Introduction
Navigating the complexities of medical device studies in Chile presents unique challenges that require a thorough understanding of the regulatory landscape. With key regulatory bodies such as the Instituto de Salud Pública (ISP) and the Agencia Nacional de Medicamentos (ANAMED) at the helm, researchers must be well-versed in the classification of medical devices and the implications it has on the regulatory pathway.
Compliance with Good Clinical Practice (GCP) guidelines is essential, as it ensures ethical standards are upheld throughout the research process. This article delves into the critical steps necessary for conducting clinical studies, including:
- Defining study objectives
- Engaging stakeholders
While emphasizing the importance of ethical considerations and effective risk management strategies, researchers can enhance their understanding of regulatory requirements and improve the feasibility of their studies by leveraging comprehensive clinical trial management services. Ultimately, this paves the way for successful outcomes in the field of medical research.
Navigating the Regulatory Landscape for Medical Device Studies in Chile
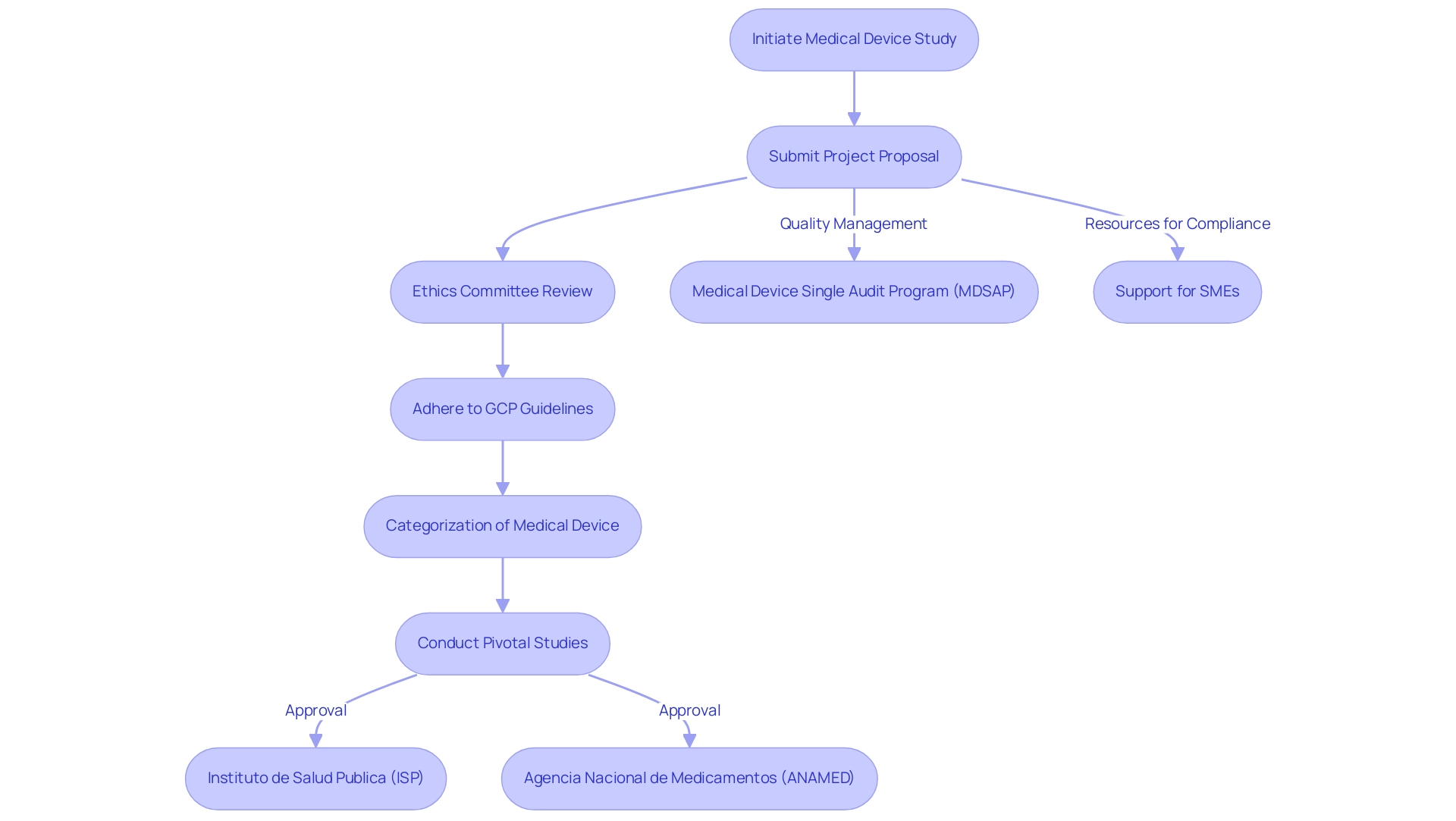
Navigating the compliance landscape for pivotal studies for medical device approval in Chile requires a comprehensive understanding of the key governing bodies, notably the Instituto de Salud Publica (ISP) and the Agencia Nacional de Medicamentos (ANAMED). Acquaintance with the categorization of the medical device is essential, as it directly affects the approval process and determines the types of pivotal studies for medical device approval in Chile required for compliance. The Medical Device Single Audit Program (MDSAP) allows a single audit of a medical device manufacturer’s Quality Management System (QMS), satisfying multiple regulatory jurisdictions, which can streamline processes for manufacturers.
Researchers must also adhere to the Good Clinical Practice (GCP) guidelines, established by the World Health Organization (WHO) and complemented by local regulations, as these are pivotal studies for medical device approval in Chile, emphasizing ethical considerations, informed consent, and data integrity. Before starting any research, it is essential to submit a detailed project proposal to the relevant ethics committee. This proposal should encompass research objectives, methodologies, and ethical considerations.
By taking this proactive approach, researchers can facilitate a more efficient approval process while ensuring strict adherence to ethical standards. Moreover, utilizing extensive clinical trial management services, like those provided by bioaccess®, can improve the feasibility and choice of research locations, compliance with regulations, and project management. This encompasses essential services such as trial set-up and start-up processes, along with continuous reporting on project status and any serious or non-serious adverse events.
The case study on support for Small and Medium Enterprises (SMEs) illustrates how providing resources can improve compliance understanding and market readiness, which is particularly advantageous for navigating the complexities of pivotal studies for medical device approval in Chile. Recent updates from the ISP emphasize ongoing enhancements in the governance framework, reinforcing the importance for researchers to stay informed about pivotal studies for medical device approval in Chile. With specialists like Katherine Ruiz, who has extensive experience in compliance affairs for medical devices, organizations can confidently navigate these challenges to ensure successful clinical trial outcomes.
A Comprehensive Step-by-Step Guide to Conducting Clinical Studies
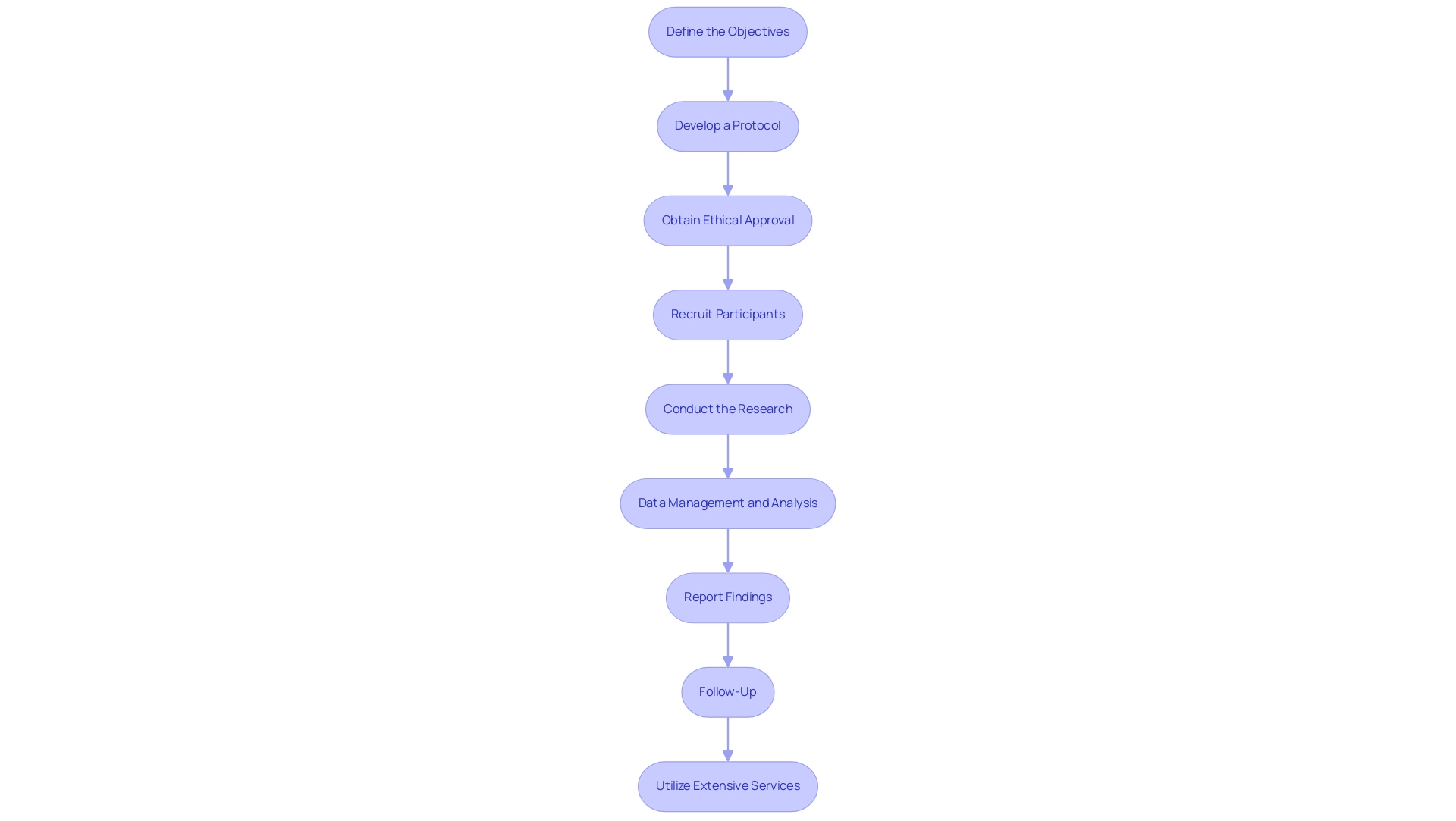
- Define the Objectives: Begin by clearly articulating both the primary and secondary aims of the research. Aligning these objectives with compliance expectations and scientific validity is crucial to ensure a firm foundation for the research. This alignment not only directs the research design but also enhances smoother interactions with oversight entities, such as INVIMA, Colombia's National Food and Drug Surveillance Institute, which ensures adherence to health regulations.
- Develop a Protocol: Construct a comprehensive research protocol that outlines the methodology, participant selection criteria, data collection methods, and statistical analysis plans. This document will serve as the framework for the research, ensuring that all team members are aligned and that compliance standards are consistently met throughout the process.
- Obtain Ethical Approval: Submit the meticulously developed protocol to an Institutional Review Board (IRB) or Ethics Committee for thorough review. It is vital to address all ethical considerations, particularly the informed consent processes, as informed consent is required for publishing identifiable information about participants. Early engagement with ethics officers can streamline the approval process and reduce the number of revisions needed.
- Recruit Participants: Implement a recruitment strategy that adheres to regulatory guidelines. It is essential that potential participants are fully informed and provide their consent prior to enrollment. Researchers have observed that tailored information delivery, particularly through face-to-face interactions, significantly enhances rapport and participant understanding. This approach has been proven effective, as demonstrated by partnerships like GlobalCare Clinical Trials, which reported over a 50% reduction in subject recruitment time and a 95% retention rate in Colombia.
- Conduct the Research: Execute the research strictly according to the approved protocol, maintaining detailed records of all procedures and data collected. Adherence to Good Clinical Practice (GCP) guidelines throughout the research duration is crucial to ensure data integrity and participant safety.
- Data Management and Analysis: Systematically collect and manage data, employing appropriate statistical methods to analyze outcomes as outlined in the predefined analysis plan. Strong data management practices will aid precise interpretation of results and adherence to compliance expectations.
- Report Findings: Prepare a comprehensive report detailing the study findings, methodology, and conclusions. Submitting this report to oversight authorities is crucial, as is considering publication in a peer-reviewed journal. Sharing insights with the broader medical community can contribute to ongoing research and improve clinical practices.
- Follow-Up: Conduct necessary follow-up assessments to monitor long-term outcomes and ensure continued compliance with regulatory requirements. This step is vital for assessing the lasting impact of the research and maintaining transparency with stakeholders. Additionally, anonymized data from the research can be made available to bona fide researchers upon application to the corresponding author, fostering further research and collaboration.
- Utilize Extensive Services: Collaborate with bioaccess™ for extensive trial management services, including feasibility assessments, site selection, and compliance reviews. Their expertise, particularly through professionals like Katherine Ruiz, a Regulatory Affairs expert with significant experience at INVIMA, can enhance the effectiveness of your study. Moreover, reflect on the implications of collaborations, such as with Idx Technologies, which illustrate the potential for innovative solutions in research within Latin America.
Ensuring Compliance with Ethical Standards
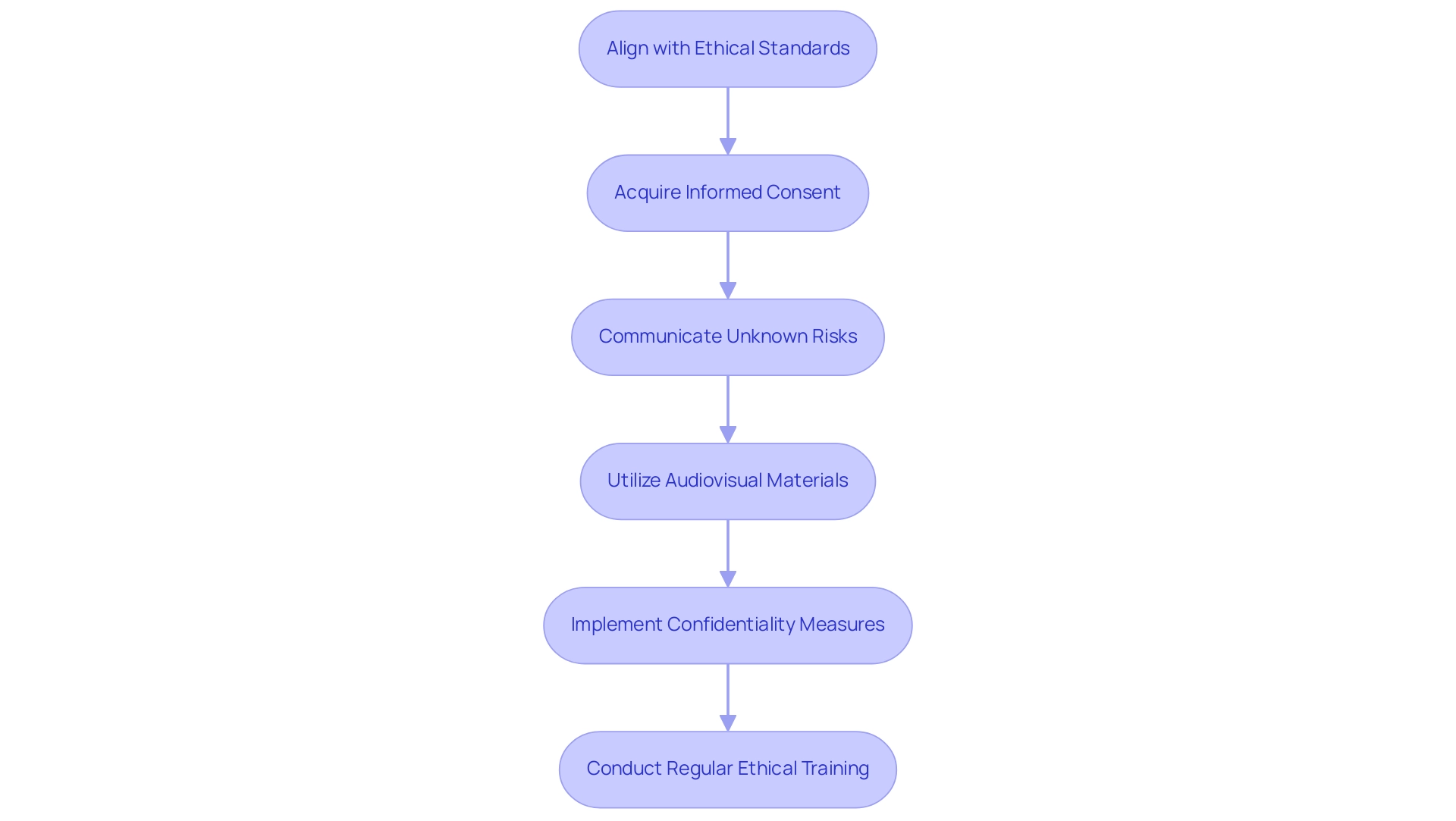
To uphold ethical standards in clinical research, it is imperative for researchers to align with the principles delineated in the Declaration of Helsinki as well as local ethical guidelines. Fundamental to these principles is the necessity for acquiring informed consent from all participants, ensuring they possess a thorough understanding of the project's purpose, procedures, and advantages. Notably, a statistic reveals that only 22 out of 100 adolescents (22.0%) answered all 8 questions correctly regarding informed consent, highlighting the need for effective communication.
In situations where uncertainties remain unknown, it is essential that informed consent documents explicitly communicate this ambiguity. As Yen-Hong Kuo, a PhD candidate and biostatistician, articulates,
When addressing research where there are no data and percentages associated with danger, the informed consent document should state the hazards are unknown.
This approach fosters transparency and honesty in the informed consent process, which is vital for maintaining trust.
The case analysis titled 'Handling Unknown Risks' emphasizes the importance of stating unknown risks in informed consent documents, ensuring participants are fully informed. Furthermore, recent discussions suggest that audiovisual materials can aid understanding, particularly for detained adults and adolescents, who should have a choice regarding informed consent materials. Researchers must also implement rigorous measures to safeguard participant confidentiality and data privacy.
Regular ethical training for all team members involved in the research is critical to uphold these high standards consistently. Furthermore, keeping open channels of communication with oversight organizations and ethics committees about any modifications or problems that occur during the research is essential for ensuring adherence and ethical integrity throughout the research process.
Implementing Effective Risk Management Strategies
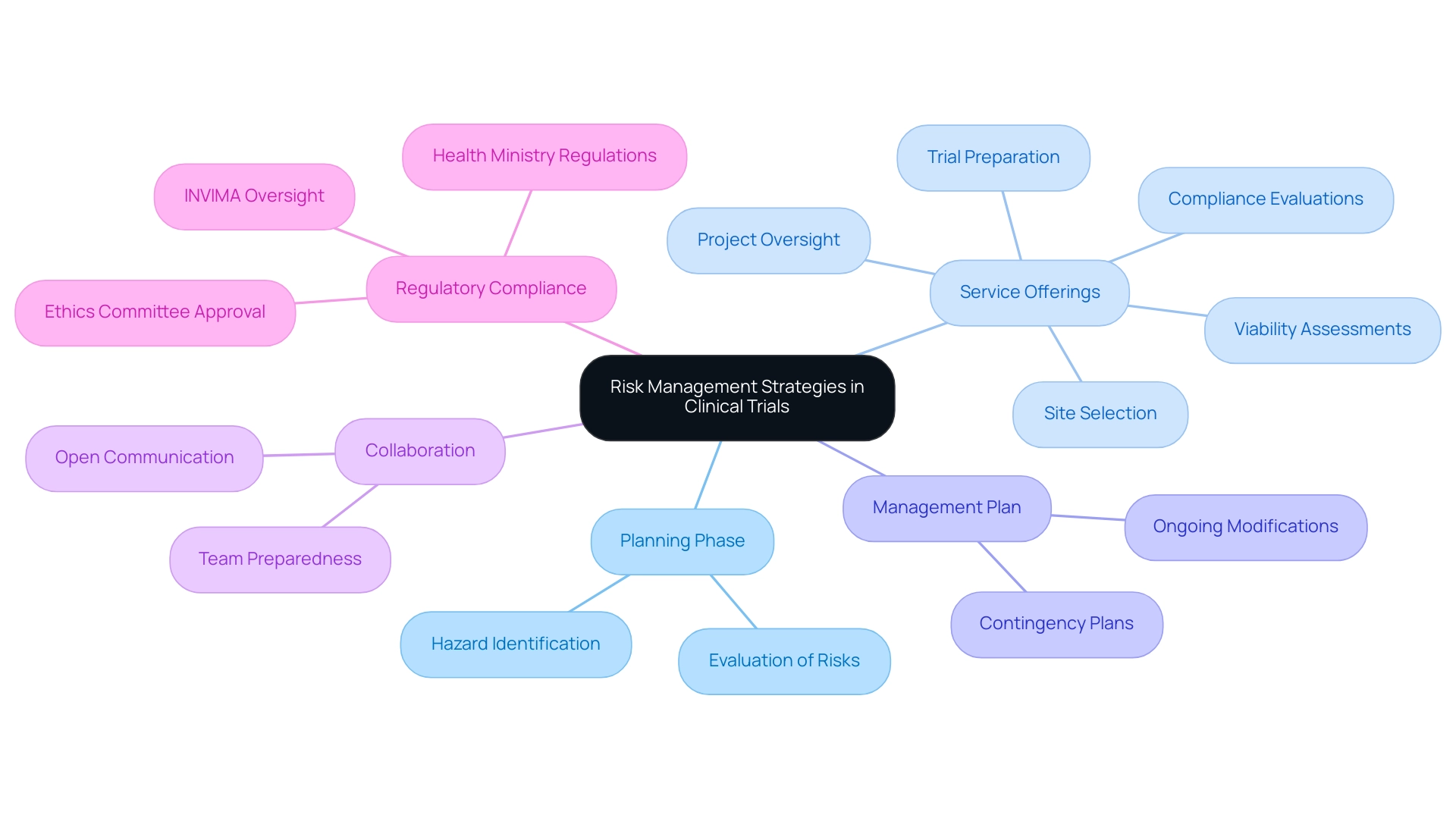
Efficient hazard management in research necessitates a comprehensive evaluation conducted during the planning phase. This evaluation should concentrate on recognizing possible hazards associated with participant safety, data integrity, and regulatory compliance—essential elements for the success of any clinical trial. Our service offerings encompass:
- Viability assessments
- Site selection
- Compliance evaluations
- Trial preparation
- Project oversight
This guarantees that all elements of the trial are carefully organized to minimize uncertainties.
A well-structured management plan is essential, detailing strategies for addressing identified challenges, including contingency plans for adverse events. Consistent oversight of the project's advancement enables prompt modifications to the management plan, ensuring that unexpected challenges are addressed proactively. Collaborating with the research team is essential; promoting open communication about potential issues helps guarantee that all team members are sufficiently prepared to address any challenges that may occur during the study.
Moreover, documenting all risk management activities provides a clear record for oversight, enhancing transparency and compliance. In Colombia, the INVIMA plays a crucial role in overseeing medical device regulations, ensuring that all trials adhere to stringent safety and efficacy standards. Approval processes involving ethics committees and health ministries are essential in this context, as they ensure compliance with national regulations.
As Regulatory Affairs expert Katherine Ruiz emphasizes, understanding these regulations is fundamental to obtaining market clearance for innovations in Colombia. Moreover, including pertinent statistics on negative occurrences in research studies would further improve the conversation on management strategies. As Robert Kiyosaki aptly stated, 'to be a winner in life, one must constantly go beyond their best,' a sentiment that resonates strongly in the context of medical research.
Moreover, effective risk management can help businesses comply with relevant laws and regulations, highlighting its critical role in medical trials.
Engaging Stakeholders and Building Collaborations
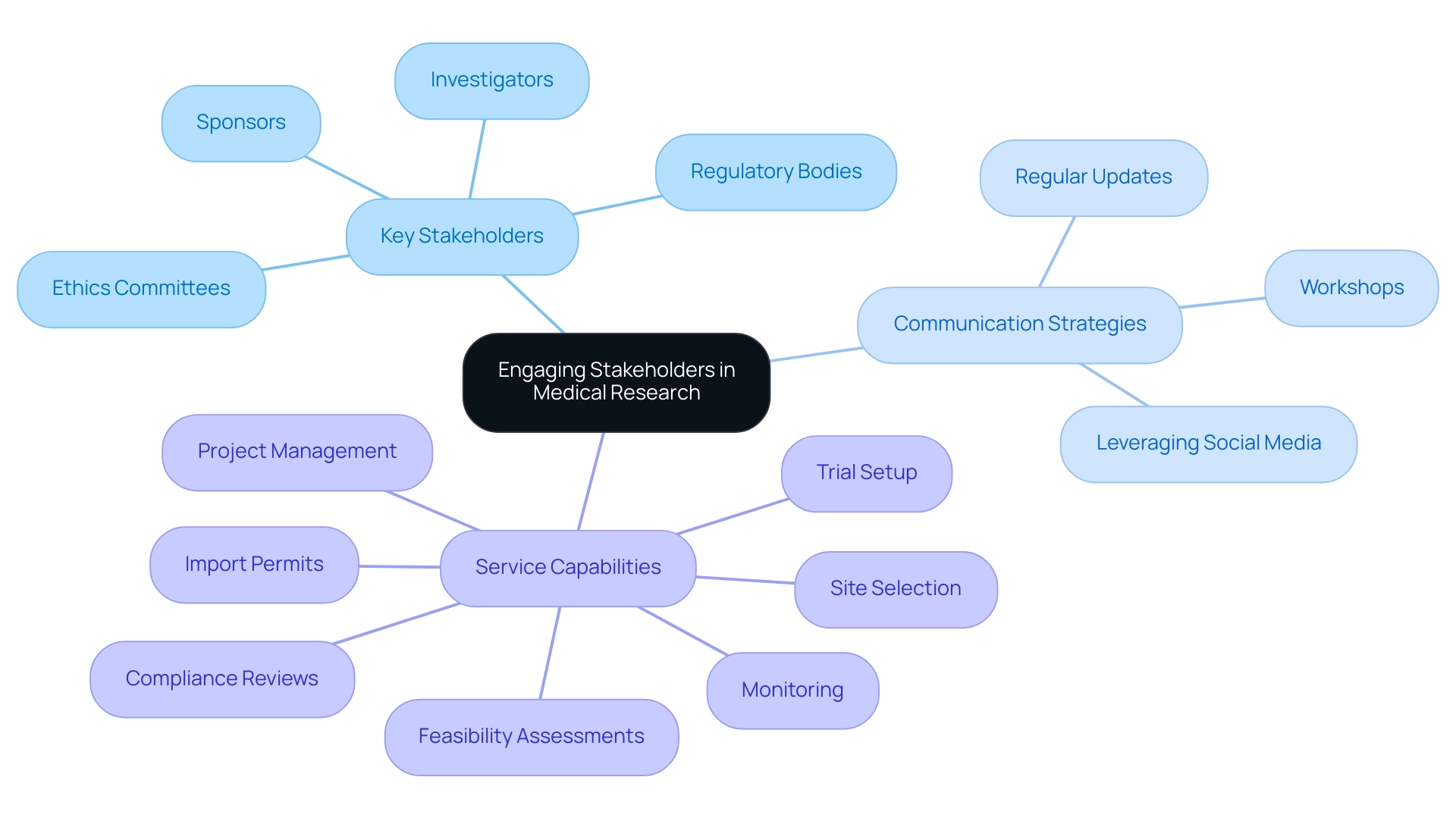
Successful stakeholder involvement is essential in medical research, especially in critical trials. Researchers must begin by identifying and mapping out key individuals and organizations involved in the research, including:
- Investigators
- Sponsors
- Regulatory bodies
- Ethics committees
In Latin America, where research trials can significantly impact local economies, establishing regular communication channels is essential to keep stakeholders informed about progress and any obstacles encountered along the way.
Regular updates through meetings or workshops can significantly foster collaboration, allowing stakeholders to provide feedback that may prove invaluable in refining research protocols. Establishing trust and upholding openness with stakeholders will not only enhance collaborations but also aid in the effective implementation of research projects. Leveraging social media and professional networks can further enhance visibility and garner support for the research initiative.
As noted by Mr. James Velez Ting, 'The establishment of this research laboratory marks a new chapter for Cebu, reinforcing its role as a vital contributor to international health research and development.' This statement reflects the growing trend of collaboration in medical research, highlighting that effective communication can lead to successful partnerships. For example, BioPharma Diagnostics partnered with a global diagnostics firm, leading to research that confirmed a serum metabolite constellation for early detection of hepatocellular carcinoma (HCC) in patients with liver cirrhosis.
This collaboration aimed to enhance the detection of early HCC lesions, demonstrating how effective stakeholder engagement can lead to tangible results in research. Given bioaccess®'s extensive experience in managing various types of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Their expertise in conducting Pivotal Studies for Medical Device Approval in Chile enables them to navigate the complexities of regulatory compliance and project management effectively. bioaccess® also offers comprehensive service capabilities, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Monitoring
This ensures that clinical trials are conducted efficiently and in accordance with local regulations.
Conclusion
Navigating the regulatory landscape for medical device studies in Chile requires a meticulous approach that encompasses a thorough understanding of the key regulatory bodies, including the Instituto de Salud Pública (ISP) and the Agencia Nacional de Medicamentos (ANAMED). The classification of medical devices significantly influences the regulatory pathway, necessitating adherence to Good Clinical Practice (GCP) guidelines to uphold ethical standards throughout the research process. Researchers must:
- Define clear study objectives
- Engage stakeholders effectively
- Leverage comprehensive clinical trial management services to enhance the feasibility and success of their studies
The importance of ethical considerations cannot be overstated; obtaining informed consent and safeguarding participant confidentiality are paramount. Implementing rigorous risk management strategies and conducting comprehensive risk assessments during the planning phase can mitigate potential challenges, ensuring participant safety and data integrity. Continuous engagement with stakeholders fosters collaboration and trust, which are essential for the successful execution of clinical trials.
In conclusion, staying informed about the evolving regulatory framework and maintaining open lines of communication with regulatory bodies and ethics committees are crucial for researchers. By adhering to these principles and strategies, researchers can navigate the complexities of medical device studies in Chile, paving the way for innovative solutions and successful clinical trial outcomes that contribute to the advancement of medical research.
Frequently Asked Questions
What are the key governing bodies involved in the medical device approval process in Chile?
The key governing bodies are the Instituto de Salud Publica (ISP) and the Agencia Nacional de Medicamentos (ANAMED).
How does the categorization of a medical device affect the approval process in Chile?
The categorization of the medical device directly affects the approval process and determines the types of pivotal studies required for compliance.
What is the Medical Device Single Audit Program (MDSAP)?
The MDSAP allows a single audit of a medical device manufacturer’s Quality Management System (QMS), satisfying multiple regulatory jurisdictions to streamline processes for manufacturers.
What guidelines must researchers adhere to during pivotal studies for medical device approval in Chile?
Researchers must adhere to Good Clinical Practice (GCP) guidelines established by the World Health Organization (WHO) and local regulations, focusing on ethical considerations, informed consent, and data integrity.
What is required before starting any research in Chile?
A detailed project proposal must be submitted to the relevant ethics committee, including research objectives, methodologies, and ethical considerations.
How can researchers improve the efficiency of the approval process?
By taking a proactive approach to compliance and utilizing clinical trial management services, such as those provided by bioaccess®, researchers can enhance feasibility, compliance, and project management.
What role does the case study on support for Small and Medium Enterprises (SMEs) play in compliance?
The case study illustrates how providing resources can improve understanding of compliance and market readiness, which is beneficial for navigating pivotal studies for medical device approval in Chile.
Why is it important for researchers to stay informed about recent updates from the ISP?
Staying informed about ongoing enhancements in the governance framework is crucial for successfully navigating the complexities of pivotal studies for medical device approval in Chile.
What services does bioaccess® provide to support researchers?
Bioaccess® offers extensive clinical trial management services, including trial set-up, compliance reviews, project oversight, and continuous reporting on project status and adverse events.




