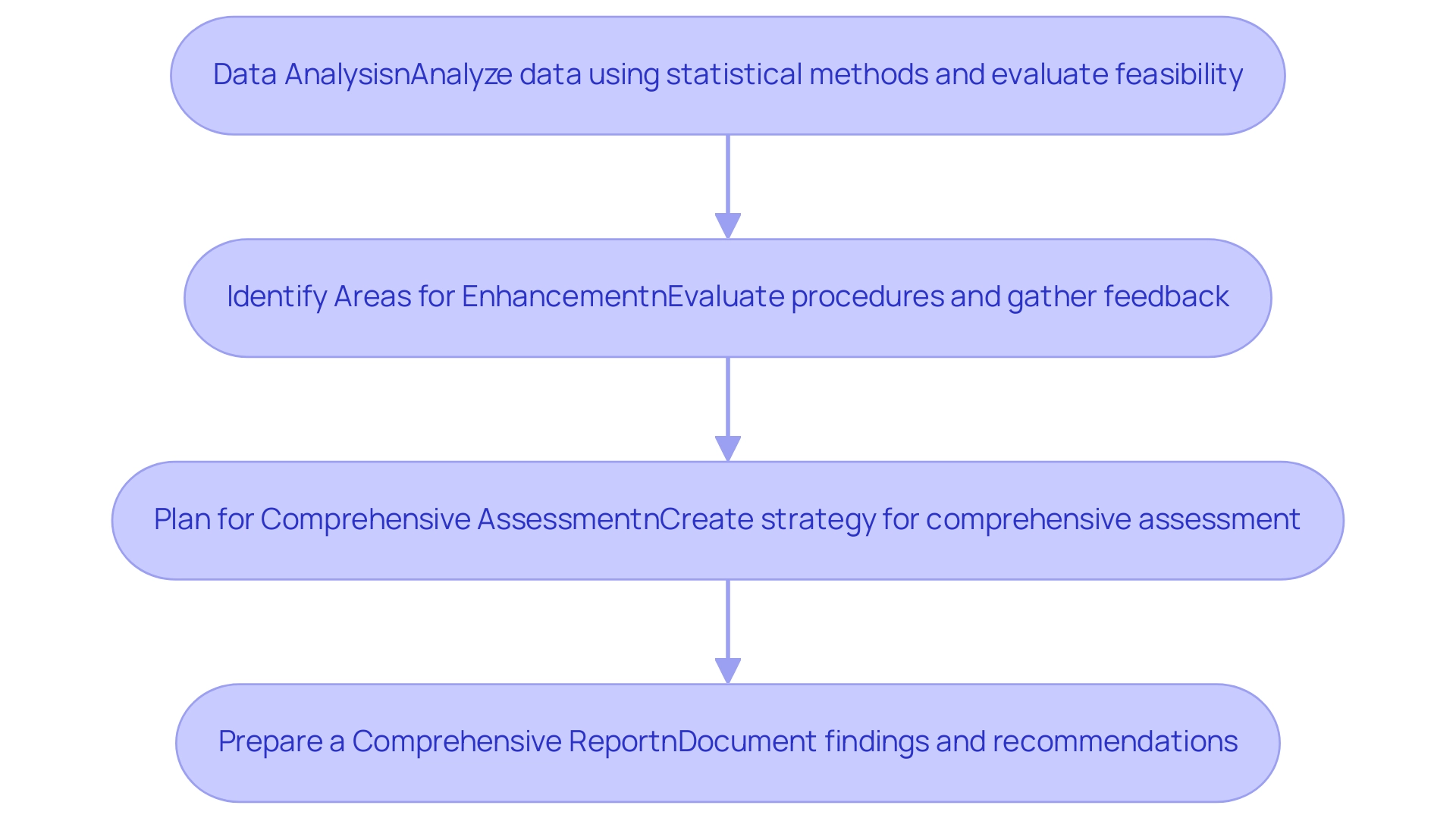
Analyzing Pilot Study Results and Preparing for Full-Scale Trials
After carrying out the preliminary research, follow these steps to analyze results and prepare for full-scale trials:
-
Data Analysis: Analyze the collected data using appropriate statistical methods. Ensure that you evaluate both primary and secondary outcomes as defined in your protocol.
- Evaluate Feasibility: Assess the viability of the research based on participant recruitment, retention rates, and any challenges faced during the initial phase. This evaluation will inform decisions about scaling up and is essential for aligning with the regulatory framework set by INVIMA.
-
Identify Areas for Enhancement: Evaluate the initial project procedures and pinpoint areas that need modifications or enhancements before initiating large-scale experiments. Consider feedback from participants and research staff, and leverage the expertise of bioaccess® in study management, including their services in site selection and monitoring, to enhance these processes.
- Prepare a Comprehensive Report: Document the findings in a comprehensive report that includes methodology, results, and recommendations. This report will be critical for stakeholders and regulatory bodies, particularly in the context of compliance reviews and import permits.
-
Plan for Comprehensive Assessment: Using the preliminary research findings, create a thorough strategy for the comprehensive assessment, integrating insights gained and tackling any recognized deficiencies. Utilize bioaccess®'s services in trial setup and project management to ensure a smooth transition to the next phase of Pilot Clinical Studies for Medtech in Colombia.
By following these steps, researchers can effectively analyze their pilot study results and strategically prepare for subsequent phases of Pilot Clinical Studies for Medtech in Colombia, ensuring adherence to regulatory standards and leveraging specialized knowledge in the field.
Conclusion
Colombia presents a unique opportunity for researchers looking to conduct pilot clinical studies, thanks to its regulatory efficiency and commitment to high-quality healthcare. By understanding the critical steps involved—such as navigating INVIMA's regulatory framework, preparing essential documentation, and obtaining ethics committee approval—researchers can ensure compliance and foster a successful study environment.
The importance of best practices in site selection and participant recruitment cannot be overstated. By leveraging local expertise and resources, including established networks and patient registries, researchers can optimize their recruitment strategies, ultimately leading to more effective pilot studies. Designing a robust study protocol further enhances the likelihood of success, as it lays a clear foundation for objectives, methodologies, and data management.
Quality control measures are essential to maintain the integrity of the study and ensure accurate results. Training research staff, conducting regular monitoring, and implementing stringent data verification processes are all vital components that contribute to the reliability of findings.
Finally, the analysis of pilot study results is crucial for preparing for full-scale trials. By evaluating feasibility, identifying areas for improvement, and documenting findings comprehensively, researchers can effectively transition to subsequent phases of their clinical research endeavors.
In conclusion, Colombia stands out as a strategic location for pilot clinical studies, where researchers can leverage its regulatory advantages and local expertise. By adhering to best practices and maintaining a focus on quality and compliance, the potential for impactful clinical research in this vibrant market is indeed significant.
Frequently Asked Questions
What is the first step to initiate pilot clinical studies for Medtech in Colombia?
The first step is to understand the regulatory requirements by reviewing the guidelines provided by the Institute Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) regarding clinical trials, including necessary documentation and ethical approvals.
What essential documents are required for submitting a clinical trial to INVIMA?
Essential documents include the Clinical Trial Protocol, Investigator’s Brochure, and informed consent forms, all of which must comply with Colombian regulations and international standards.
What should researchers do after preparing their documents?
Researchers should submit the compiled documentation to INVIMA for review and be prepared to respond to any queries or requests for additional information.
Why is it important to obtain ethics committee approval?
Obtaining ethics committee approval is crucial for ensuring ethical compliance and participant safety throughout the trial.
How can researchers stay informed about regulatory changes?
Researchers can stay updated by routinely reviewing INVIMA’s publications, industry news, and engaging in forums that address research advancements in Colombia.
What services can researchers utilize to streamline the trial process?
Researchers can leverage comprehensive trial management services such as feasibility assessments, site selection, project management, and compliance reviews provided by specialized organizations.
What financial incentives are available for R&D in Colombia?
Colombia offers R&D tax incentives, including a 100% tax deduction and other financial benefits for investments in science, technology, and innovation projects.
What factors should be considered when selecting research locations for pilot trials?
Suitable research locations should be assessed based on prior experience with studies, available facilities, and access to the target patient group.
How can researchers enhance participant recruitment for their studies?
Researchers can develop a comprehensive recruitment plan, utilize patient registries, incentivize participation, and regularly monitor recruitment progress to adapt strategies as needed.
What steps should be taken to safeguard the integrity of a pilot clinical study?
Implement robust quality control measures, including training for research staff, regular monitoring, data verification, feedback mechanisms, and meticulous documentation of all study-related activities.
What should researchers do after completing preliminary research?
Researchers should analyze collected data, evaluate feasibility, identify areas for enhancement, prepare a comprehensive report, and plan for a thorough assessment for the next phases of their studies.

