Introduction
In the dynamic landscape of clinical research, Argentina presents unique opportunities and challenges for initiating pilot studies. As the healthcare sector continues to evolve, understanding the structured steps required to successfully navigate this process is paramount.
From defining clear study objectives to securing ethical approvals and funding, each phase plays a critical role in ensuring the integrity and efficacy of clinical trials. Moreover, as regulatory frameworks become increasingly complex, researchers must be equipped with effective strategies for participant recruitment and data management to optimize their studies.
By adhering to best practices and leveraging expert support, stakeholders can enhance their chances of achieving successful outcomes in this burgeoning field.
Essential Steps for Initiating Pilot Clinical Studies in Argentina
To successfully commence pilot medical trials in Argentina, it is essential to follow these structured steps:
- Define the Objectives: Begin by clearly articulating the goals of the pilot project, including the specific medical device being tested and the anticipated clinical outcomes. A well-defined objective provides clarity and direction throughout the research process.
- Develop a Research Protocol: Construct a comprehensive research protocol outlining the methodology, eligibility criteria, and statistical analysis plans. It is imperative that the protocol adheres to local regulations to ensure integrity and compliance throughout the study.
- Obtain Ethical Approval: Submit the meticulously prepared research protocol to an ethics committee for rigorous review and approval. This step is critical in safeguarding the rights and well-being of participants, thereby reinforcing the ethical foundation of the research.
- Secure Funding: Identify and pursue potential funding sources, such as grants, sponsorships, or institutional support. Adequate financial backing is crucial for facilitating the smooth execution of the study.
- Recruit a Qualified Research Team: Assemble a group of skilled researchers and medical personnel, ensuring they possess the necessary expertise and experience to effectively carry out the investigation. A competent team enhances the credibility and execution of the trial.
- Conduct Pre-Study Activities: Execute site assessments and training sessions to prepare the research team for the study protocol and device usage. These activities guarantee that the team is well-prepared, enabling a smooth commencement of the study.
Moreover, it is significant that when suitable patients are educated about research studies by their doctors, they show a willingness to participate more than 50% of the time. This statistic underscores the importance of effective communication and engagement strategies in recruitment efforts. Healthcare experts, especially nurses, play an essential role in this process by informing patients about research studies and addressing personal factors and beliefs that may impede participation, as emphasized by Hillyer et al.
By employing evidence-based interventions, such as the teach-back method, nurses can enhance patient understanding and comfort with study participation. By systematically following these steps and utilizing the comprehensive research management services provided by bioaccess®, including feasibility studies, site selection, compliance reviews, and project management, researchers can establish a solid foundation for pivotal studies for medical device approval in Argentina. Additionally, bioaccess® specializes in Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), offering crucial assistance in navigating the regulatory framework to ensure compliance and successful results in clinical research.
Navigating the Regulatory Framework for Medical Device Trials in Argentina
Navigating the regulatory framework for medical device trials in Argentina requires a strategic approach that encompasses several essential considerations:
- Understand ANMAT Regulations: It is crucial to familiarize yourself with the regulations established by the National Administration of Medicines, Food and Medical Technology (ANMAT), the governing body responsible for the approval of medical devices in Argentina. Staying informed about these regulations ensures compliance and facilitates a smoother process. For instance, in 2023, approximately 75% of medical device submissions were approved within the standard timeline, showcasing the effectiveness of adhering to ANMAT guidelines.
- Classify the Medical Device: Accurately classifying the medical device as Class I, II, or III is vital, as this classification dictates the level of regulatory scrutiny and the specific documentation required for approval. Understanding the nuances of each class helps streamline the submission process. Recent statistics indicate that Class II devices account for nearly 60% of all submissions, highlighting the need for precise classification.
- Prepare Submission Dossier: Assemble a comprehensive submission dossier that encompasses all necessary technical documentation, clinical data, and risk assessments. Adhering to ANMAT guidelines in your dossier preparation is imperative to meet regulatory standards. An analysis involving a recent Class II device examination demonstrated that thorough documentation reduced approval delays by 30% compared to less detailed submissions.
- Engage with Regulatory Authorities: Establish and maintain open lines of communication with ANMAT throughout the approval process. Proactively addressing any queries or additional requirements helps foster a collaborative relationship that can expedite the approval timeline. Regulatory expert Dr. Juan Pérez emphasizes, "Maintaining transparency with ANMAT can significantly enhance the chances of a timely approval," which aligns with bioaccess®'s commitment to supporting clients through effective communication.
- Monitor Compliance: Once the experiment is underway, it is essential to continuously monitor compliance with all regulatory requirements. Vigilance in this area helps avoid breaches that could jeopardize the integrity of the study. According to a recent report, 20% of tests encountered compliance issues due to insufficient monitoring, highlighting the significance of this step.
By utilizing bioaccess®'s knowledge, which extends beyond 20 years in overseeing studies—including Early-Feasibility, First-In-Human, Pilot, and Post-Market Follow-Up Studies—researchers can improve their chances of a smooth approval process for pivotal studies for medical device approval in Argentina. Our service capabilities encompass project management, feasibility assessments, site selection, and compliance reviews, ensuring a comprehensive approach to study management. As noted by the Argentine Chamber of Medical Specialties, “Travel expenses must be reasonable and limited to the days on which the scientific or professional event is planned to be held,” highlighting the importance of aligning research activities with ethical standards.
Strategies for Effective Participant Recruitment in Clinical Trials
To enhance participant recruitment for research trials, several effective strategies should be employed:
- Define Target Population: GlobalCare Clinical Trials clearly delineated the characteristics of their target population, aligning with research objectives and eligibility criteria. This focus helped them identify suitable participants and enhance recruitment efficiency, addressing common issues like overoptimistic recruitment estimates.
- Utilize Multiple Recruitment Channels: By employing a variety of recruitment channels, including social media and community outreach, Global Care broadened its reach to potential participants. Their recent campaigns on social media demonstrated a growing effectiveness in engaging diverse populations in clinical research.
- Engage with Patient Advocacy Groups: Collaborating with patient advocacy organizations allowed GlobalCare to access established networks and foster trust within the community. These groups provided insights into the needs of potential participants, facilitating better communication and recruitment efforts.
- Provide Clear Information: GlobalCare created informative materials that succinctly explained the project's purpose, procedures, and potential benefits. By addressing participants' concerns in an accessible manner, they alleviated apprehensions and encouraged involvement, as emphasized in their structured checklist for trial feasibility assessments.
- Offer Incentives: Global Care offered incentives such as compensation for time and travel, motivating individuals to participate and remain engaged. This approach not only aided recruitment but also supported participant retention, contributing to their remarkable reduction in recruitment time by over 50% and a retention rate exceeding 95%.
- Build Trust: Establishing rapport with potential participants was vital for Global Care. By demonstrating transparency regarding the research goals and commitment to participant well-being, they fostered a trusting environment. Bridging the gap between protocol writers and implementation teams was crucial in overcoming recruitment challenges.
By implementing these strategies, GlobalCare Clinical Trials significantly improved participant recruitment and retention, contributing to more reliable research outcomes. Their collaboration with bioaccess™ not only expanded service offerings but also substantially enhanced their operational efficiency in Latin America, showcasing the effectiveness of these strategies.
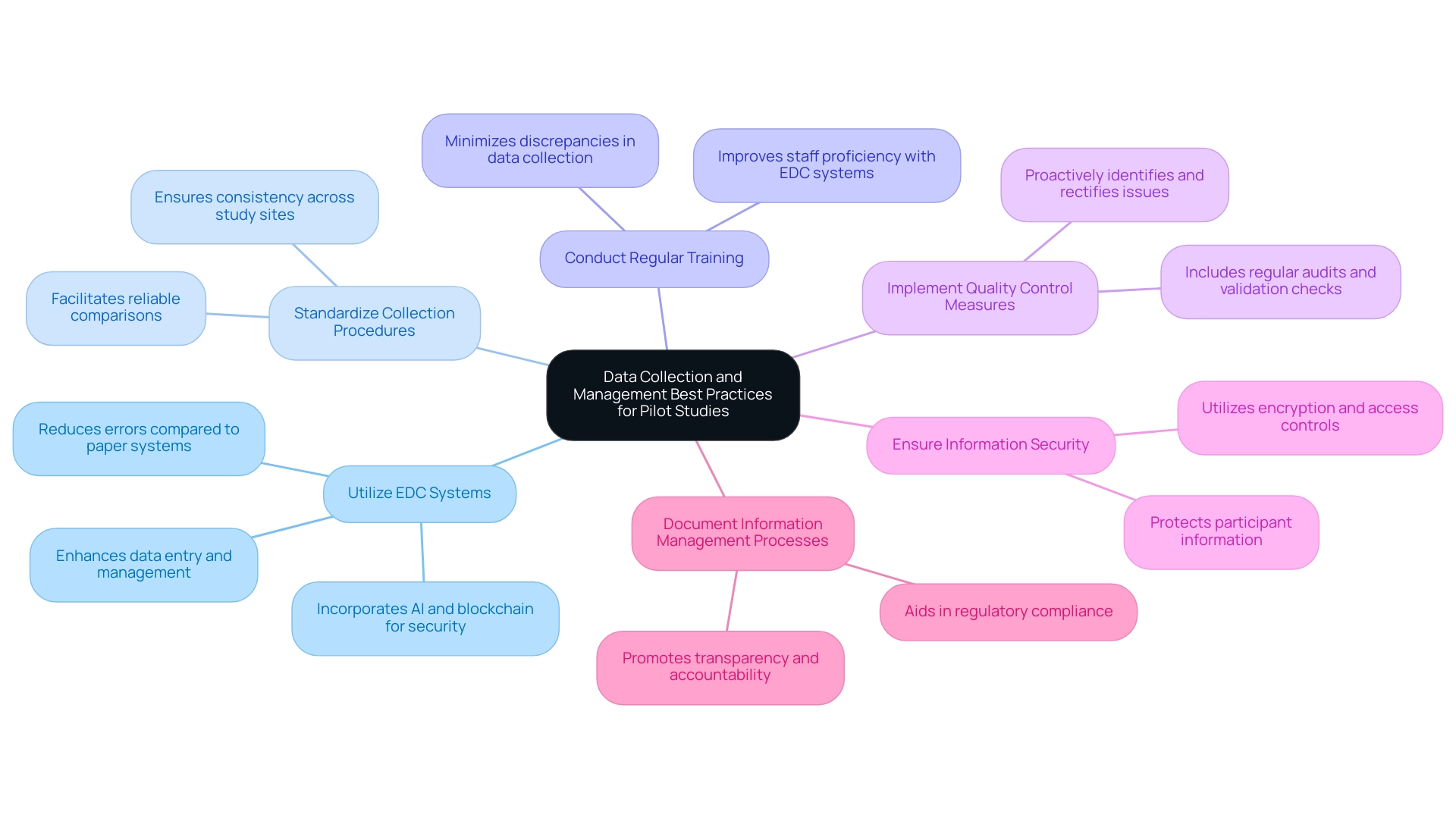
Data Collection and Management Best Practices for Pilot Studies
To uphold rigorous standards in information collection and management during pilot studies, it is essential to implement the following best practices:
- Utilize Electronic Information Capture (EDC) Systems: Leveraging EDC systems significantly enhances entry, management, and storage capabilities. This shift from traditional paper-based systems reduces the likelihood of errors and fosters a more accurate dataset, which is critical for the integrity of research outcomes. Recent advancements, such as AI-driven information analysis and blockchain security, further enhance the functionality of EDC systems, ensuring more secure and efficient handling.
- Standardize Collection Procedures: It is imperative to develop and strictly adhere to standardized collection protocols. This consistency across all study sites ensures reliable comparisons and more robust results, aiding in the overall success of the study.
- Conduct Regular Training: Ongoing training sessions for research staff on effective information collection techniques and EDC system utilization are vital. Such training minimizes discrepancies and bolsters information accuracy, which is crucial for achieving reliable findings.
- Implement Quality Control Measures: Establishing comprehensive quality control processes, including regular audits and thorough information validation checks, is necessary to swiftly identify and rectify any emerging issues. This proactive approach is essential for upholding high information quality standards.
- Ensure Information Security: Protecting participant information is paramount. Implementing robust security measures—such as encryption protocols and strict access controls—safeguards sensitive information and enhances the trustworthiness of the research process.
- Document Information Management Processes: Keeping detailed records of all information management procedures promotes transparency and accountability throughout the research. This practice not only aids in adhering to regulations but also improves the trustworthiness of the information gathered.
Given recent reports emphasizing how EDC systems have revolutionized studies by improving information precision and oversight, following these best practices becomes even more essential. By implementing these strategies, researchers can ensure the integrity and reliability of the data collected during pilot projects, ultimately contributing to the success of clinical trials. Furthermore, statistics indicate that the use of EDC systems in research has increased significantly, highlighting their effectiveness and necessity in contemporary pilot projects.
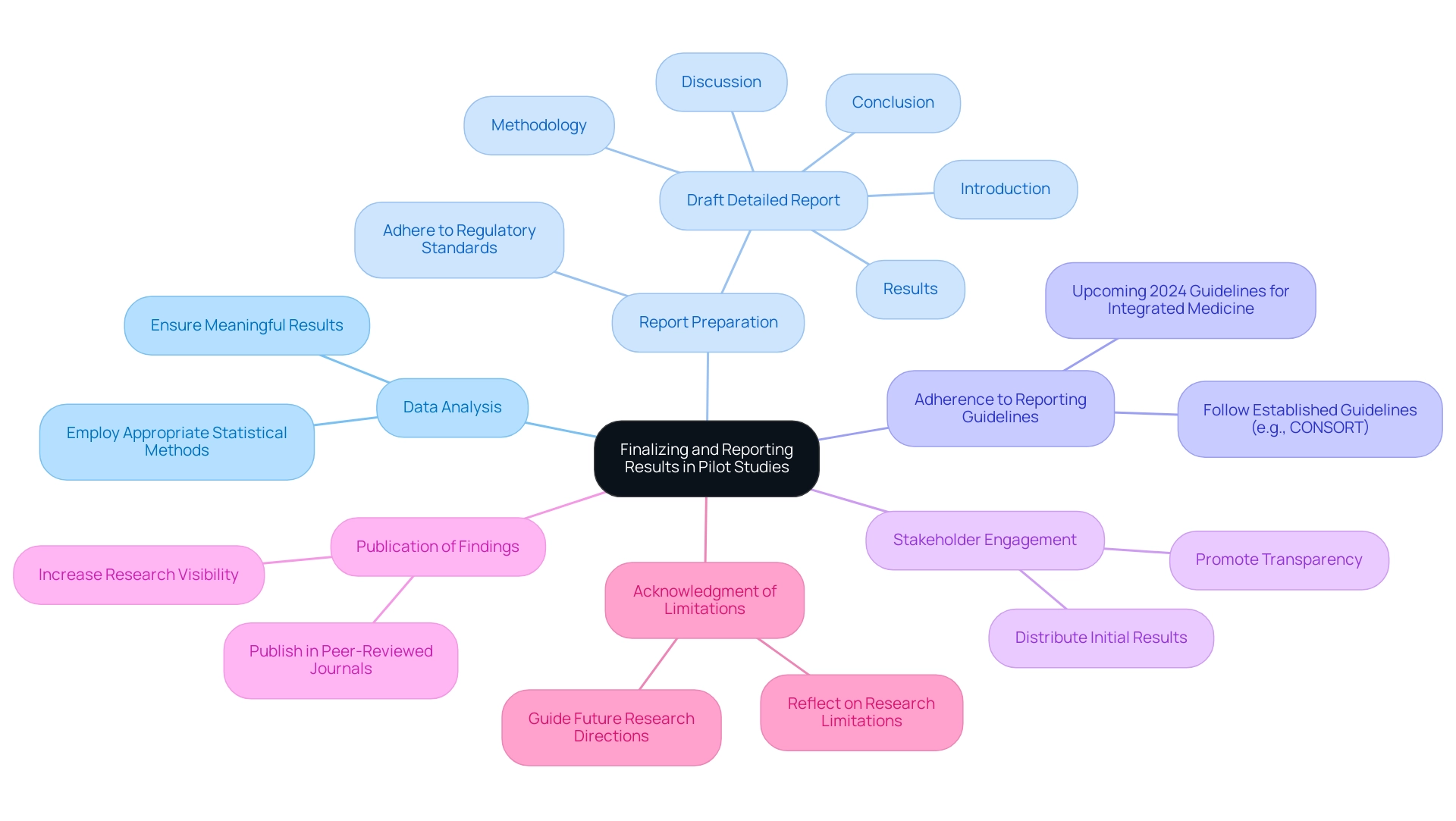
Finalizing and Reporting Results: Key Considerations for Pilot Studies
Finalizing and reporting results from pilot studies requires careful attention to several key aspects:
- Thorough Data Analysis: It is crucial to employ appropriate statistical methods that align with the goals of the research. Accurate analysis ensures that the results are both meaningful and actionable.
- Comprehensive Report Preparation: Drafting a detailed report is essential. This report should encompass an introduction, methodology, results, discussion, and conclusion, presenting a clear narrative of the findings, while also adhering to the rigorous standards set by regulatory bodies.
- Adherence to Reporting Guidelines: Following established reporting guidelines, such as CONSORT, is vital for enhancing the transparency and comparability of results. Recent developments suggest that new guidelines for reporting medical trials, particularly those integrating Chinese and Western medicine, are scheduled to be published in 2024. As Zhao-Xiang Bian notes, "The group plans to publish the guidance as an open access document, which the group plans for 2024," underscoring the importance of these guidelines for the field.
- Stakeholder Engagement: Keeping stakeholders informed is important. Distributing initial results with funding organizations and regulatory bodies promotes transparency and can enable future partnerships, especially in local economies affected by clinical research.
- Publication of Findings: Publishing results in peer-reviewed journals not only contributes to the scientific community but also increases the visibility of the research, prompting further inquiry and application. This aligns with the efforts of bioaccess® in promoting international collaboration and recognition.
- Acknowledgment of Research Limitations: It is essential to reflect on and acknowledge any limitations within the research. This transparency helps to contextualize the findings and can guide future research directions.
Additionally, bioaccess® specializes in various research types, including Early-Feasibility Evaluations (EFS), First-In-Human Trials (FIH), Pilot Trials, and Pivotal Studies for Medical Device Approval Argentina, as well as Post-Market Clinical Follow-Up Evaluations (PMCF). The case study titled "Guidelines for Reporting Clinical Trials on Integrated Chinese and Western Medicine" exemplifies the efforts being made to standardize reporting in this area. Furthermore, the Reporting Guideline for Environmental Outcomes in Clinical Trials: SPIRIT Extension is set to be published in 2027, highlighting the ongoing developments in reporting guidelines.
Focusing on these considerations can significantly enhance the quality of reporting in clinical trials, ultimately contributing to the advancement of medical knowledge and improved patient outcomes.
Conclusion
Successful pilot studies in Argentina depend on a structured approach that incorporates essential steps for effective research. Key actions include:
- Defining clear study objectives
- Developing comprehensive protocols
- Obtaining ethical approvals
- Securing adequate funding
Assembling a qualified research team and employing effective communication and participant recruitment strategies are also vital for building trust within the community.
Navigating the regulatory framework established by ANMAT is crucial for medical device trials. Key considerations include:
- Understanding device classification
- Preparing detailed submission dossiers
- Maintaining open communication with regulatory authorities
These actions can significantly expedite the approval process. Continuous compliance monitoring throughout the trial is essential for safeguarding the integrity of the research.
Best practices in data collection and management are fundamental for ensuring high standards in pilot studies. Effective strategies include:
- Utilizing electronic data capture systems
- Standardizing procedures
- Implementing quality control measures
These practices enhance data accuracy and reliability. Additionally, finalizing and reporting results with thorough analysis, adherence to reporting guidelines, and stakeholder engagement further enrich the impact of the research.
In conclusion, following these structured steps and leveraging expert support will enable stakeholders in Argentina to effectively navigate the complexities of clinical research. This comprehensive approach not only enhances the likelihood of successful outcomes but also contributes to advancing medical knowledge and improving patient care, positioning Argentina as a significant player in the evolving landscape of clinical trials.
Frequently Asked Questions
What are the essential steps to commence pilot medical trials in Argentina?
The essential steps include defining the objectives, developing a research protocol, obtaining ethical approval, securing funding, recruiting a qualified research team, and conducting pre-study activities.
Why is it important to define the objectives of a pilot project?
Clearly articulating the goals of the pilot project provides clarity and direction throughout the research process.
What should be included in a research protocol?
A comprehensive research protocol should outline the methodology, eligibility criteria, and statistical analysis plans, and it must adhere to local regulations.
How is ethical approval obtained for a pilot study?
A meticulously prepared research protocol must be submitted to an ethics committee for rigorous review and approval to safeguard the rights and well-being of participants.
What role does funding play in pilot medical trials?
Adequate financial backing is crucial for facilitating the smooth execution of the study, which involves identifying and pursuing potential funding sources.
What qualifications should the research team possess?
The research team should consist of skilled researchers and medical personnel with the necessary expertise and experience to effectively carry out the investigation.
What are pre-study activities, and why are they important?
Pre-study activities include site assessments and training sessions to prepare the research team for the study protocol and device usage, ensuring a smooth commencement of the study.
How can healthcare experts assist in participant recruitment?
Healthcare experts, especially nurses, can educate patients about research studies, addressing personal factors and beliefs that may impede participation.
What strategies can enhance participant recruitment for research trials?
Strategies include defining the target population, utilizing multiple recruitment channels, engaging with patient advocacy groups, providing clear information, offering incentives, and building trust.
What is the significance of using Electronic Information Capture (EDC) systems in pilot studies?
EDC systems enhance entry, management, and storage capabilities, reducing errors and fostering a more accurate dataset critical for the integrity of research outcomes.
What best practices should be followed for information collection and management?
Best practices include utilizing EDC systems, standardizing collection procedures, conducting regular training, implementing quality control measures, ensuring information security, and documenting information management processes.
What key aspects must be addressed when finalizing and reporting results from pilot studies?
Key aspects include thorough data analysis, comprehensive report preparation, adherence to reporting guidelines, stakeholder engagement, publication of findings, and acknowledgment of research limitations.




