Overview
The article emphasizes the essential steps and considerations for designing clinical trials for medical devices in Colombia. It highlights the critical need to understand the local healthcare landscape, regulatory framework, and demographic diversity. This understanding is vital for creating effective trials that not only meet regulatory requirements but also address patient needs. Insights from industry experts and successful case studies further support this approach, demonstrating the importance of collaboration in overcoming challenges within the Medtech landscape.
Introduction
In the rapidly evolving world of clinical research, Colombia emerges as a beacon of opportunity for medical device trials. Its diverse patient population, advanced healthcare infrastructure, and a regulatory framework that fosters innovation position the country as an increasingly attractive destination for U.S. companies looking to conduct clinical studies.
As the landscape continues to develop, grasping the nuances of trial design, regulatory processes, and collaboration with local stakeholders becomes essential for success.
This article explores the intricacies of conducting clinical trials in Colombia, emphasizing the strategic advantages, challenges, and future trends that shape this promising environment for medical device research.
Understanding the Colombian Clinical Trials Landscape
Creating studies for medical devices in Colombia necessitates the design of clinical trials that are deeply informed by the local research environment. This encompasses an understanding of the rich diversity of the patient population, the availability of healthcare facilities, and the expertise of local investigators. Colombia is increasingly recognized for its medical research capabilities, bolstered by a robust healthcare system and a regulatory framework that fosters innovation.
As Julio G. Martinez-Clark, CEO of bioaccess, asserts, "The nation’s combination of a large and diverse population, experienced clinical research sites, efficient regulatory processes, a cost-competitive environment, a regionally respected association of clinical research professionals (AVANZAR) collaborating with the government to increasingly attract more foreign investment in clinical research, and a track record of successful medtech clinical studies since 2010 make it an appealing choice for U.S. medical device companies." By familiarizing yourself with these local dynamics, you can effectively tailor your research design to meet regulatory requirements and address patient needs.
Key aspects to consider include:
- Patient Demographics: Colombia's diverse population provides a broad spectrum of participants, enhancing the generalizability of trial results. This diversity is vital for designing clinical trials for medical devices in Colombia, as it allows for a more comprehensive understanding of how various demographics respond to treatments.
- Healthcare Infrastructure: Evaluate the availability of hospitals and clinics equipped to support your study. Colombia boasts a well-established healthcare system, with numerous facilities that excel in conducting high-quality medical research. Bioaccess offers extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting, ensuring that your study is well-supported.
- Local Expertise: Collaborating with local researchers is essential. Their familiarity with the regulatory environment and cultural nuances can significantly streamline the testing process and enhance participant recruitment. The partnership between bioaccess and Caribbean Health Group aims to establish Barranquilla as a premier location for designing clinical trials for medical devices in Colombia, supported by the nation's Minister of Health, further strengthening the local knowledge available for your research.
- Regulatory Framework: Understanding the role of INVIMA, the National Food and Drug Surveillance Institute, is crucial. INVIMA oversees the regulatory procedures for research studies and medical devices, ensuring compliance with local laws and facilitating the authorization of investigational products.
In 2025, the research study landscape in Colombia is projected to sustain its growth trajectory, with Phase III studies anticipated to be the largest revenue-generating segment. Meanwhile, Phase I studies are expected to emerge as the fastest-growing segment, reflecting a rising interest in early-stage research. The support services market for medical studies is forecasted to expand from USD 79.7 million in 2024 to USD 126.7 million by 2030, demonstrating a compound annual growth rate (CAGR) of 8.1% from 2025 to 2030.
Notably, the country is projected to account for 0.3% of the global market in 2024.
Understanding these trends, alongside the patient demographics and healthcare framework, will empower you to design effective studies for clinical trials for medical devices in Colombia that not only comply with local regulations but also align with the needs of the Colombian population.
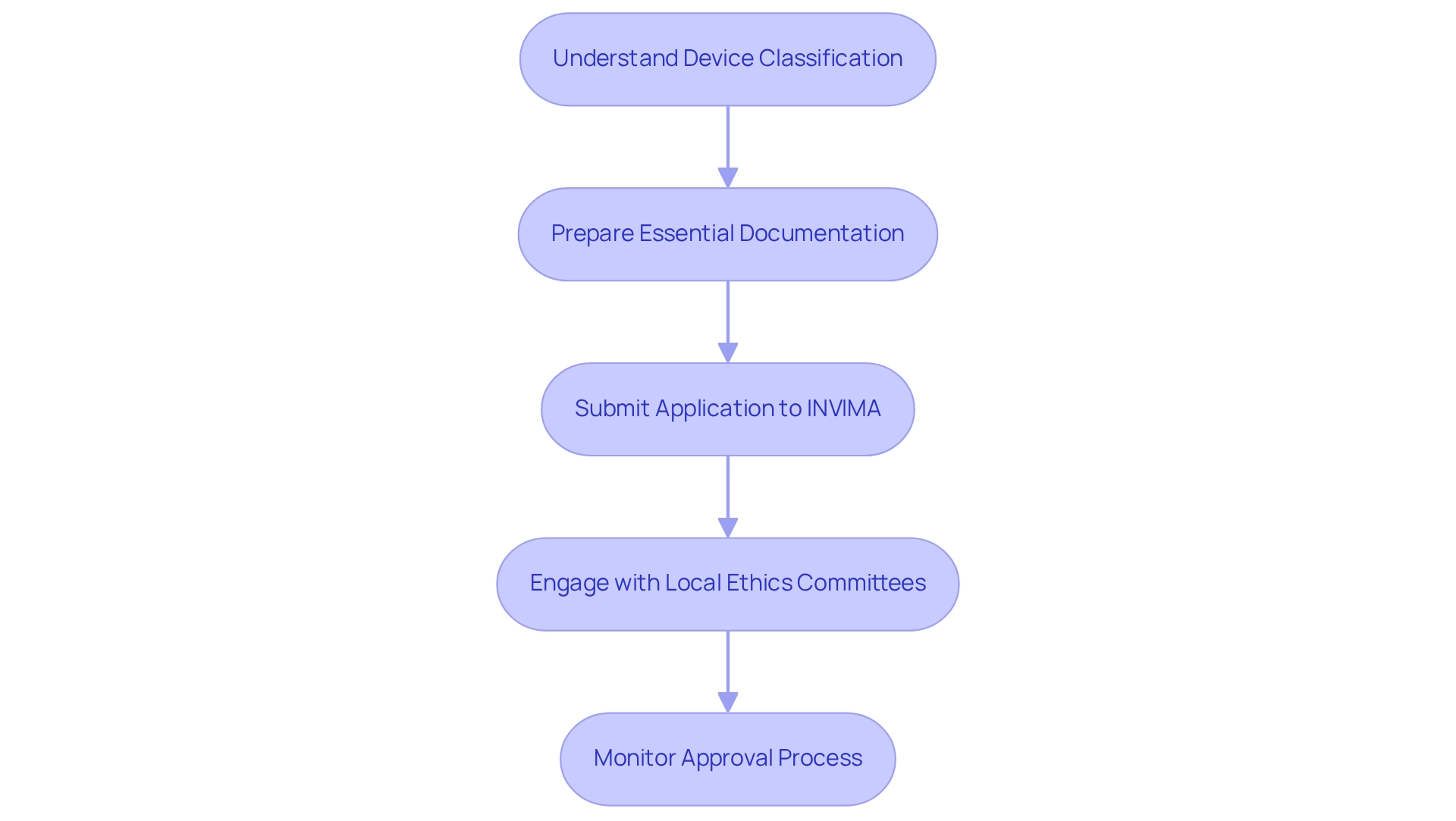
Navigating Colombia's Regulatory Framework for Medical Device Trials
Colombia's regulatory framework for medical device evaluations is primarily overseen by the National Food and Drug Surveillance Institute (INVIMA). To navigate this framework effectively, consider the following steps:
-
Understand the Classification of Your Device: Begin by determining whether your device falls into Class I, II, or III. This classification is crucial as it dictates the regulatory pathway and the level of scrutiny your application will face. bioaccess® can assist in this classification process by providing insights based on their extensive experience in the Medtech sector.
-
Prepare Essential Documentation: Gather all needed paperwork, including research study protocols, informed consent forms, and investigator brochures. Thorough and accurate documentation is essential for a smooth approval process. bioaccess® offers support in preparing these documents to ensure compliance with INVIMA's requirements. This includes guidance on best practices for documentation as outlined in the User Manuals context, ensuring that all necessary elements are included.
-
Submit Your Application to INVIMA: Ensure that your application is complete and adheres to local regulations. A well-prepared submission can significantly expedite the review process. With bioaccess®'s expertise, you can enhance the quality of your submission, increasing the likelihood of a timely review. bioaccess® also provides metrics on submission success rates to further bolster your confidence in the process.
-
Engage with Local Ethics Committees: Securing approval from an Institutional Review Board (IRB) or ethics committee is a prerequisite for INVIMA approval. This step is essential to guarantee that your experiment meets ethical standards and safeguards participant rights. bioaccess® can facilitate communication with these committees to streamline the approval process, leveraging their established relationships to expedite approvals.
-
Monitor the Approval Process: Maintain regular communication with INVIMA throughout the review process. Being proactive in addressing any queries or additional requirements can help facilitate a timely approval. bioaccess®'s team can help in managing this communication efficiently, ensuring that all inquiries are handled quickly.
Colombia ranks fourth in Latin America for recruiting research studies per million people, following Chile, Argentina, and Brazil. This competitive environment highlights the significance of comprehending the INVIMA regulatory framework, particularly in the context of designing clinical trials for medical devices in Colombia, as the agency has recently concentrated on improving the implementation and supervision of research studies. As observed by industry specialists, the evolving guidelines from INVIMA seek to harmonize the processes for sponsors, sites, and ethics committees, thereby simplifying the path to successful medical device study submissions.
Significantly, phase III was the largest revenue-generating phase in the country's research market in 2023, while phase I is anticipated to show the quickest growth during the forecast period. With over 15 years of experience in the Medtech sector, bioaccess® is well-positioned to assist companies in navigating these regulations effectively.
Advantages of Conducting Clinical Trials in Colombia
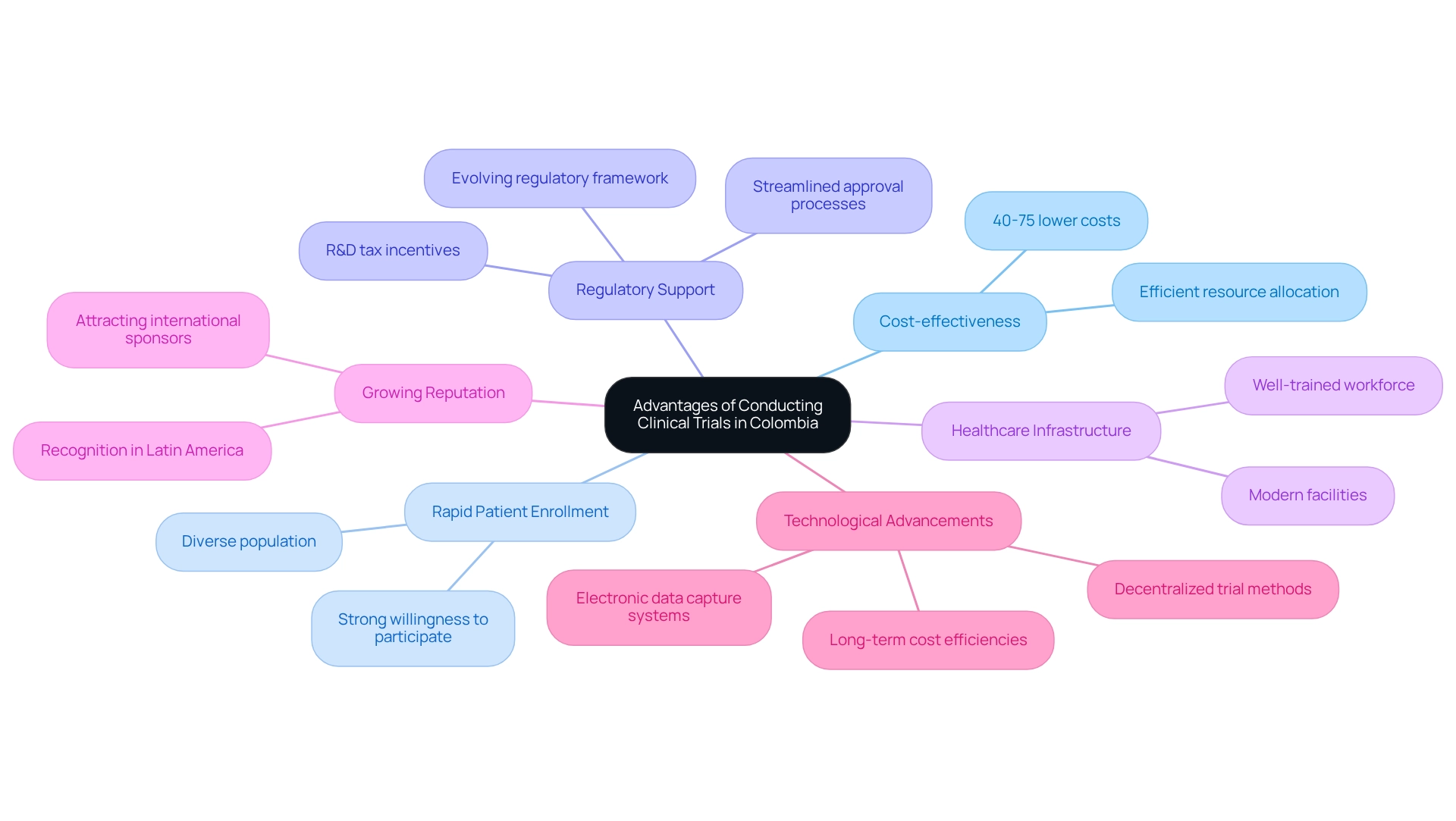
Conducting medical studies in Colombia presents numerous strategic advantages that establish it as a prime location for clinical evaluations.
- Cost-effectiveness is a significant factor; expenses for these studies can be notably lower than those in North America and Europe, often reduced by 40% to 75%. This substantial difference allows sponsors to allocate resources more efficiently while maintaining rigorous study standards.
- Additionally, the country's diverse population and strong willingness to participate in medical studies facilitate rapid patient enrollment. This efficiency is crucial for adhering to study timelines and accelerating the path to market.
- The regulatory support in Colombia is also evolving, with a framework that increasingly favors research initiatives. The flexible regulatory environment, paired with considerable R&D tax incentives, fosters innovation and supports research endeavors. Recent initiatives aim to streamline approval processes, enabling sponsors to navigate the regulatory landscape with greater ease and initiate studies promptly.
- Moreover, Colombia boasts a high-quality healthcare infrastructure, featuring modern facilities and a well-trained workforce of healthcare professionals. This infrastructure ensures that medical studies are conducted under high standards of care, which is essential for the integrity of the research.
- As Colombia continues to enhance its capabilities in medical studies, it is gaining a growing reputation as a hub for clinical evaluations in Latin America. This expanding recognition attracts international sponsors and researchers, further solidifying the nation's position in the global medical research arena.
- Looking ahead to 2025, these advantages are expected to become even more pronounced, with Colombia's regulatory framework evolving to further encourage innovation and research. The nation's commitment to fostering a supportive environment for research is evident in the increasing number of partnerships and collaborations in the sector. The integration of technological advancements, such as electronic data capture systems and decentralized research approaches, is transforming the landscape of medical studies, leading to both initial investments and long-term savings.
- A relevant case study illustrates that while technology may elevate upfront costs, it can yield significant efficiencies and cost reductions over time, making it a worthwhile investment for research sponsors.
- Patricio Ledesma, Head of Clinical Operations and Founder at Sofpromed CRO, underscores the importance of effectively planning and executing studies, expressing his commitment to assisting biotech leaders in navigating these processes across various regions, including Colombia.
- The collaborative environment in this region is further demonstrated by the presence of 266 partners who can utilize specialized purposes to safeguard and communicate privacy choices, thereby enhancing the overall research experience.
In summary, Colombia's strategic advantages—including cost-effectiveness, rapid patient recruitment, a supportive regulatory environment, and high-quality healthcare infrastructure—position it as an ideal location for designing clinical trials for medical devices. The expansion of Argentina's market further contextualizes the allure of Colombia within the broader Latin American landscape, reinforcing its potential as a prominent destination for medical research. Additionally, with bioaccess®'s expertise in overseeing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies, sponsors can confidently rely on a trusted CRO and consulting partner to navigate the complexities of research in Colombia.
Key Design Considerations for Medical Device Clinical Trials
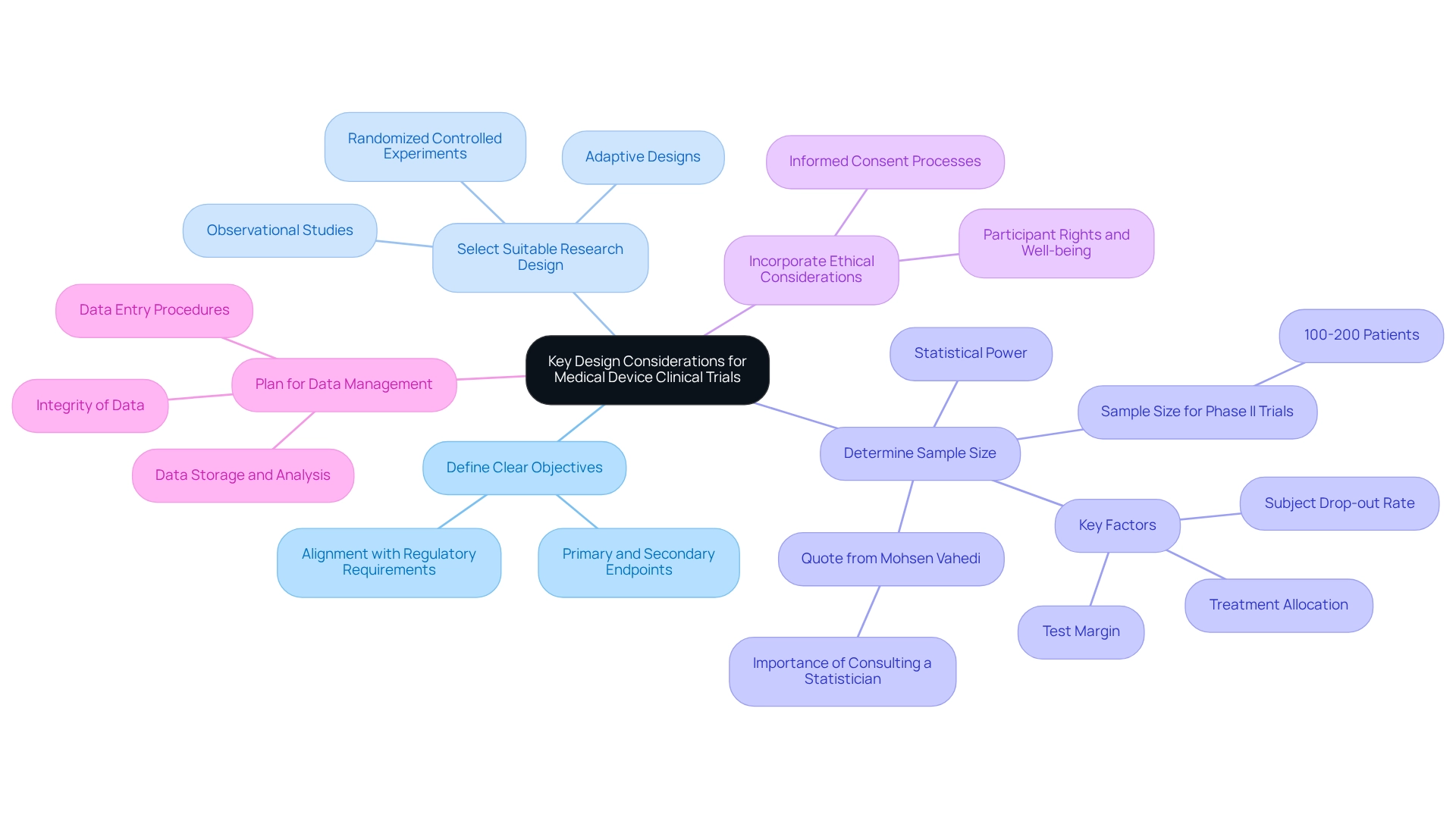
Designing clinical trials for medical devices necessitates meticulous consideration of several key elements to ensure successful outcomes and compliance with regulatory standards. This is particularly vital in Latin America, where bioaccess® offers accelerated medical device clinical study services, drawing on over 20 years of Medtech experience.
- Define Clear Objectives: Establishing well-defined primary and secondary endpoints is crucial. These objectives must align with regulatory requirements and reflect clinical relevance, ensuring that the study effectively addresses significant health outcomes.
- Select Suitable Research Design: The choice of research design—be it randomized controlled experiments, observational studies, or adaptive designs—should be guided by specific research objectives. Each design possesses unique strengths; for instance, randomized controlled experiments are often preferred for their ability to minimize bias, while observational studies can yield valuable insights in real-world contexts.
- Determine Sample Size: Calculating the necessary sample size is essential for ensuring statistical power and the validity of results. For example, Phase II studies typically require a sample size of 100-200 patients. Factors such as test margin, subject drop-out rates, and treatment allocation must be considered to achieve reliable outcomes. Mohsen Vahedi emphasizes that "the determination of sufficient sample size is a crucial aspect of any research studies, and a qualified statistician is the ideal person to consult for assistance during the planning of a research project." A recent study titled "Challenges in Sample Size Calculation for Diagnostic Models" underscores the complexities involved in calculating sample sizes, particularly in ensuring that the sample is representative of the target population and that the metrics used for evaluation are clinically significant.
- Incorporate Ethical Considerations: Ethical considerations are paramount in clinical research design. It is essential to respect the rights and well-being of participants, which includes implementing robust informed consent processes. This not only safeguards participants but also enhances the credibility of the study.
- Plan for Data Management: A comprehensive data management plan is vital for accurate and secure data collection and analysis. This plan should delineate procedures for data entry, storage, and analysis, ensuring that the integrity of the data is preserved throughout the experiment.
By focusing on these factors, researchers can significantly enhance the process of designing clinical trials for medical devices in Colombia. Such efforts lead to efficient studies that not only meet regulatory requirements but also yield valuable insights for the medical device sector. Leveraging bioaccess®'s expertise in overseeing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, combined with a tailored strategy to navigate the complexities of INVIMA regulations and the broader Latin American Medtech landscape, can greatly improve the success of studies in the region.
Fostering Collaboration: Academia, Industry, and Government in Clinical Trials
Cooperation among academia, industry, and government is essential for the successful design of clinical trials for medical devices in Colombia. To foster such collaboration, stakeholders should consider the following strategies:
- Engage with Academic Institutions: Forge partnerships with universities and academic centers to leverage their expertise and resources. A recent analysis of 200 phase III and IV studies published in high-impact medical journals revealed that industry employees co-authored 87% of these publications, highlighting the critical role of academic collaboration in study design and reporting. This partnership enhances the quality of investigations and drives innovation in medical study methodologies.
- Involve Government Agencies: Collaborate with regulatory bodies such as INVIMA to ensure compliance with local regulations and gain insights into the evolving regulatory landscape. This partnership can streamline the approval process and bolster the credibility of medical studies, particularly given the importance of designing clinical trials for medical devices in Colombia. The support from the Minister of Health for initiatives like the partnership between bioaccess™ and Caribbean Health Group to introduce medical trials in Barranquilla exemplifies the significance of governmental involvement.
- Create Public-Private Partnerships: Establish alliances with industry stakeholders to share resources, knowledge, and funding. Successful public-private partnerships in Colombia, which have expedited the development of medical technologies, illustrate the potential for designing clinical trials for medical devices to enhance access to innovative solutions. For instance, the collaboration between GlobalCare Clinical Trials and bioaccess™ has achieved over a 50% reduction in recruitment duration and 95% retention rates, showcasing the effectiveness of joint efforts.
- Engage in Local Networks: Participate in medical study networks and associations to connect with other scholars and organizations in the field. Involvement in these networks can facilitate knowledge exchange and foster collaborative opportunities.
- Leverage Collaborative Statistics: Utilize data from recent studies to inform your strategies. The partnership between academia and industry in research has shown that while most academic authors view these collaborations positively, some encounter challenges such as publication delays and conflicts with industry sponsors. Understanding these dynamics is crucial for developing more effective collaboration strategies. Furthermore, maintaining sufficient mental health, as noted by Gabriela Puentes, enhances work performance in healthcare organizations, underscoring the importance of a supportive environment in research settings. By implementing these strategies, stakeholders can improve the efficiency of research studies in the region, particularly in designing clinical trials for medical devices in Colombia, ultimately facilitating quicker market entry for innovative medical devices and contributing to local economic development.
Challenges and Opportunities in Colombia's Clinical Trial Ecosystem
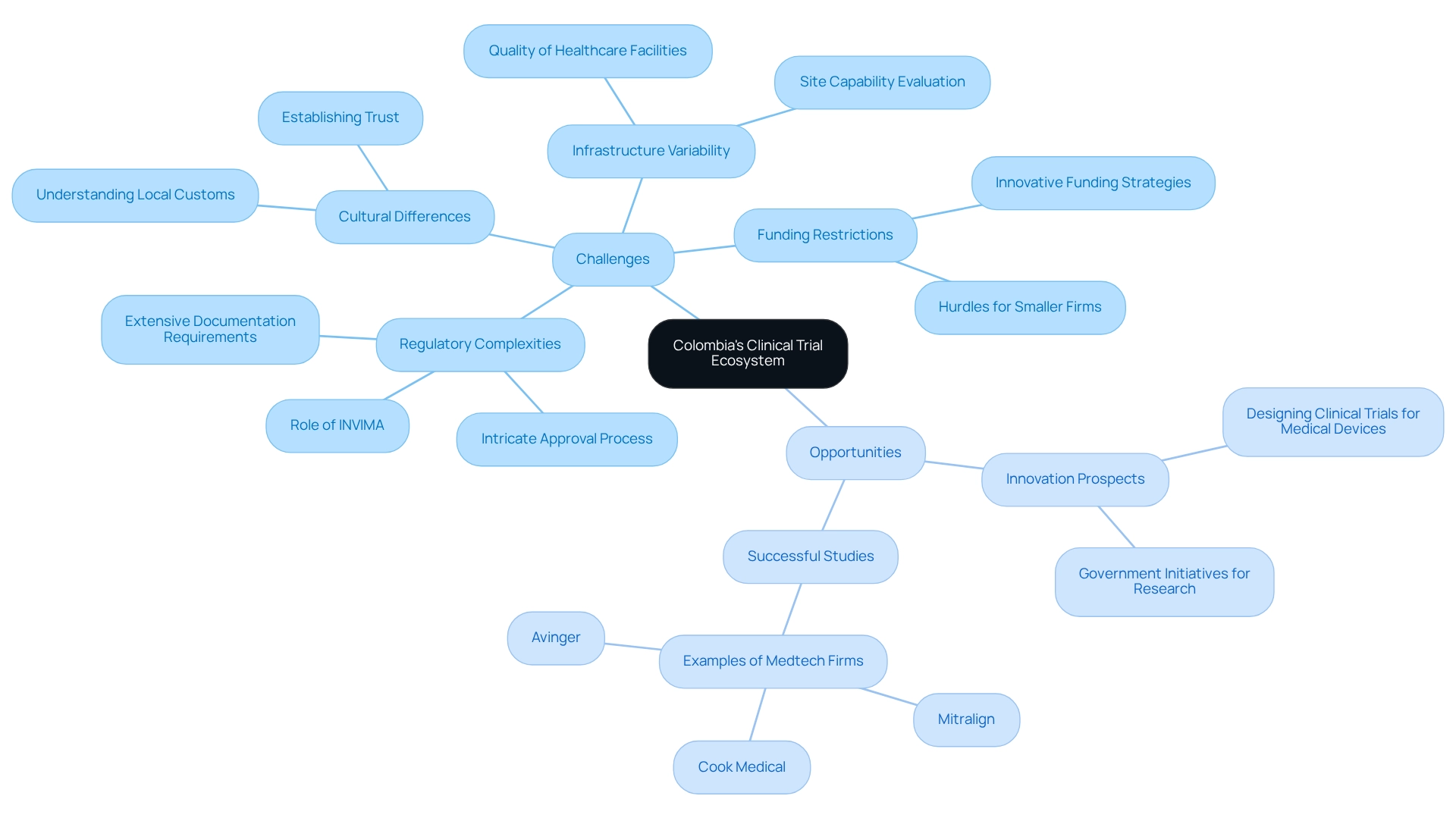
Colombia presents a wealth of opportunities for clinical trials; however, researchers must navigate several challenges that can impact their success.
- Regulatory Complexities: The approval process in Colombia can be intricate and time-consuming, often requiring extensive documentation. Understanding the specific regulatory environment, including the role of INVIMA, is essential when designing clinical trials for medical devices in Colombia. bioaccess offers comprehensive services, including review and feedback on study documents to ensure compliance with country requirements, streamlining this process.
- Cultural Differences: Effective patient recruitment and retention hinge on a deep understanding of local customs and practices. Cultural factors play an important role in establishing trust and ensuring participant involvement throughout the study.
- Infrastructure Variability: The quality of healthcare facilities across Colombia can vary significantly. This inconsistency may impact the implementation of experiments, making it essential for researchers to evaluate site capabilities thoroughly before proceeding. bioaccess aids in the feasibility and choice of research locations and principal investigators (PIs) to ensure optimal study conditions.
- Funding Restrictions: Obtaining sufficient financing for research studies continues to be a hurdle, especially for smaller firms. Innovative funding strategies and partnerships can help mitigate this issue.
In spite of these challenges, there are significant prospects for innovation in designing clinical trials for medical devices in Colombia within the research ecosystem of the nation. The country's large metropolitan areas facilitate robust enrollment and retention, contributing to lower dropout rates compared to the U.S. and EU. Notably, 52% of worldwide studies now take place beyond the U.S., with low- and middle-income nations becoming more appealing for all stages of research due to their larger participant pools and fewer regulatory obstacles.
Several medtech firms, such as Mitralign, Avinger, and Cook Medical, have successfully carried out studies in the country, demonstrating the potential for effective study execution.
As the Colombian government actively aims to draw more medical studies by focusing on designing clinical trials for medical devices as part of its initiative to transform into a knowledge economy by 2031, researchers can utilize this favorable environment to improve the research landscape. Julio G. Martinez-Clark, CEO, observes, "The administration in that country seems to be the sole nation in Latin America with a proactive effort to draw more research studies as part of its strategy to evolve into a knowledge-based economy by 2031." By tackling the current obstacles and leveraging the extensive management services provided by bioaccess, which encompass import permits and nationalization of investigational devices, along with reporting on study progress and adverse events, researchers can promote a more efficient and effective research process, ultimately aiding the advancement of medical devices in the region.
Case Studies: Successful Medical Device Trials in Colombia
The nation has emerged as a significant center for effective medical equipment evaluations, showcasing its robust competencies in healthcare studies. As Julio G. Martinez-Clark, CEO of bioaccess, observes, "The country's combination of a large and diverse population, experienced clinical research sites, efficient regulatory processes, a cost-competitive environment, and a regionally respected association of clinical research professionals (AVANZAR) collaborating with the government to increasingly attract more foreign investment in clinical research, along with a track record of successful medtech clinical studies since 2010, make it an appealing choice for U.S. medical device companies." Notable examples include:
- Cardiac Implantable Devices: A recent trial involving a novel cardiac device yielded impressive results, culminating in regulatory approval and subsequent market entry. This success underscores the country's potential as a testing ground for innovative cardiac solutions.
- Orthopedic Implants: Research on orthopedic implants revealed significant enhancements in patient recovery times, showcasing the device's effectiveness and the country's capacity to conduct impactful studies.
- Diabetes Management Devices: Trials centered on innovative diabetes management devices received favorable patient responses and resulted in better health outcomes, further demonstrating the advantages of conducting medical studies in the country.
In 2023, the nation represented 0.2% of the worldwide medical studies market, emphasizing its importance in the broader context of health investigations. These case studies not only highlight the successful implementation of medical experiments in a South American country but also offer valuable insights and lessons for future investigative efforts. The collaboration between bioaccess and Welwaze Medical Inc. for the launch of the Celbrea® medical device, along with partnerships with GlobalCare Clinical Trials to enhance ambulatory services, exemplifies the strategic alliances that are propelling success in this sector.
Moreover, Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, shared his favorable experience with bioaccess during its initial human trial in South America, reinforcing the effectiveness of these collaborations. Furthermore, Hancock Jaffe Laboratories' choice of bioaccess for the first-in-human Venovalve® study further illustrates the company's crucial role in promoting medical studies in South America. The nation's blend of a varied population, effective regulatory procedures, and a supportive research environment positions it as an attractive location for medical device firms aiming to advance their innovations.
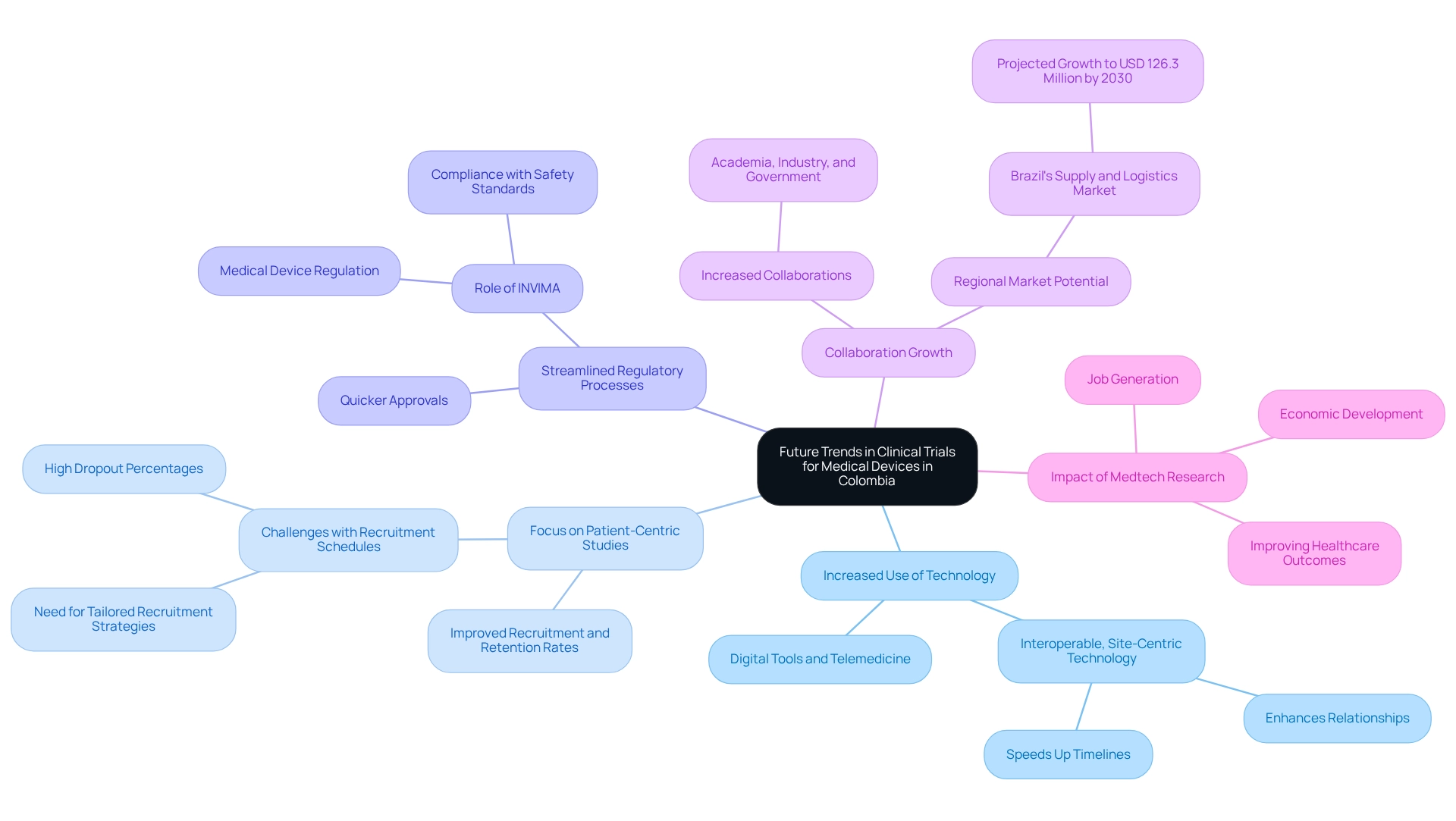
Future Trends in Clinical Trials for Medical Devices in Colombia
The clinical trial landscape in Colombia is undergoing significant transformation, driven by several emerging trends that are set to shape its future:
- Increased Use of Technology: The adoption of digital tools and telemedicine is revolutionizing patient recruitment and data collection processes. This technological integration not only simplifies operations but also improves the overall efficiency of medical studies. As Ryan Jones, CEO & Co-Founder of Florence Healthcare, highlights, "By prioritizing interoperable, site-centric technology, sites, sponsors, and CROs can enhance relationships, speed up timelines, and pave the way in an increasingly digital clinical research landscape."
- Focus on Patient-Centric Studies: There is a growing emphasis on designing studies that prioritize the needs and experiences of patients. This shift towards patient-centric study designs is particularly relevant in designing clinical trials for medical devices in Colombia, as supported by statistics indicating that such approaches can lead to improved recruitment and retention rates, ultimately accelerating the development of medical devices. Nonetheless, the biopharma sector encounters considerable obstacles with recruitment schedules and dropout percentages, underscoring the necessity for more captivating and tailored recruitment strategies to enhance study delivery times and retention rates.
Ongoing efforts to streamline regulatory processes are making Colombia an increasingly appealing location for designing clinical trials for medical devices. These reforms are anticipated to enable quicker approvals, thus accelerating the timeline from initiation to market access. Furthermore, performing a clear country preparedness assessment can assist in prioritizing nations for research allocation, offering a strategic viewpoint for scholars and institutions. In the context of designing clinical trials for medical devices in Colombia, the role of INVIMA, the National Food and Drug Surveillance Institute of the country, is crucial as it oversees medical device regulation and classification, ensuring compliance with safety and efficacy standards.
- Collaboration Growth: The research study environment in the country is experiencing an increase in collaborations among academia, industry, and government organizations. This cooperative method promotes creativity and enhances the framework essential for designing clinical trials for medical devices in Colombia. Moreover, the anticipated expansion of the Brazil research supply and logistics market, predicted to hit USD 126.3 million by 2030, highlights the regional landscape's potential and its implications for Colombia.
Companies like bioaccess® are leading this change by providing services that include designing clinical trials for medical devices in Colombia and offering expedited medical device research study services in Latin America. With over 20 years of expertise in the Medtech field, bioaccess® specializes in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Their tailored method for designing clinical trials for medical devices in Colombia ensures that medical studies are conducted efficiently and effectively, meeting the evolving demands of the market. Furthermore, the influence of Medtech research studies on local economies, including job generation and economic development, emphasizes the wider importance of these assessments in improving healthcare results.
By remaining attuned to these trends, researchers and organizations can strategically position themselves for success in Colombia's dynamic clinical trial environment, ultimately enhancing the advancement of medical devices and improving patient outcomes.
Conclusion
Colombia emerges as a leading destination for clinical trials, particularly in the realm of medical devices, due to its diverse patient population, advanced healthcare infrastructure, and a regulatory framework that fosters innovation. This article underscores the strategic advantages of conducting trials in Colombia, including cost-effectiveness, swift patient recruitment, and growing support from regulatory bodies such as INVIMA. A thorough understanding of the local landscape—including patient demographics and healthcare capabilities—is essential for designing successful trials that comply with regulatory standards.
The collaboration among academia, industry, and government significantly enriches the clinical trial ecosystem, paving the way for innovative solutions and streamlined processes. By engaging with local experts and forming partnerships, companies can navigate the complexities of clinical trials more proficiently. While challenges like regulatory intricacies and cultural differences persist, they can be effectively managed through comprehensive planning and collaboration with experienced local entities.
Looking to the future, emerging trends such as the increased utilization of technology, a focus on patient-centric trial designs, and ongoing regulatory reforms are poised to redefine the landscape of clinical trials in Colombia. As this environment evolves, stakeholders are urged to remain informed and adaptable, ensuring they seize the opportunities presented by this burgeoning market. Ultimately, Colombia's commitment to enhancing its clinical research capabilities positions it as a vital player on the global stage, making it an ideal choice for U.S. medical device companies aiming to advance their innovations and improve patient outcomes.
Frequently Asked Questions
What is necessary for creating studies for medical devices in Colombia?
Creating studies in Colombia requires designing clinical trials that are informed by the local research environment, which includes understanding the patient population, healthcare facilities, and local investigator expertise.
Why is Colombia considered an appealing choice for U.S. medical device companies?
Colombia is appealing due to its large and diverse population, experienced clinical research sites, efficient regulatory processes, cost-competitive environment, and a respected association of clinical research professionals that collaborates with the government to attract foreign investment.
What key aspects should be considered when designing clinical trials in Colombia?
Key aspects include patient demographics, healthcare infrastructure, local expertise, and understanding the regulatory framework governed by INVIMA.
How does patient diversity in Colombia benefit clinical trials?
The diverse population enhances the generalizability of trial results, allowing for a better understanding of how different demographics respond to treatments.
What role does healthcare infrastructure play in clinical trials in Colombia?
A well-established healthcare system with numerous facilities supports high-quality medical research, which is crucial for the success of clinical studies.
Why is local expertise important in clinical trials?
Collaborating with local researchers helps navigate the regulatory environment and cultural nuances, streamlining the testing process and improving participant recruitment.
What is INVIMA's role in the regulatory framework for medical devices in Colombia?
INVIMA oversees the regulatory procedures for research studies and medical devices, ensuring compliance with local laws and facilitating the authorization of investigational products.
What are the projected trends for clinical research studies in Colombia by 2025?
The research study landscape is expected to grow, with Phase III studies being the largest revenue-generating segment, while Phase I studies are anticipated to be the fastest-growing segment.
What steps should be taken to navigate the INVIMA regulatory framework?
Steps include understanding the classification of your device, preparing essential documentation, submitting your application, engaging with local ethics committees, and monitoring the approval process.
How can bioaccess assist in the regulatory process for medical devices?
Bioaccess provides support in classification, documentation preparation, application submission, communication with ethics committees, and managing interactions with INVIMA to facilitate a timely approval process.




