Introduction
The landscape of medical device regulation is evolving, and Post-Market Clinical Follow-Up (PMCF) has emerged as a vital component in ensuring the safety and efficacy of devices once they are introduced to the market. As healthcare systems increasingly prioritize patient outcomes, Medtech companies face the dual challenge of navigating complex regulatory frameworks while implementing effective PMCF strategies. This is particularly evident in Latin America, where diverse regulatory environments and unique challenges necessitate a tailored approach.
By systematically gathering and analyzing clinical data post-launch, manufacturers can not only comply with stringent regulations but also enhance their understanding of device performance in real-world settings. This article delves into the intricacies of PMCF, offering insights into its significance, methodologies, and the critical role of ethical compliance in fostering trust and transparency within the healthcare community.
Understanding Post-Market Clinical Follow-Up (PMCF) in Medical Devices
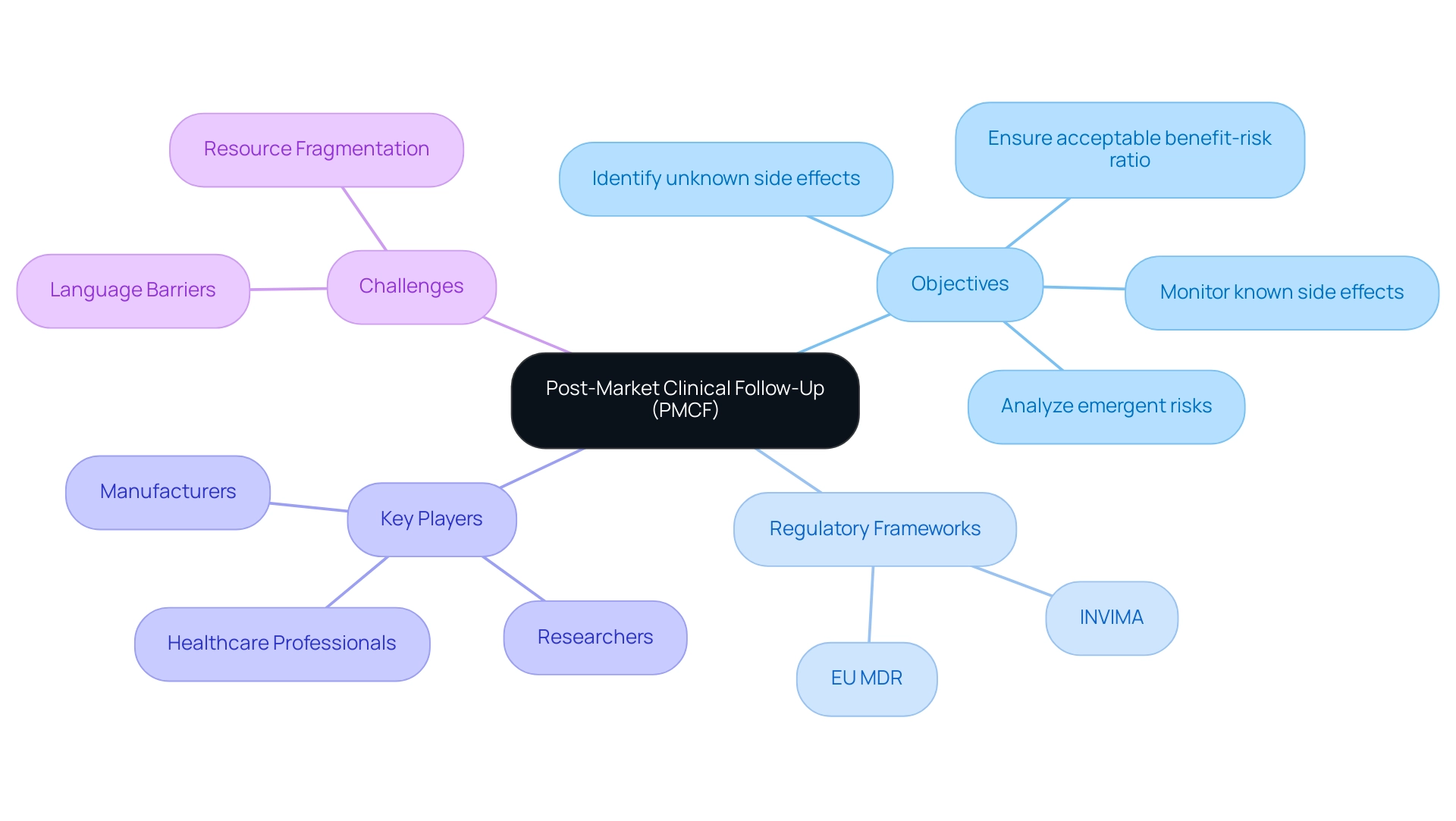
Post-Market Clinical Follow-Up is a crucial component of medical equipment lifecycle management, involving the systematic collection and assessment of clinical data concerning the safety and performance of products after market introduction. The main aim of post-market clinical follow-up is to confirm the ongoing safety and effectiveness of products as they are used in real-world settings, which is especially vital for Medtech firms traversing the intricate landscape of Latin America, where obstacles like language barriers and resource fragmentation can impede successful execution.
Regulatory frameworks, particularly the European Union Medical Device Regulation (EU MDR), emphasize the necessity of post-market clinical follow-up as part of a comprehensive post-market surveillance strategy. In Latin America, regulatory agencies such as INVIMA play a vital role, offering oversight and ensuring that the post-market clinical follow-up activities align with local ethical standards and the specific risk class of each device. This continuous assessment is essential for identifying potential risks, ensuring compliance with regulatory standards, and enhancing patient outcomes.
Producers are obligated to record a post-marketing clinical follow-up Plan that details the procedure for information gathering, which must consider local ethical approval standards. The program aims to:
- Identify unknown side effects
- Monitor known side effects
- Analyze emergent risks
- Ensure the benefit-risk ratio remains acceptable
By continuously collecting clinical data, post-market clinical follow-up assists manufacturers in validating the ongoing safety and performance of their products, aiding clinical assessments in a strong regulatory environment.
As emphasized by the partnership between Greenlight Guru and bioaccess™, which seeks to expedite Medtech innovations and clinical trials in Latin America, the importance of post-market clinical follow-up cannot be overstated. This partnership addresses the challenges faced by Medtech companies by providing streamlined processes and support for navigating regulatory complexities. According to Etienne Nichols, Head of Industry Insights & Education at Greenlight Guru, "As a Mechanical Engineer and Medical Device Guru, I specialize in simplifying complex ideas, teaching system integration, and connecting industry leaders."
His insights highlight the significance of post-market clinical follow-up in promoting a deeper comprehension of device performance and safety.
As this process evolves, it is essential for manufacturers, researchers, and healthcare professionals in regions like the Dominican Republic to understand the significance of Post-Market Clinical Follow-Up Dominican Republic in ensuring the highest standards of patient care, particularly in light of successful case studies like PAVmed's first-in-human study in Colombia, which exemplifies the potential of clinical research in this vibrant market.
Step-by-Step Guide to Conducting PMCF in the Dominican Republic
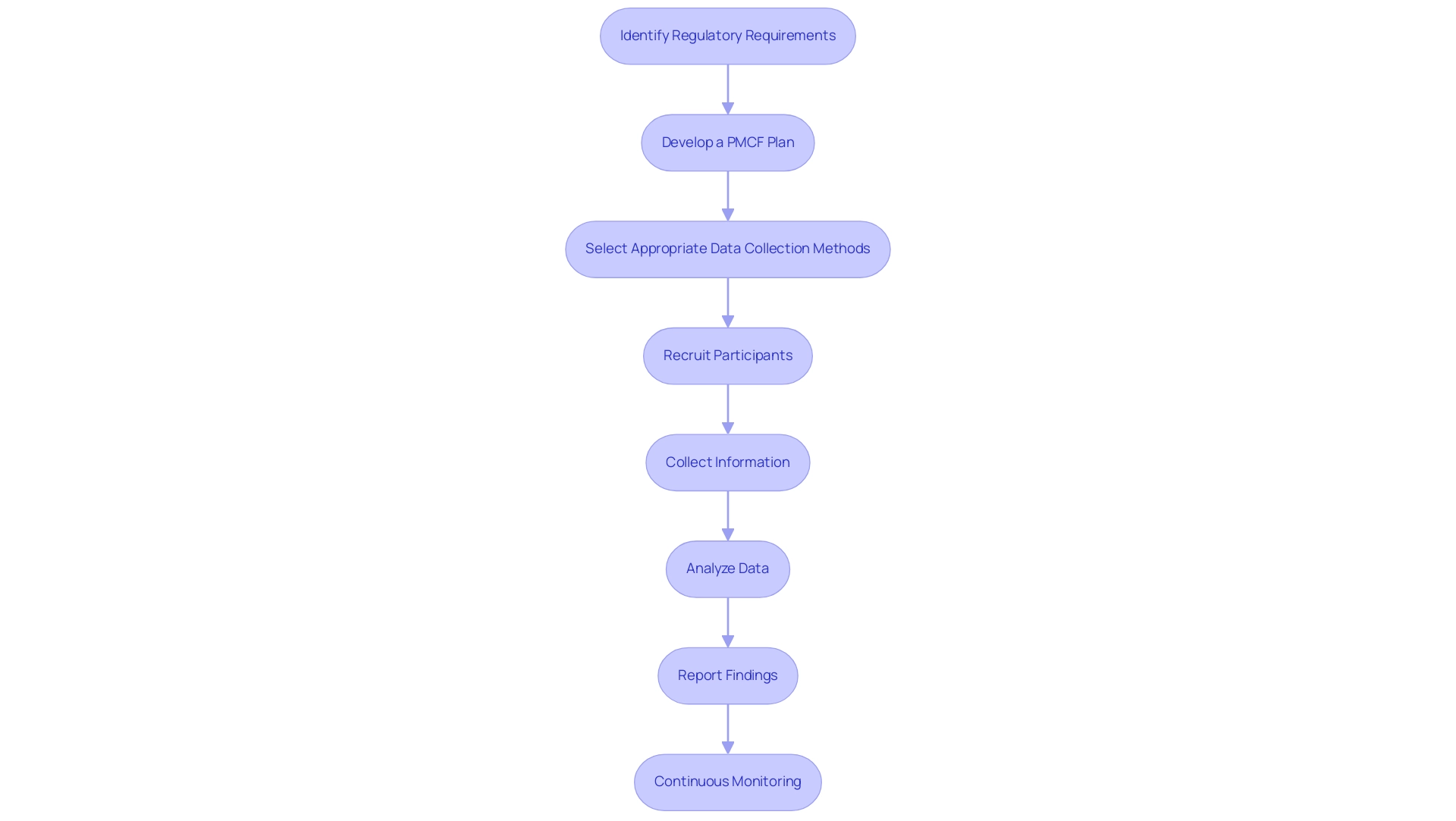
Utilizing the knowledge of experts can improve your strategy for effectively carrying out Post-Market Clinical Follow-Up Dominican Republic. Follow these steps:
-
Identify Regulatory Requirements: Begin by familiarizing yourself with local regulations governing post-market clinical follow-up, including compliance with the Dominican Republic's Ministry of Public Health directives and relevant international guidelines.
The service assists in navigating these regulations to ensure compliance.
-
Develop a PMCF Plan: Create a comprehensive PMCF plan that outlines objectives, methodology, and timelines for information collection, ensuring alignment with regulatory expectations and ethical considerations like informed consent.
The organization offers guidance in developing these plans to meet all necessary standards.
-
Select Appropriate Data Collection Methods: Choose suitable methods for data collection, such as surveys, clinical evaluations, or existing databases, to effectively capture relevant clinical information regarding the product's performance and safety.
The company provides expertise in selecting the most effective methods tailored to your study.
-
Recruit Participants: Identify and recruit a representative sample of patients or healthcare providers using the medical device, ensuring recruitment strategies comply with ethical standards and regulatory requirements.
The platform can assist in site selection and recruitment strategies to optimize participant enrollment.
-
Collect Information: Implement the information collection process as outlined in your PMCF plan, maintaining rigorous adherence to protocols to ensure integrity and reliability.
The company offers project management services to oversee this process.
-
Analyze Data: After data collection, conduct thorough analysis to evaluate the performance and identify safety concerns, utilizing appropriate statistical methods for meaningful conclusions.
This tool can support this analysis, ensuring robust methodologies are applied.
-
Report Findings: Prepare a comprehensive report detailing findings, methodologies, and recommendations for enhancements or additional studies, and submit this report to relevant regulatory authorities.
bioaccess® assists in compliance reviews and ensures that reporting meets all regulatory requirements.
-
Continuous Monitoring: Establish a system for ongoing monitoring of the device's performance and safety, incorporating feedback from users and stakeholders.
Regularly update your Post-Market Clinical Follow-Up Dominican Republic plan based on new data and regulatory changes.
This company offers ongoing assistance to guarantee that monitoring conforms to optimal practices.
By collaborating with them, you gain from over 20 years of Medtech knowledge, ensuring your Post-Market Clinical Follow-Up Dominican Republic is executed with a tailored method that fulfills all necessary standards.
Ensuring Compliance with Ethical Standards
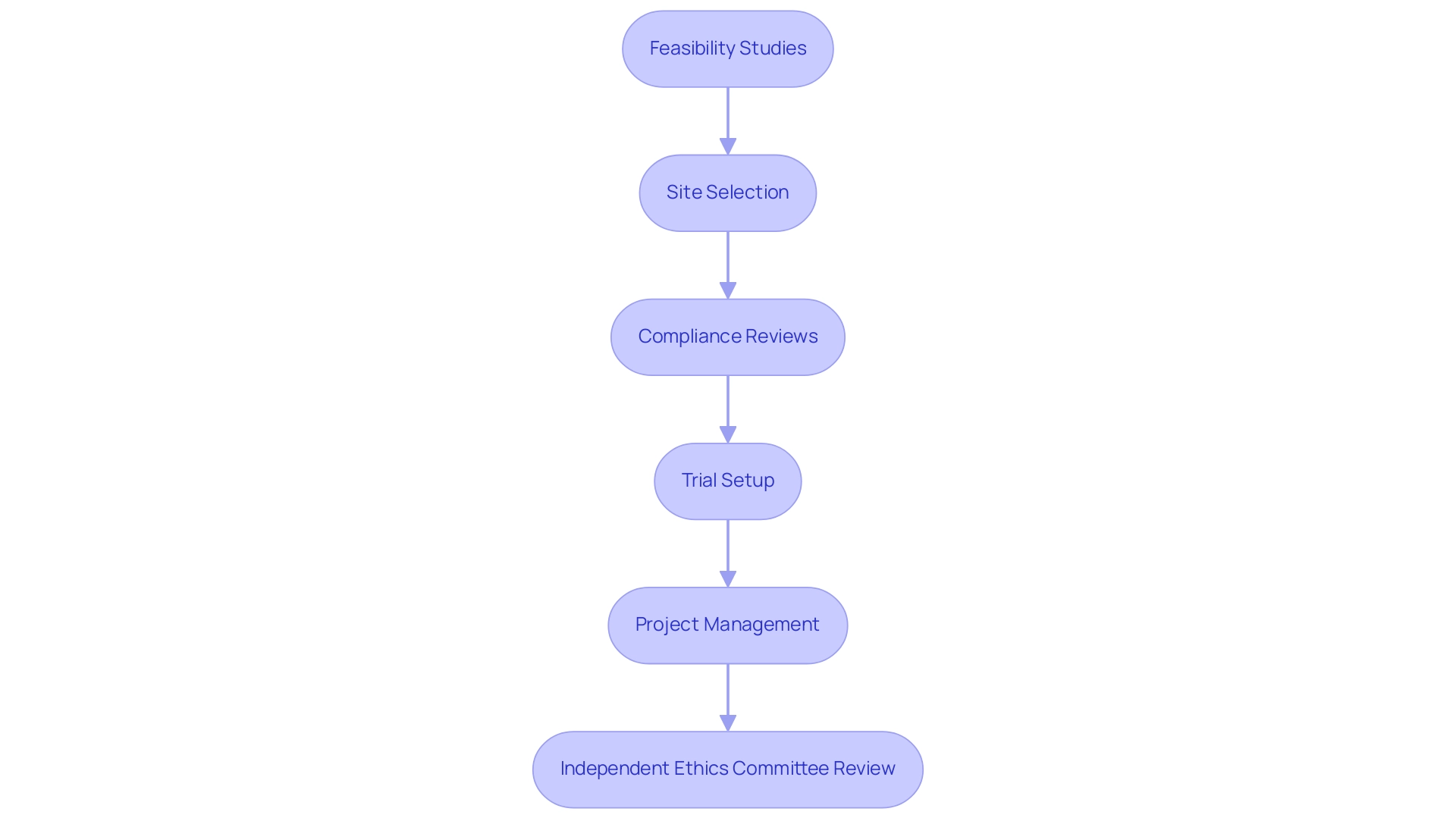
Ethical compliance serves as a fundamental element in the implementation of Post-Market Clinical Follow-Up Dominican Republic studies at the company. Our extensive services, which include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Project management
ensure that all research activities strictly adhere to established ethical guidelines, with informed consent being a fundamental requirement. Participants receive comprehensive information regarding the study's objectives, methodologies, potential risks, and anticipated benefits.
According to Young Shin Lee, MD, PhD, 'Publication of clinical research must be based in truth, honesty, and the responsibility to achieve the best care for patients.' This sentiment underscores the necessity for transparency in the informed consent process, which is crafted to meet the highest ethical standards. Moreover, it is imperative that post-market clinical follow-up activities undergo thorough review and approval by an independent ethics committee or institutional review board.
Such oversight not only safeguards participants' rights but also reinforces the integrity of the study. With over 20 years of expertise in Medtech, bioaccess® emphasizes starting with clear end goals and developing a reusable post-market clinical follow-up plan template to streamline the ethical review process, ensuring that ethical considerations are addressed from the outset. Regularly revisiting and addressing these considerations throughout the Post-Market Clinical Follow-Up Dominican Republic journey is vital, particularly in light of evolving regulatory standards and emerging concerns, ensuring compliance and fostering trust within the research community.
The case study titled 'Basic Knowledge of Endoscopic Retrograde Cholangiopancreatography' serves as a significant resource, offering foundational knowledge that improves understanding of ethical compliance in the Post-Market Clinical Follow-Up Dominican Republic studies. Additionally, insights from Emanuel EJ et al. emphasize that ethical clinical research must prioritize the welfare of participants, reinforcing the significance of informed consent and ethical standards in post-market clinical follow-up research.
Leveraging Technology for PMCF Data Collection
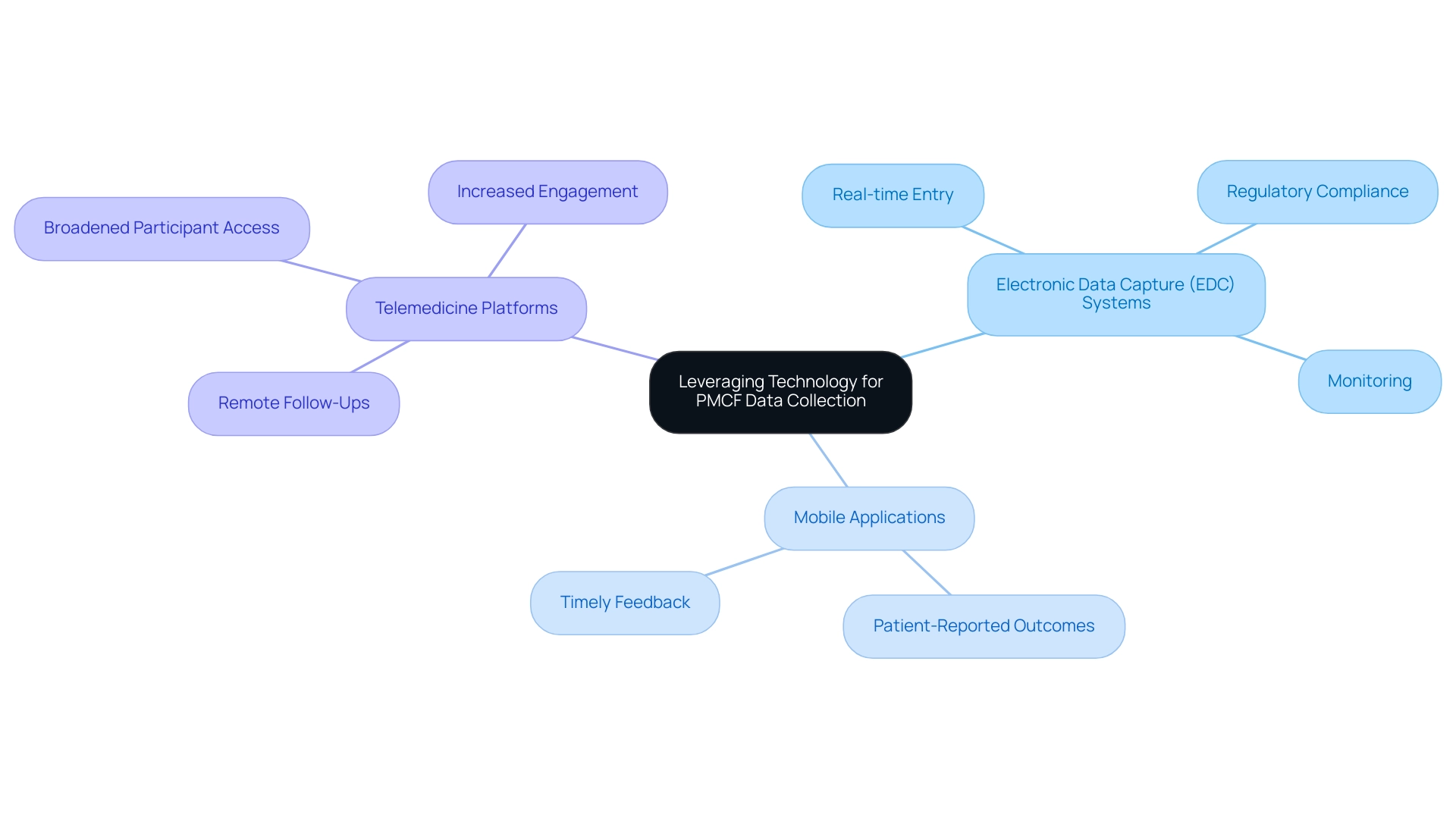
Incorporating technology into PMCF information collection can significantly enhance efficiency and accuracy, particularly when supported by comprehensive clinical trial management services offered by bioaccess®. Utilizing electronic information capture (EDC) systems facilitates real-time entry and monitoring, ensuring compliance with regulatory standards. Mobile applications can be employed to collect patient-reported outcomes, allowing for timely feedback on device performance, which is crucial in post-market evaluations.
Additionally, telemedicine platforms enable remote follow-ups, broadening participant access and engagement. By utilizing these technologies together with bioaccess®'s knowledge in overseeing various studies, such as:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
Researchers can optimize data collection processes, minimize errors, and improve the overall quality of their Post-Market Clinical Follow-Up Dominican Republic activities. Our service capabilities also encompass feasibility studies, site selection, compliance reviews, trial setup, and project management, ensuring a comprehensive approach to clinical trials.
Engaging Stakeholders Throughout the PMCF Process
Involving stakeholders throughout the process is essential for success. This includes collaborating with healthcare providers, regulatory bodies such as INVIMA—Colombia's National Food and Drug Surveillance Institute responsible for ensuring compliance and safety in medical device oversight—and patient advocacy groups. Regular communication helps ensure that all parties are informed and aligned with the study's objectives.
Consider holding stakeholder meetings to discuss progress, share findings, and solicit feedback. By fostering a collaborative environment, you can enhance the quality of your Post-Market Clinical Follow-Up Dominican Republic efforts, build trust among stakeholders, and contribute to meaningful impacts on local economies through job creation and improved healthcare outcomes, ultimately leading to better patient results. Moreover, it is essential to incorporate specific service capabilities, such as:
- Trial setup
- Project management
- Reporting
to ensure compliance with INVIMA's regulations.
INVIMA plays a vital role in overseeing the selection of research sites and principal investigators, conducting compliance reviews, and monitoring the overall progress of clinical trials, thereby ensuring that all regulatory requirements are met.
Conclusion
The significance of Post-Market Clinical Follow-Up (PMCF) in the medical device sector cannot be overstated, particularly as it relates to ensuring ongoing safety and efficacy after product launch. By systematically collecting and analyzing clinical data in real-world environments, manufacturers not only comply with regulatory requirements but also enhance their understanding of device performance. This is especially crucial in diverse markets like Latin America, where unique regulatory challenges necessitate a tailored approach.
Implementing an effective PMCF strategy involves a multi-faceted process, including:
- Developing a comprehensive PMCF plan
- Selecting appropriate data collection methods
- Engaging stakeholders
- Ensuring ethical compliance
By utilizing technology and fostering collaboration among healthcare providers, regulatory bodies, and patient advocacy groups, Medtech companies can streamline their PMCF efforts. This collaborative approach is essential for building trust and improving patient outcomes, ultimately leading to a more transparent and efficient healthcare system.
As the landscape of medical device regulation continues to evolve, embracing PMCF as a core component of post-market surveillance will play a pivotal role in advancing patient care and fostering innovation. The commitment to ethical standards and continuous monitoring not only safeguards participants but also reinforces the integrity of clinical research, ensuring that the benefits of medical devices are realized while minimizing potential risks. The path forward lies in the diligent execution of PMCF strategies, which will undoubtedly contribute to the enhancement of patient safety and the overall efficacy of medical technologies.
Frequently Asked Questions
What is Post-Market Clinical Follow-Up (PMCF)?
PMCF is a systematic process for collecting and assessing clinical data regarding the safety and performance of medical products after they have been introduced to the market. Its main aim is to confirm the ongoing safety and effectiveness of these products in real-world settings.
Why is PMCF particularly important for Medtech firms in Latin America?
In Latin America, Medtech firms face challenges such as language barriers and resource fragmentation that can hinder successful execution of PMCF. Continuous assessment is vital to identify potential risks, ensure compliance with regulatory standards, and enhance patient outcomes.
What regulatory frameworks support PMCF?
The European Union Medical Device Regulation (EU MDR) emphasizes the necessity of PMCF as part of a comprehensive post-market surveillance strategy. In Latin America, agencies like INVIMA provide oversight to ensure PMCF activities align with local ethical standards and the specific risk class of each device.
What are the objectives of a PMCF plan?
The PMCF plan aims to: Identify unknown side effects, Monitor known side effects, Analyze emergent risks, Ensure the benefit-risk ratio remains acceptable.
What steps are involved in effectively carrying out PMCF in the Dominican Republic?
The steps include: Identify regulatory requirements, Develop a PMCF plan, Select appropriate data collection methods, Recruit participants, Collect information, Analyze data, Report findings, Continuous monitoring.
How does ethical compliance play a role in PMCF?
Ethical compliance is fundamental in PMCF studies, ensuring that all research activities adhere to established ethical guidelines, including obtaining informed consent from participants. Oversight by independent ethics committees is also essential to safeguard participant rights.
What technologies can enhance PMCF information collection?
Technologies such as electronic data capture (EDC) systems, mobile applications for patient-reported outcomes, and telemedicine platforms can significantly improve the efficiency and accuracy of data collection in PMCF.
Why is stakeholder involvement crucial in PMCF?
Involving stakeholders, including healthcare providers, regulatory bodies, and patient advocacy groups, is essential for ensuring alignment with study objectives, enhancing the quality of PMCF efforts, and building trust among all parties involved.
What role does INVIMA play in PMCF?
INVIMA oversees the selection of research sites and principal investigators, conducts compliance reviews, and monitors the overall progress of clinical trials to ensure that all regulatory requirements are met.

