Introduction
In the realm of medical devices, ensuring ongoing safety and efficacy post-market approval is paramount. Post-Market Clinical Follow-Up (PMCF) serves as a critical mechanism for evaluating these devices in real-world settings, allowing manufacturers to monitor performance and identify potential risks that may arise after initial market release.
This article delves into the intricacies of PMCF, exploring its essential components, regulatory considerations, and best practices for implementation. By understanding the significance of PMCF and the methodologies involved, stakeholders can better navigate the complexities of clinical research, ultimately enhancing patient safety and device quality in an ever-evolving healthcare landscape.
Introduction to Post-Market Clinical Follow-Up (PMCF)
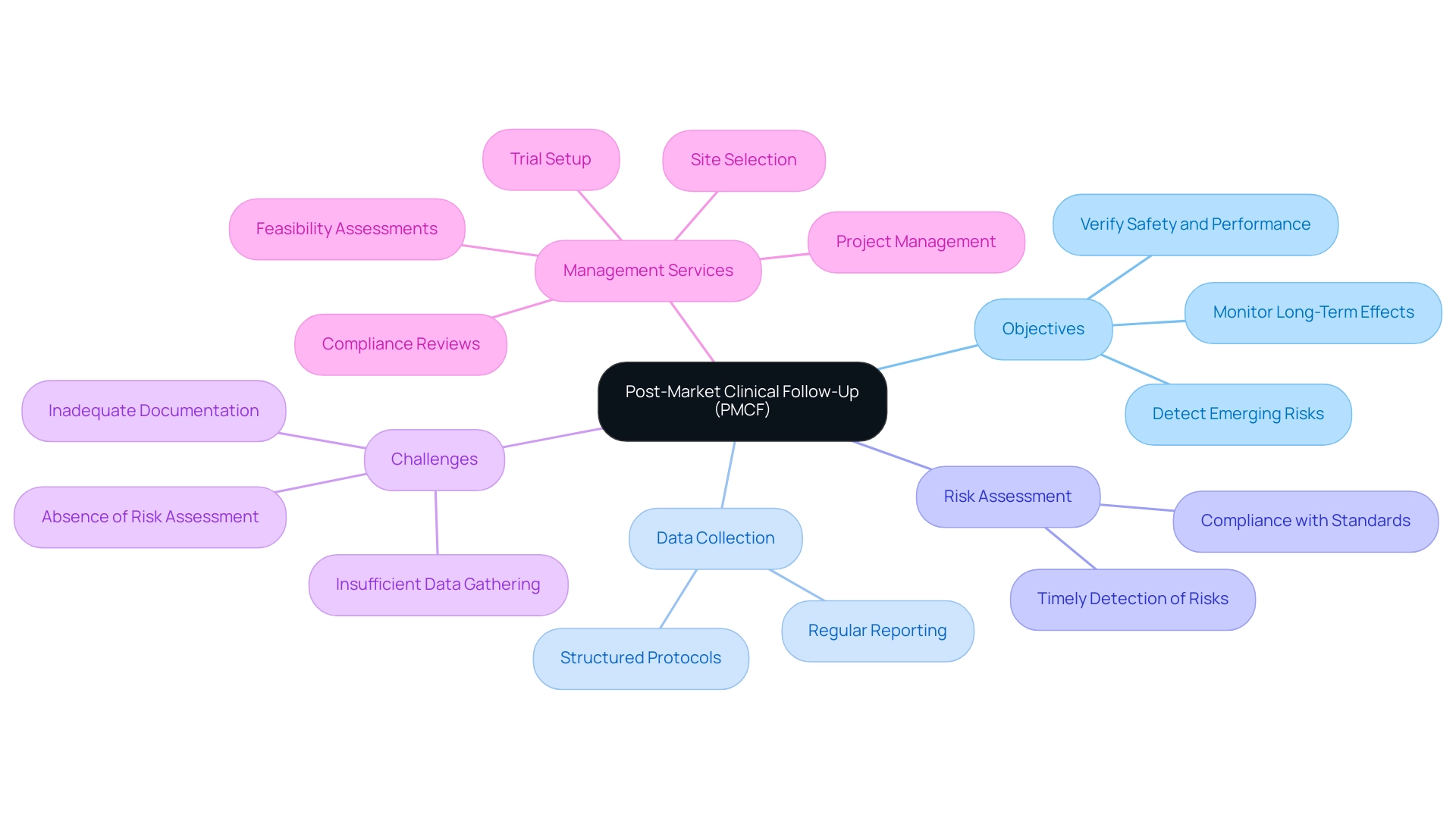
Post-Market Clinical Follow-Up is a fundamental aspect of the clinical research lifecycle, emphasizing the continuous assessment of medical products after market authorization. Its primary objective is to verify that these instruments consistently meet established safety and performance criteria in real-world environments. As Jasper Van de Sande points out, 'More recently, he conducted real-world evidence analyses and emphasized the importance of post-market clinical follow-up,' highlighting the significance of this continuous assessment.
Post-Market Clinical Follow-Up entails systematic data collection and rigorous analysis to monitor the long-term effects of a device, thus enabling the timely detection of any emerging risks or adverse events that may not have been evident during pre-market evaluations. Bioaccess® brings over 20 years of Medtech expertise to the table, offering comprehensive clinical trial management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
Our approaches in Post-Market Clinical Follow-Up include structured data collection protocols, regular risk assessments, and thorough reporting mechanisms to ensure compliance with regulatory standards.
Frequent problems in Post-Market Clinical Follow-Up evaluations include:
- Insufficient data gathering
- Absence of risk assessment
- Inadequate documentation of adverse events
These issues are emphasized in the case analysis 'Common Non-Conformities and Tips.' Establishing clear protocols and prompt reporting can reduce non-compliance risks and improve the effectiveness of Post-Market Clinical Follow-Up studies. This process is not simply a regulatory obligation; it is essential for ensuring compliance with evolving standards and enhancing product quality.
By promoting ongoing enhancement, the organization offers essential insights to stakeholders concerning the real-world effectiveness and safety of medical instruments, ultimately reinforcing their dedication to patient safety.
Distinguishing Between PMCF and PMCF Studies
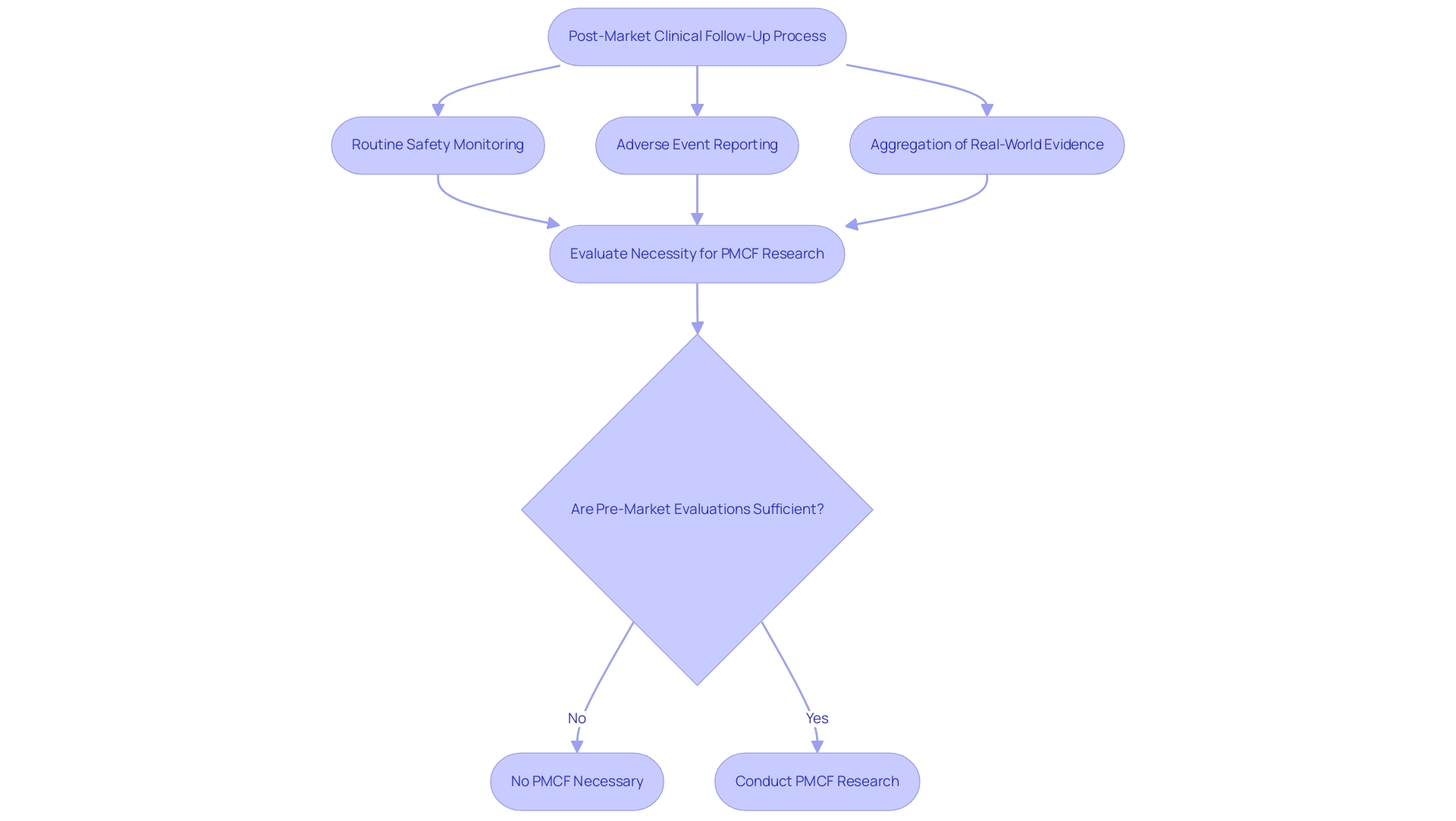
The activities involved in Post-Market Clinical Follow-Up focus on assessing the performance of medical instruments after they have been launched into the market. Within this framework, these investigations represent specific research initiatives aimed at systematically assessing a device's safety and effectiveness over time. These investigations typically involve rigorous data collection and analysis methods, which may include clinical trials or observational research tailored to capture real-world performance.
Essential elements of the post-market clinical follow-up process include:
- Routine safety monitoring
- Adverse event reporting
- Aggregation of real-world evidence
These elements are crucial for ensuring ongoing compliance and safety. Understanding the difference between Post-Market Clinical Follow-Up and post-market clinical follow-up analyses is essential for researchers, as it allows them to align their research designs with regulatory expectations. As noted in an article from BoneZone, 'Post-Market Studies in Lieu of Clinical Studies,' this alignment not only enhances the credibility of the findings but also ensures the necessary evidence is gathered to support the ongoing safety and efficacy of medical devices in a rapidly evolving healthcare landscape.
Moreover, a structured approach for evaluating the necessity for Post-Market Clinical Follow-Up research, as detailed in the case analysis 'Step-by-Step Recommendations for Post-Market Clinical Follow-Up,' includes:
- Assessing risk categories from guidance documents
- Determining if pre-market evaluations are sufficient
This process may result in the conclusion that 'no post-market clinical follow-up is necessary' if pre-market risk controls are confirmed as sufficient. Additionally, various procedures and training related to Post-Market Clinical Follow-Up, including the Post-Market Surveillance Procedure and the Webinar on this topic, are available for purchase, providing valuable resources for clinical research directors.
Bioaccess® stands out as a leader in this field, providing accelerated medical equipment clinical study services throughout Latin America, leveraging expertise in Early-Feasibility, First-In-Human, Pilot, and Pivotal studies. Katherine Ruiz, a specialist in compliance matters for medical equipment and in vitro diagnostics in Colombia, exemplifies the caliber of professionals dedicated to ensuring adherence and innovation in this crucial area. For further insights and tailored solutions, we invite you to BOOK A MEETING with our team.
Key Components of a PMCF Plan
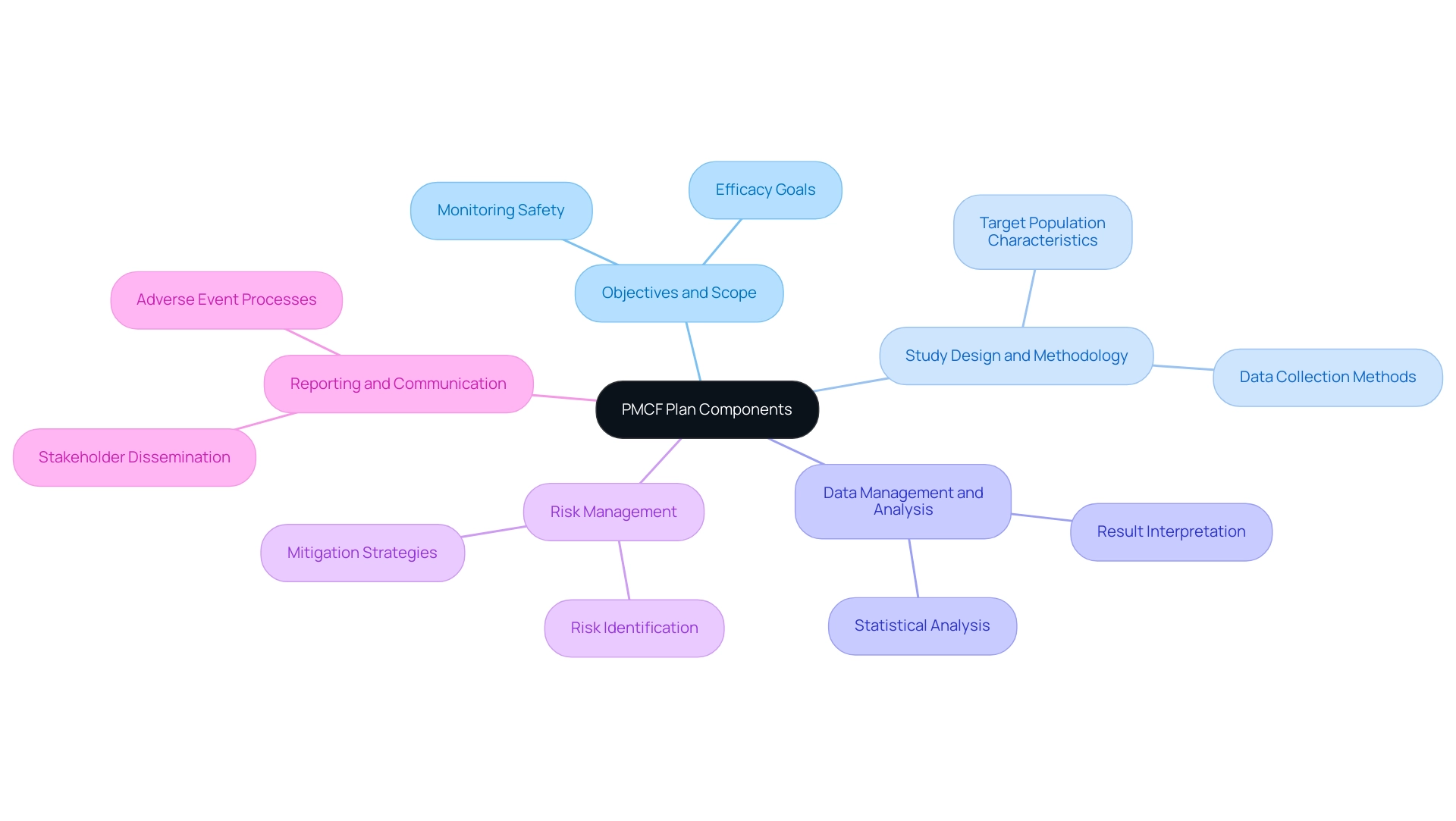
A comprehensive strategy for Post-Market Clinical Follow-Up includes several essential elements crucial for maintaining regulatory compliance and effective oversight of product performance. These include:
- Objectives and Scope: This section outlines clearly defined goals for PMCF activities, emphasizing which aspects of performance will be closely monitored to ensure safety and efficacy.
- Study Design and Methodology: This component provides a thorough description of data collection methods, detailing the type of study—whether observational or randomized—and the characteristics of the target population.
- Data Management and Analysis: Strategies for data handling, including robust statistical analysis and interpretation of results, are crucial for deriving meaningful insights from collected data.
- Risk Management: Identification of potential risks associated with the device's use in a post-market context is essential, alongside strategies for their mitigation.
- Reporting and Communication: This component includes plans for disseminating findings to stakeholders, such as oversight organizations, and outlines processes for addressing any adverse events promptly.
Significantly, the announcement of the clinical study must be made by the sponsor at least 30 days prior to the commencement of the clinical investigation, emphasizing the importance of timely adherence to compliance requirements. By leveraging expert insights, such as those from the Celegence team who assert that by utilizing their services, manufacturers can make Post-Market Clinical Follow-Up activities less time-consuming and costly, organizations can enhance the efficiency and effectiveness of their efforts. Moreover, Katherine Ruiz, a Regulatory Affairs specialist with substantial experience at INVIMA, highlights the significance of following regulatory details in Colombia, ensuring that Post-Market Clinical Follow-Up not only showcases the safety and effectiveness of medical products throughout their lifecycle but also conforms to local regulations.
Bioaccess® provides extensive clinical trial management services, including feasibility evaluations, site selection, compliance assessments, trial setup, import permits, project management, and reporting, all of which are crucial for successful Post-Market Clinical Follow-Up activities. A well-organized method guarantees that these activities adhere to applicable regulations and aid in the continuous evaluation of medical equipment safety and efficacy, especially in the context of Early-Feasibility Research, First-In-Human Trials, and other critical investigations managed by bioaccess®.
Regulatory Considerations for PMCF
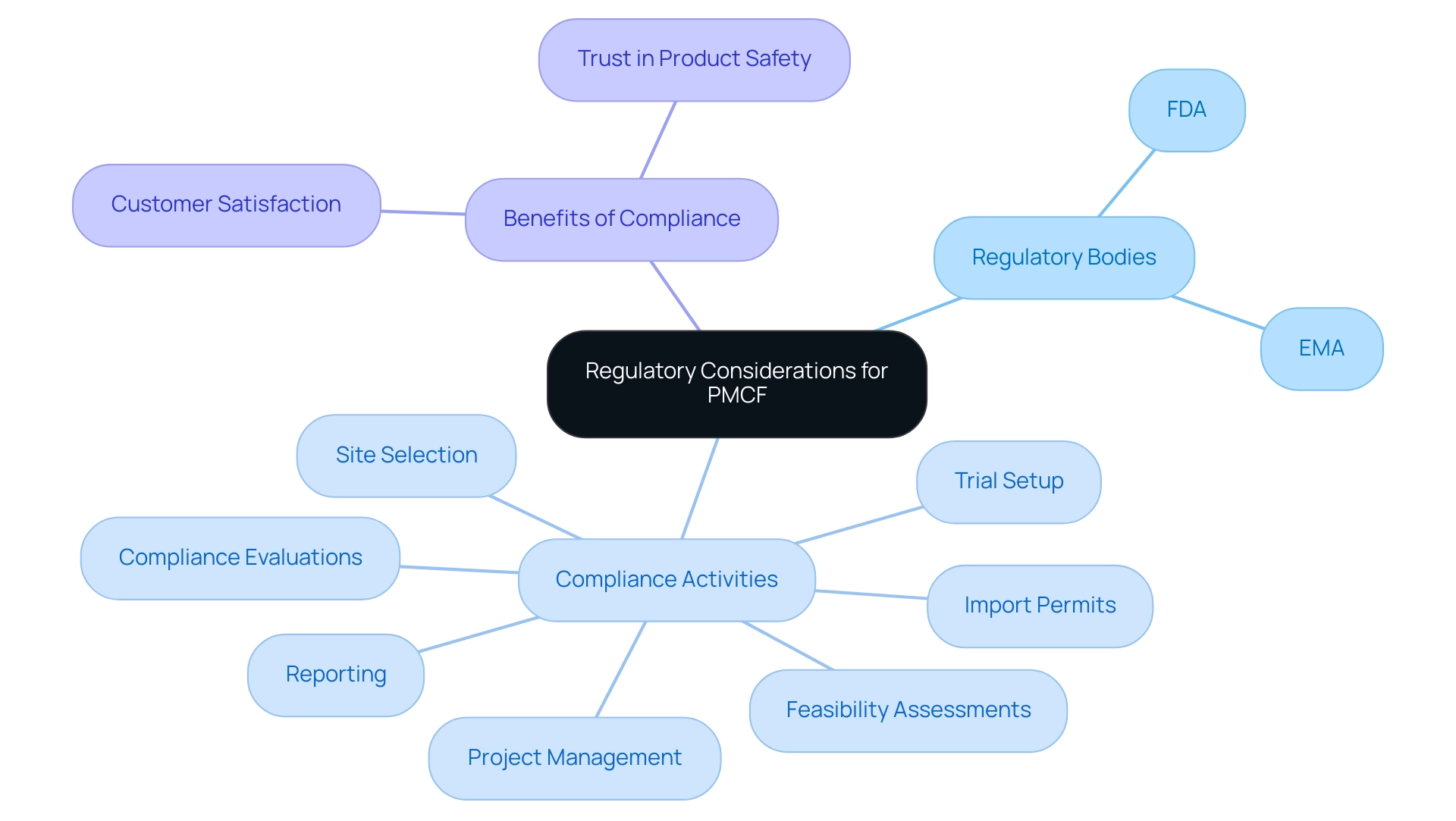
Regulatory considerations for Post-Market Clinical Follow-Up are critical and vary significantly across different regions, primarily overseen by regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for studies. Central to these regulations is the requirement for manufacturers to conduct Post-Market Clinical Follow-Up activities that validate the ongoing safety and efficacy of medical products after their release. Within the European Union, the Medical Device Regulation (MDR) establishes a stringent framework for post-market surveillance, requiring that manufacturers prepare and submit reports on clinical follow-up, document any adverse events meticulously, and provide updates to stakeholders regarding the device's performance over time.
Importantly, the conclusions drawn from the Post-Market Clinical Follow-Up data must be documented in the evaluation report, which assesses the impact on technical documentation and compliance. Compliance with these regulations is crucial—not only does it safeguard patient safety, but it also enhances the credibility of the research data collected. With bioaccess®’s extensive clinical trial management offerings, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial setup
- Import permits
- Project management
- Reporting
clients can navigate the complexities of Post-Market Clinical Follow-Up with confidence.
Bioaccess® brings over 20 years of experience in Medtech, ensuring specialized knowledge and flexibility in managing clinical trials. User surveys, for instance, are a cost-effective method for rapid data collection, typically conducted within weeks or, at most, months. These surveys enable prompt recognition of safety issues and enhance overall product usability, as demonstrated in the case study titled 'Benefits of User Surveys for Post-Market Clinical Follow-Up.'
This proactive approach to compliance can substantially elevate customer satisfaction and reinforce trust in the product's safety profile. Katherine Ruiz, a compliance affairs specialist with extensive experience in medical device and in vitro diagnostics guidelines in Colombia, emphasizes that maintaining adherence to evolving Post-Market Clinical Follow-Up requirements is essential for the integrity of clinical research and the protection of patient welfare.
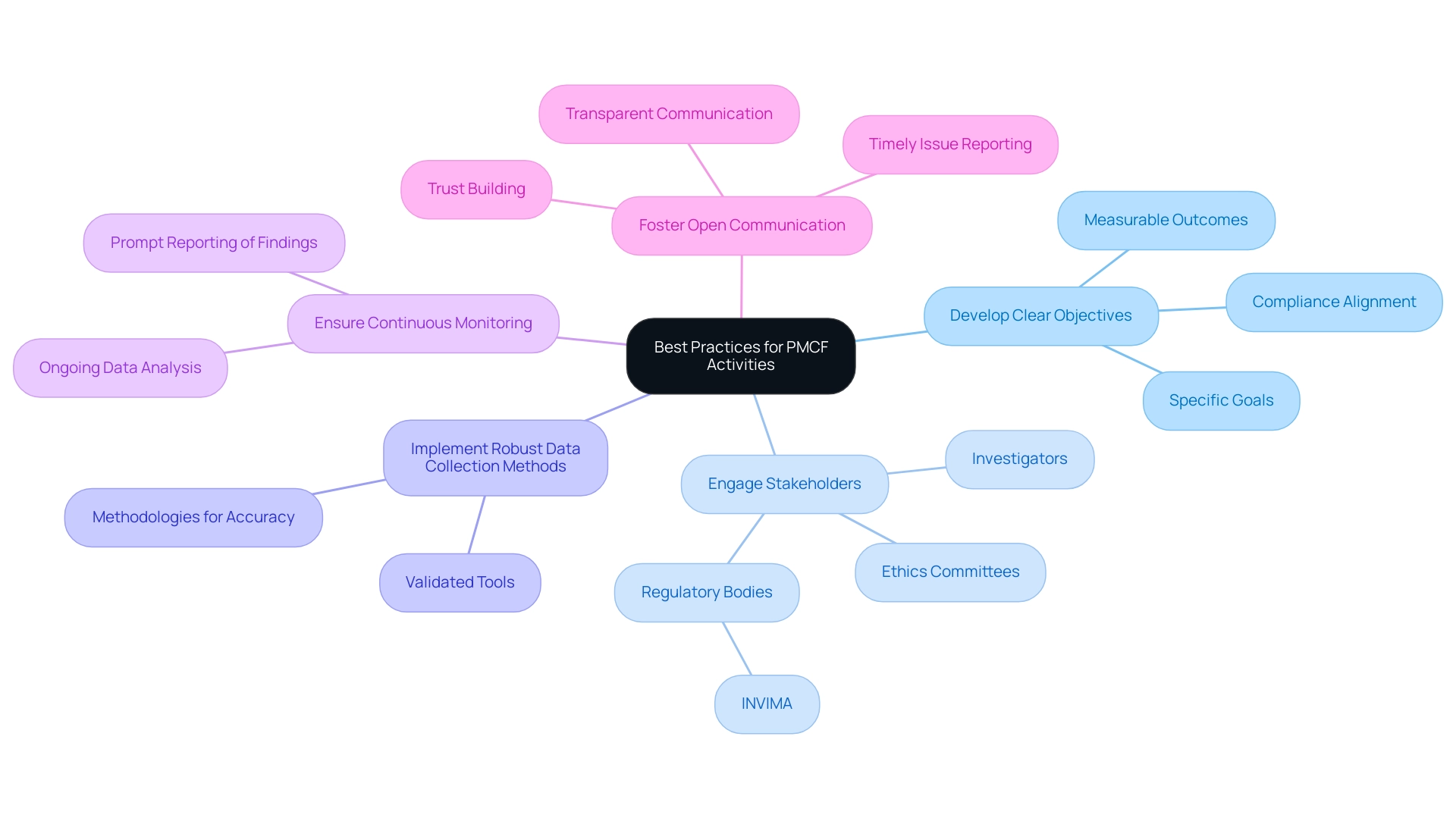
Best Practices for Conducting PMCF Activities
To ensure the effectiveness of Post-Market Clinical Follow-Up activities, researchers should adhere to several best practices:
- Develop Clear Objectives: Establish specific, measurable goals for Post-Market Clinical Follow-Up that align with compliance expectations and clinical relevance, guiding the research direction and outcomes.
- Engage Stakeholders: Collaboration with key stakeholders—including investigators, regulatory bodies like INVIMA, and ethics committees—is vital for comprehensive oversight and a supportive study environment.
- Implement Robust Data Collection Methods: Utilize validated tools and methodologies for data gathering to enhance reliability and accuracy.
The evolving landscape of Post-Market Clinical Follow-Up necessitates rigorous data collection practices.
- Ensure Continuous Monitoring: Regularly assess device performance through ongoing data analysis, promptly reporting concerning findings. This proactive approach is crucial for addressing emerging challenges related to post-market adverse event patterns.
According to recent statistics, these studies play an essential role in mitigating these challenges effectively.
- Foster Open Communication: Maintain transparent communication with all stakeholders to build trust and facilitate timely issue reporting. As outlined in the Medical Device Regulation (MDR), the Post-Market Clinical Follow-Up is defined as 'a continuous process that updates the clinical evaluation,' emphasizing effective communication throughout.
The conclusions of the Post-Market Clinical Follow-Up evaluation report are critical for clinical assessment and risk management, identifying preventive and/or corrective measures based on findings. Furthermore, planning for the Post-Market Clinical Follow-Up involves essential commercial decisions regarding the continuation of products in European markets. By following these best practices, researchers not only uphold the integrity of Post-Market Clinical Follow-Up activities but also contribute to advancing medical knowledge and enhancing patient safety.
Bioaccess® specializes in managing various studies, including Early-Feasibility Studies, First-In-Human Studies, and Pilot Studies, ensuring that all aspects of clinical trials are meticulously handled. Katherine Ruiz, a Regulatory Affairs specialist with extensive experience at INVIMA, plays a crucial role in navigating the regulatory landscape, ensuring compliance, and facilitating successful Post-Market Clinical Follow-Up processes.
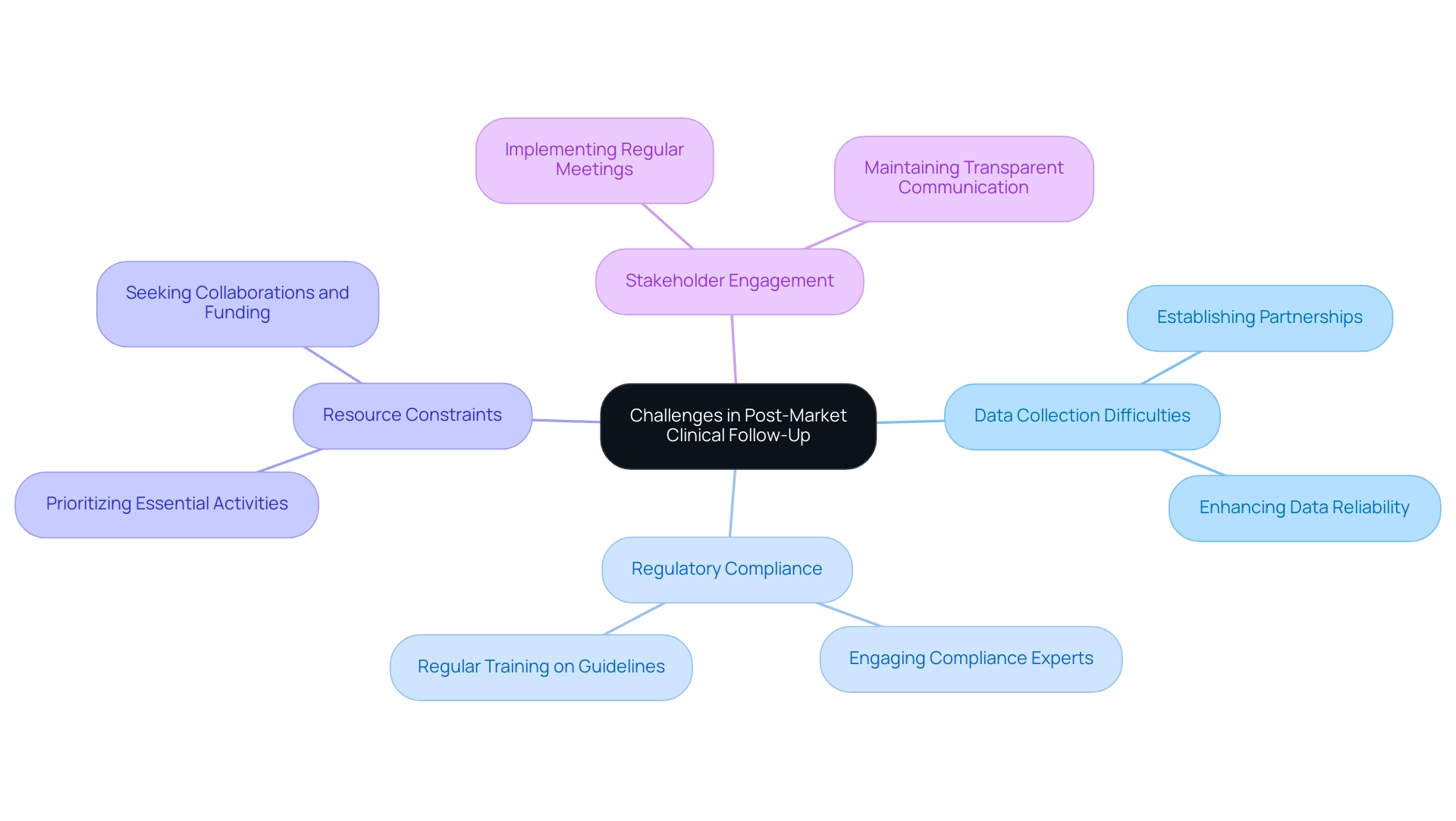
Challenges in PMCF and How to Overcome Them
The challenges presented by Post-Market Clinical Follow-Up activities require strategic solutions to ensure efficacy and safety. Among these challenges are:
- Data Collection Difficulties: One of the primary hurdles researchers face is patient recruitment and ensuring data reliability. Establishing robust partnerships with healthcare providers is crucial, as it facilitates access to diverse patient populations, ultimately enhancing data collection efforts. As Marcus Torr, who has overseen over 400 PMCF surveys, notes, the right partnerships can significantly streamline the recruitment process.
- Compliance with Regulatory Changes: The landscape of medical device regulations is constantly evolving, presenting a daunting task for researchers. To navigate these complexities, engaging with experts like Katherine Ruiz, a compliance affairs specialist with over 20 years in Medtech, is essential. Regular training and staying updated on the latest regulatory guidelines ensure ongoing compliance and reduce risks associated with non-adherence.
- Resource Constraints: Often, project monitoring and control efforts are hindered by limited budgets and personnel. To address this issue, prioritizing essential activities is vital. Researchers should also seek collaborations and funding opportunities to bolster their resources, which will help in fostering a more effective Post-Market Clinical Follow-Up strategy.
- Stakeholder Engagement: Maintaining stakeholder participation throughout the process can be challenging. Implementing regular meetings and maintaining transparent communication strategies can significantly enhance collaboration and support from stakeholders, which is critical for the success of project management initiatives.
To efficiently address these challenges, bioaccess® offers a range of services tailored for Post-Market Clinical Follow-Up, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial organization
- Import permits
- Project management
- Detailed reporting
By leveraging these services, researchers can enhance their Post-Market Clinical Follow-Up strategies while ensuring adherence to regulatory requirements and optimizing resource allocation.
The recent case study titled "Expert Insights from Industry Leaders" highlights how engaging with professionals can provide valuable perspectives on overcoming these challenges. Participants of the webinar will have the chance to take part in a live Q&A session, discussing particular questions and concerns related to Post-Market Clinical Follow-Up activities.
By recognizing these challenges and proactively applying effective solutions, researchers can significantly enhance the impact of their Post-Market Clinical Follow-Up activities, thereby contributing to continuous advancements in medical safety and efficacy. As Gavin Quigley, Senior Internal Clinician at BSI, emphasizes, tackling these hurdles head-on is essential for successful PMCF implementation, supported by the comprehensive clinical trial management services offered by bioaccess®.
Conclusion
Post-Market Clinical Follow-Up (PMCF) is an indispensable aspect of the medical device lifecycle, ensuring that products maintain their safety and effectiveness long after their initial market approval. This article has explored the multifaceted components of PMCF, including its objectives, methodologies, and the critical role of regulatory compliance. By maintaining a systematic approach to data collection and risk management, stakeholders can effectively monitor device performance in real-world settings, thereby safeguarding patient welfare.
The distinction between PMCF and PMCF studies is crucial for researchers, as it informs the design and execution of studies that align with regulatory expectations. A well-structured PMCF plan, incorporating clear objectives and robust data management strategies, is essential for not only fulfilling regulatory obligations but also driving continuous improvement in device quality. Furthermore, best practices such as engaging stakeholders and fostering open communication can significantly enhance the overall effectiveness of PMCF activities.
Despite the challenges that may arise, including data collection difficulties and resource constraints, proactive strategies and expert collaborations can provide the necessary support for successful PMCF implementation. By leveraging the insights and services offered by experienced organizations like Bioaccess®, stakeholders can navigate the complexities of PMCF, ultimately contributing to enhanced medical device safety and efficacy.
In conclusion, the ongoing evaluation of medical devices through PMCF is not merely a regulatory requirement; it is a vital commitment to patient safety and the advancement of healthcare quality. Embracing the principles and best practices of PMCF will ensure that medical devices not only meet but exceed safety and performance standards, fostering trust and confidence in the ever-evolving healthcare landscape.
Frequently Asked Questions
What is Post-Market Clinical Follow-Up (PMCF)?
Post-Market Clinical Follow-Up is a continuous assessment process that evaluates medical products after market authorization to ensure they meet established safety and performance criteria in real-world environments.
What are the primary objectives of PMCF?
The primary objectives of PMCF include verifying the ongoing safety and effectiveness of medical devices, monitoring long-term effects, and detecting any emerging risks or adverse events that may not have been identified during pre-market evaluations.
What activities are involved in PMCF?
PMCF activities involve systematic data collection, rigorous analysis, routine safety monitoring, adverse event reporting, and the aggregation of real-world evidence.
What are common challenges faced in PMCF evaluations?
Common challenges include insufficient data gathering, absence of risk assessment, and inadequate documentation of adverse events.
How can organizations improve the effectiveness of PMCF studies?
Organizations can improve PMCF studies by establishing clear protocols, ensuring timely reporting, and adhering to regulatory standards, which enhances compliance and product quality.
What essential elements should be included in a PMCF strategy?
A comprehensive PMCF strategy should include objectives and scope, study design and methodology, data management and analysis, risk management, and reporting and communication plans.
What regulatory considerations are important for PMCF?
Regulatory considerations vary by region and typically require manufacturers to conduct PMCF activities that validate the ongoing safety and efficacy of medical products, with specific guidelines set by bodies like the FDA and EMA.
What best practices should researchers follow for effective PMCF?
Researchers should develop clear objectives, engage stakeholders, implement robust data collection methods, ensure continuous monitoring, and foster open communication.
What are some strategic solutions to overcome challenges in PMCF?
Strategic solutions include forming partnerships for better data collection, staying updated on regulatory changes, prioritizing essential activities, and maintaining stakeholder engagement through regular communication.
How does Bioaccess® support PMCF activities?
Bioaccess® provides a range of clinical trial management services, including feasibility assessments, site selection, compliance evaluations, trial organization, and detailed reporting, to enhance PMCF strategies while ensuring regulatory compliance.

