Introduction
In Chile, the burgeoning Medtech sector is poised for significant advancements, yet navigating the regulatory landscape can be a formidable challenge for researchers. With a framework governed primarily by the Chilean Ministry of Health and the National Commission for Scientific and Technological Research, understanding the intricacies of conducting pilot clinical studies is essential.
This article delves into the key regulatory requirements, step-by-step processes, and the importance of stakeholder engagement, while also highlighting effective budgeting and quality control strategies. By providing a comprehensive overview of these critical components, researchers can better position themselves to successfully execute their studies and contribute to the evolving field of medical technology in Chile.
Understanding the Regulatory Framework for Medtech in Chile
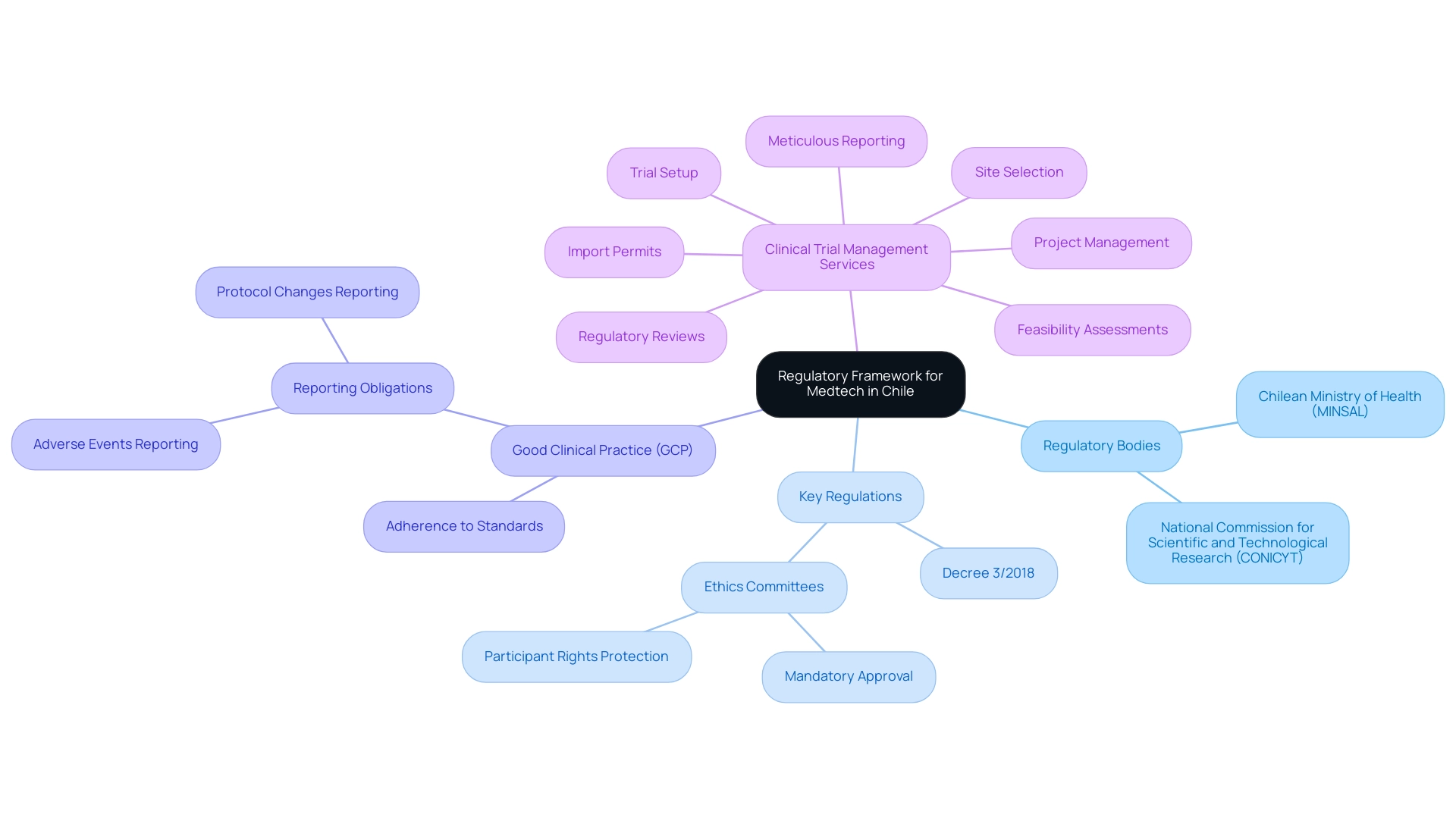
In Chile, pilot clinical studies for Medtech require navigating a regulatory framework primarily overseen by the Chilean Ministry of Health (Ministerio de Salud, MINSAL) and the National Commission for Scientific and Technological Research (CONICYT). To successfully manage this research, researchers must understand the following key regulations:
- Decree 3/2018: This decree outlines the guidelines for conducting medical research in Chile, including essential requirements for ethical approval and informed consent.
- Ethics Committees: Approval from a recognized ethics committee is mandatory, ensuring the protection of participant rights and welfare throughout the study.
- Good Clinical Practice (GCP): Following GCP guidelines is essential, involving adherence to globally acknowledged standards for the design, execution, documentation, and reporting of trials.
- Reporting Obligations: Researchers are required to promptly report adverse events and any significant changes to the protocol to the regulatory authorities.
To navigate this complex landscape effectively, leveraging comprehensive clinical trial management services like those offered by bioaccess® can be invaluable. Their expertise includes feasibility assessments, site selection, regulatory reviews, trial setup, import permits, project management, and meticulous reporting. Bioaccess® specializes in various research types, including Pilot Clinical Studies for Medtech in Chile, Early-Feasibility Assessments, First-In-Human Trials, and Post-Market Clinical Follow-Up Evaluations (PMCF).
Furthermore, Katherine Ruiz, a Regulatory Affairs expert with extensive experience in medical devices and in vitro diagnostics, can provide guidance in overcoming regulatory hurdles, competition, and recruitment challenges often faced by Medtech startups. Comprehending these regulations and seeking advice from specialists is crucial for guaranteeing adherence and promoting the successful implementation of trials.
Step-by-Step Process for Conducting Pilot Clinical Studies in Chile
Carrying out preliminary clinical trials in Chile with bioaccess® involves several key steps:
-
Define the Research Objective: Clearly outline the purpose of the pilot project, including the hypothesis, objectives, and expected outcomes.
-
Develop the Research Protocol: Create a detailed research protocol that includes the design, methodology, participant criteria, and data collection methods, ensuring alignment with regulatory requirements.
- Obtain Ethical Approval: Submit the research protocol to an ethics committee for review and approval. Address any feedback provided by the committee to ensure compliance with ethical standards.
-
Recruit Participants: Create a recruitment strategy that details how participants will be chosen and notified about the research, ensuring that informed consent is obtained from all participants.
-
Conduct the Research: Implement the research according to the approved protocol. Monitor the research closely to ensure adherence to Good Clinical Practice (GCP) guidelines and promptly address any issues that arise during the research.
-
Data Management and Analysis: Collect and manage data systematically. Utilize statistical methods to analyze the results and draw conclusions based on the findings.
-
Reporting Results: Prepare a comprehensive report detailing the research's findings, methodologies, and conclusions. Share the results with stakeholders, including regulatory bodies and the ethics committee.
-
Follow-Up and Ongoing Enhancement: After the research, carry out follow-up evaluations to gauge the effect of the findings and pinpoint areas for enhancement in future investigations.
By utilizing bioaccess®'s expertise in preliminary trials and comprehensive trial management services—including feasibility assessments, site selection, adherence reviews, trial setup, import permits, project management, and reporting—researchers can effectively pilot clinical studies for medtech in Chile. Our adaptability and specialized expertise in medical device research guarantee adherence to regulations while promoting medical knowledge and enhancing patient outcomes.
Identifying and Engaging Stakeholders
The success of pilot clinical studies for Medtech in Chile depends on involving stakeholders, which promotes an environment favorable to cooperation and adherence. Key stakeholders involved in this process include:
- Investigators: Collaborating with lead researchers who have knowledge in the pertinent medical field guarantees that the research is methodologically rigorous and follows best practices. Our extensive clinical trial management services encompass feasibility assessments and site selection to identify the most suitable investigators.
- Regulatory Bodies: Establishing transparent communication with regulatory authorities like INVIMA is crucial for ensuring compliance with applicable regulations. INVIMA's oversight, particularly in medical device matters, ensures that all trial protocols adhere to national health standards, facilitating the necessary approvals. Compliance reviews are integral to this process, ensuring that all aspects of the trial meet regulatory requirements.
- Ethics Committees: Collaborating closely with ethics committees ensures that ethical considerations are prioritized throughout the research, thereby enhancing participant protection and trust. Notably, a recent international analysis on biomedical research ethics revealed that the U.S. is unique in prohibiting institutional review boards from considering social implications, indicating a need for policy changes to incorporate these considerations into research ethics frameworks.
- Participants: Early engagement with potential participants helps to build trust and encourage their involvement. Offering clear, accessible information about the research's purpose and benefits is essential for informed consent. Furthermore, emphasizing the positive effects of Medtech research on local economies—such as job creation and healthcare enhancement—can encourage participation.
- Sponsors: For research involving external funding, maintaining regular communication with sponsors keeps them informed of progress and any challenges encountered, fostering transparency and support. This is essential for maintaining collaboration and guaranteeing the successful implementation of the project.
The importance of stakeholder engagement is underscored by recent findings that highlight how diverse perspectives contribute to richer ethical assessments in biomedical research. As noted by Mark A. Rothstein, Director of Translational Bioethics, there is a growing consensus within the international research community that the societal implications of biomedical research must be considered: "This international collaboration indicates an overwhelming consensus that the societal and long-term implications of biomedical research are extraordinarily important and should be considered in ethics assessments." By skillfully recognizing and involving these stakeholders, researchers can develop a cooperative structure that greatly improves the implementation and results of pilot clinical studies for Medtech in Chile.
Furthermore, our extensive services encompass detailed reporting on research status, inventory, and both serious and non-serious adverse events, ensuring all aspects of the trial are monitored and documented.
Budgeting and Resource Allocation
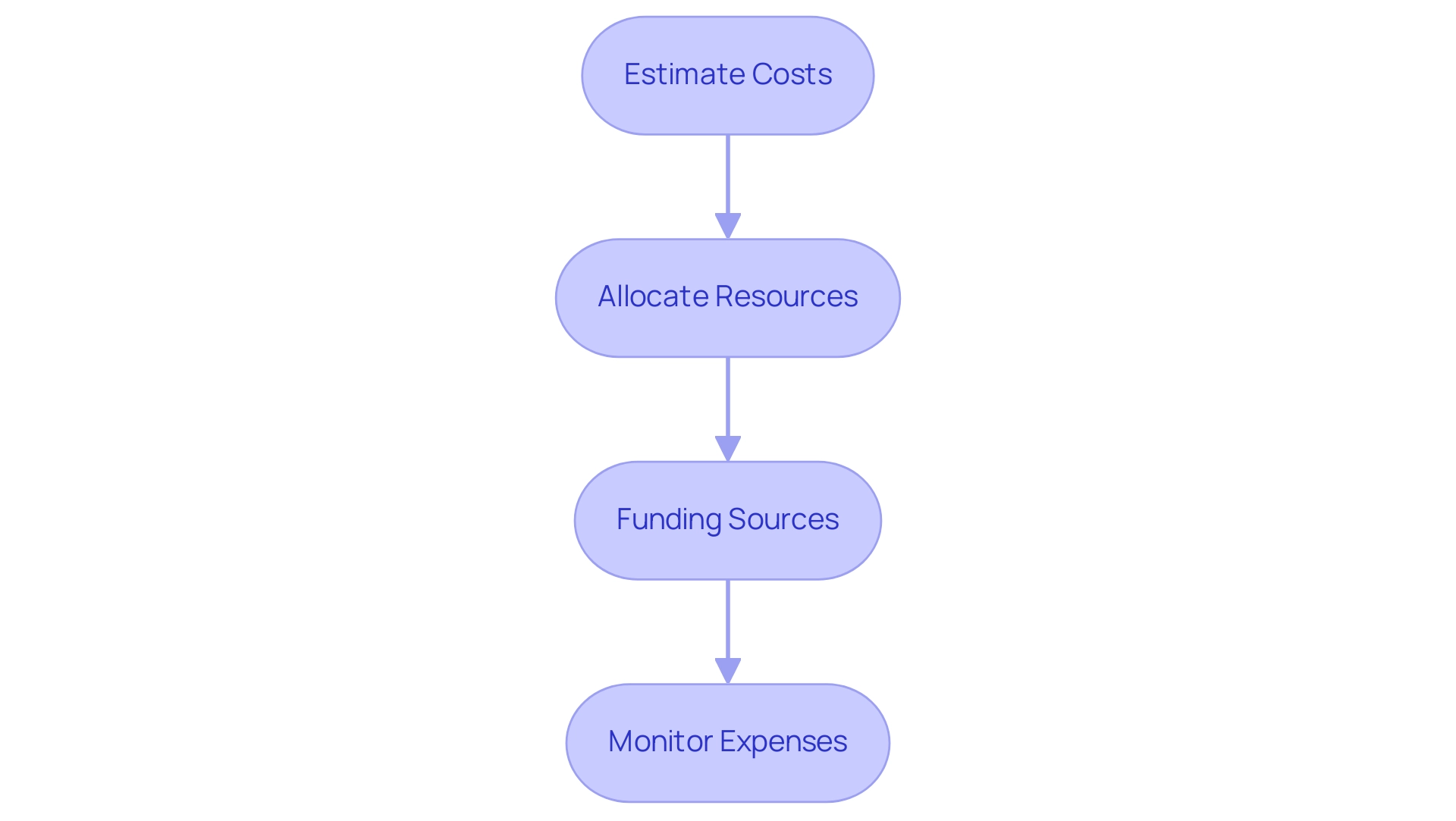
Creating a budget for a pilot clinical trial involves several steps:
-
Estimate Costs: Identify all potential costs associated with the research, including personnel, equipment, materials, participant compensation, and administrative expenses. bioaccess® provides comprehensive support in this area, leveraging over 20 years of experience in Medtech to ensure all financial aspects are thoroughly considered.
-
Allocate Resources: Determine how resources will be distributed across different phases of the research, ensuring that key areas are adequately funded. With expertise in managing Early-Feasibility, First-In-Human, and Pilot Clinical Studies for Medtech in Chile, as well as Pivotal and Post-Market Follow-Up Studies, bioaccess® helps optimize resource allocation for successful outcomes.
-
Funding Sources: Explore potential funding sources, such as grants from government agencies, private foundations, or industry partnerships. Prepare proposals as needed to secure funding. Our team is well-versed in compliance reviews and can guide you on how to align your proposals with country requirements, including the feasibility and selection of research sites and principal investigators (PIs).
-
Monitor Expenses: Implement a system for tracking expenses throughout the research to ensure that the budget is adhered to and to identify any areas where adjustments may be necessary. bioaccess® provides strong project management and reporting services, including evaluation and input on research documents to adhere to national regulations, keeping you updated on project status, inventory, and adverse events, thereby ensuring financial viability.
By meticulously planning and distributing resources, researchers can improve the chances of successfully carrying out Pilot Clinical Studies for Medtech in Chile while upholding financial sustainability. This method ultimately aids in job creation, economic expansion, and enhanced healthcare results in the local economy, as initial experiments play a crucial role in advancing medical technologies and promoting international cooperation.
Ensuring Quality Control and Compliance
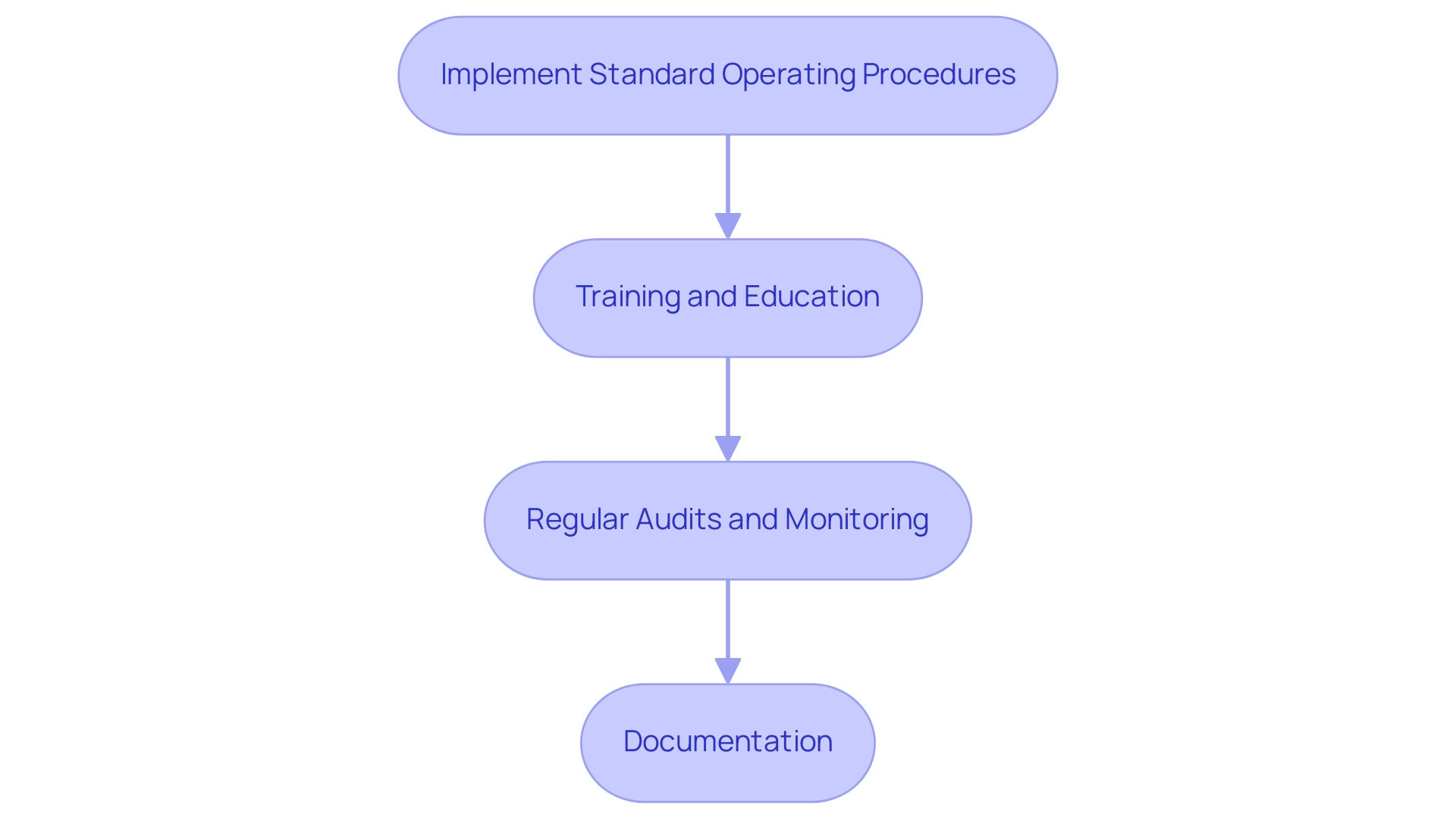
To ensure quality management and adherence during trial investigations, researchers should:
- Implement Standard Operating Procedures (SOPs): Develop and adhere to SOPs for all study-related activities to ensure consistency and compliance with regulatory requirements, as supported by bioaccess®’s expertise in managing research trials.
- Training and Education: Provide ongoing training for all team members involved in the research to ensure they are aware of GCP guidelines and the specific requirements of the protocol, facilitated by bioaccess®’s comprehensive training services tailored to clinical research.
- Regular Audits and Monitoring: Conduct regular audits and monitoring of research activities to identify any deviations from the protocol and implement corrective actions as needed. This aligns with bioaccess®’s commitment to rigorous project management and oversight, including site selection and feasibility studies.
- Documentation: Maintain thorough documentation of all research processes, including participant consent forms, data collection methods, and any adverse events. This documentation is critical for regulatory compliance and future reference, reinforcing the importance of meticulous reporting as highlighted by bioaccess®.
By prioritizing quality control and compliance, along with leveraging the comprehensive clinical trial management services of bioaccess®, including feasibility studies and site selection, researchers can enhance the credibility of their pilot clinical studies for Medtech in Chile and contribute to the advancement of medical knowledge.
Conclusion
Navigating the regulatory landscape for Medtech in Chile is a complex yet vital undertaking for researchers aiming to conduct pilot clinical studies. Understanding the key regulations, such as Decree 3/2018, and obtaining necessary ethical approvals are essential steps in ensuring compliance and protecting participant welfare. The importance of adhering to Good Clinical Practice (GCP) guidelines cannot be overstated, as these standards safeguard the integrity of the research process and the validity of the results.
Effective stakeholder engagement is another cornerstone of successful pilot studies. Collaborating with investigators, regulatory bodies, ethics committees, participants, and sponsors fosters a collaborative environment that enhances the study’s execution and outcomes. By prioritizing transparent communication and building trust among all parties involved, researchers can significantly improve the likelihood of achieving their objectives.
Additionally, meticulous budgeting and resource allocation are critical for the sustainability and success of pilot studies. By estimating costs, allocating resources wisely, and monitoring expenses, researchers can ensure that their studies are not only financially viable but also impactful in advancing medical technologies. Coupled with stringent quality control measures and ongoing training, these practices position researchers to contribute meaningfully to the Medtech sector in Chile.
In conclusion, mastering the intricacies of the regulatory framework, engaging stakeholders, and implementing robust financial and quality control strategies are all integral components that empower researchers to navigate the challenges of conducting pilot clinical studies. By embracing these elements, they can play a pivotal role in the evolution of medical technology, ultimately benefiting both the healthcare system and society at large.
Frequently Asked Questions
What regulatory bodies oversee pilot clinical studies for Medtech in Chile?
The regulatory framework for pilot clinical studies in Chile is primarily overseen by the Chilean Ministry of Health (MINSAL) and the National Commission for Scientific and Technological Research (CONICYT).
What is Decree 3/2018, and why is it important?
Decree 3/2018 outlines the guidelines for conducting medical research in Chile, including requirements for ethical approval and informed consent. It mandates approval from a recognized ethics committee to protect participant rights and welfare.
What are Good Clinical Practice (GCP) guidelines?
Good Clinical Practice (GCP) guidelines are globally acknowledged standards for the design, execution, documentation, and reporting of clinical trials. Researchers must adhere to these guidelines and report adverse events and significant protocol changes to regulatory authorities.
How can bioaccess® assist in managing pilot clinical studies in Chile?
Bioaccess® offers comprehensive clinical trial management services, including feasibility assessments, site selection, regulatory reviews, trial setup, import permits, project management, and meticulous reporting, specifically for Pilot Clinical Studies for Medtech in Chile.
What are the key steps involved in carrying out preliminary clinical trials in Chile?
The key steps include defining the research objective, developing the research protocol, recruiting participants, conducting the research, managing and analyzing data, reporting results, and following up for ongoing enhancement.
Why is stakeholder engagement important in pilot clinical studies?
Engaging stakeholders such as investigators, regulatory bodies, ethics committees, participants, and sponsors fosters cooperation, ensures compliance with regulations, enhances participant protection, and promotes transparency throughout the research process.
What factors should be considered when creating a budget for a pilot clinical trial?
Researchers should estimate costs, allocate resources, explore funding sources, and monitor expenses to ensure financial sustainability and successful execution of the trial.
What practices ensure quality management and adherence during trial investigations?
Implementing Standard Operating Procedures (SOPs), providing ongoing training, conducting regular audits and monitoring, and maintaining thorough documentation are essential practices for ensuring quality management and compliance.
How does bioaccess® support quality control in clinical studies?
Bioaccess® supports quality control through expertise in managing research trials, offering training services, conducting audits, and emphasizing the importance of meticulous documentation for regulatory compliance.




