Overview
The article outlines a comprehensive step-by-step guide for designing effective clinical trials for medical devices, emphasizing the importance of understanding clinical trial phases, regulatory requirements, and the role of technology and collaboration. It supports this by detailing the necessity of defining clear endpoints, ensuring appropriate sample sizes, and leveraging advancements like electronic data capture and mobile health applications to enhance trial efficiency and data quality.
Introduction
The landscape of clinical trials is a complex and multifaceted realm that plays a pivotal role in the advancement of medical devices. By systematically categorizing trials into distinct phases—ranging from initial safety assessments to post-market surveillance—researchers can meticulously evaluate the efficacy and safety of innovative technologies.
As the industry evolves, especially in response to the challenges posed by the COVID-19 pandemic, understanding the various types of clinical trials becomes increasingly important. This knowledge not only aids in the design and execution of trials but also addresses the unique hurdles faced by Medtech companies, particularly in regions like Latin America, where regulatory complexities and communication barriers can hinder progress.
The insights provided in this article will illuminate the critical considerations necessary for navigating the clinical trial process, ultimately fostering successful outcomes in medical device development.
Understanding the Phases and Types of Clinical Trials
Clinical studies are systematically classified into four primary phases, each fulfilling a unique role in the advancement and assessment of medical instruments.
- Phase I studies primarily focus on assessing safety and determining dosage levels.
- Phase II studies are structured to assess the effectiveness of the apparatus.
- Phase III studies adopt a comparative approach, evaluating the new device against established standard treatments. Recent data indicates that Phase III success rates have climbed to 66%, as noted by Murray Aitken, significantly surpassing the 56% average observed over the past decade prior to the pandemic, highlighting an encouraging trend in research outcomes.
- Phase IV assessments involve post-market surveillance to monitor ongoing safety and effectiveness.
In addition to these phases, clinical evaluations can be further classified into:
- Interventional studies: actively test new treatments.
- Observational studies: monitor participants without intervention.
- Registry studies: collect data on patients with specific conditions.
Comprehending these categories is essential for designing effective clinical trials for medical devices and selecting the most appropriate design and methodology for your specific study.
This method not only guarantees thorough data gathering for regulatory submissions but also supports designing effective clinical trials for medical devices to showcase the necessary effectiveness for market approval.
As the environment of studies evolves, especially considering the heightened interest in decentralized and hybrid models, as shown by the COVID-19 pandemic's influence on rare disease studies, being knowledgeable in these phases and types will be crucial for achieving successful results in research.
Moreover, Medtech companies face unique challenges in Latin America, including regulatory hurdles and communication barriers. bioaccess® tackles these challenges with its customized services, such as Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), which are essential for maneuvering the intricacies of research in this area.
Their expertise in managing Pilot, Pivotal, and Post-Market Follow-Up studies further provides essential solutions to bridge the gap between innovation and execution in Latin America. Furthermore, it is noteworthy that 50% of experiments that specified children in their inclusion criteria were open to neonates, emphasizing the importance of inclusivity and diversity in research.

Key Considerations for Designing Clinical Trials for Medical Devices
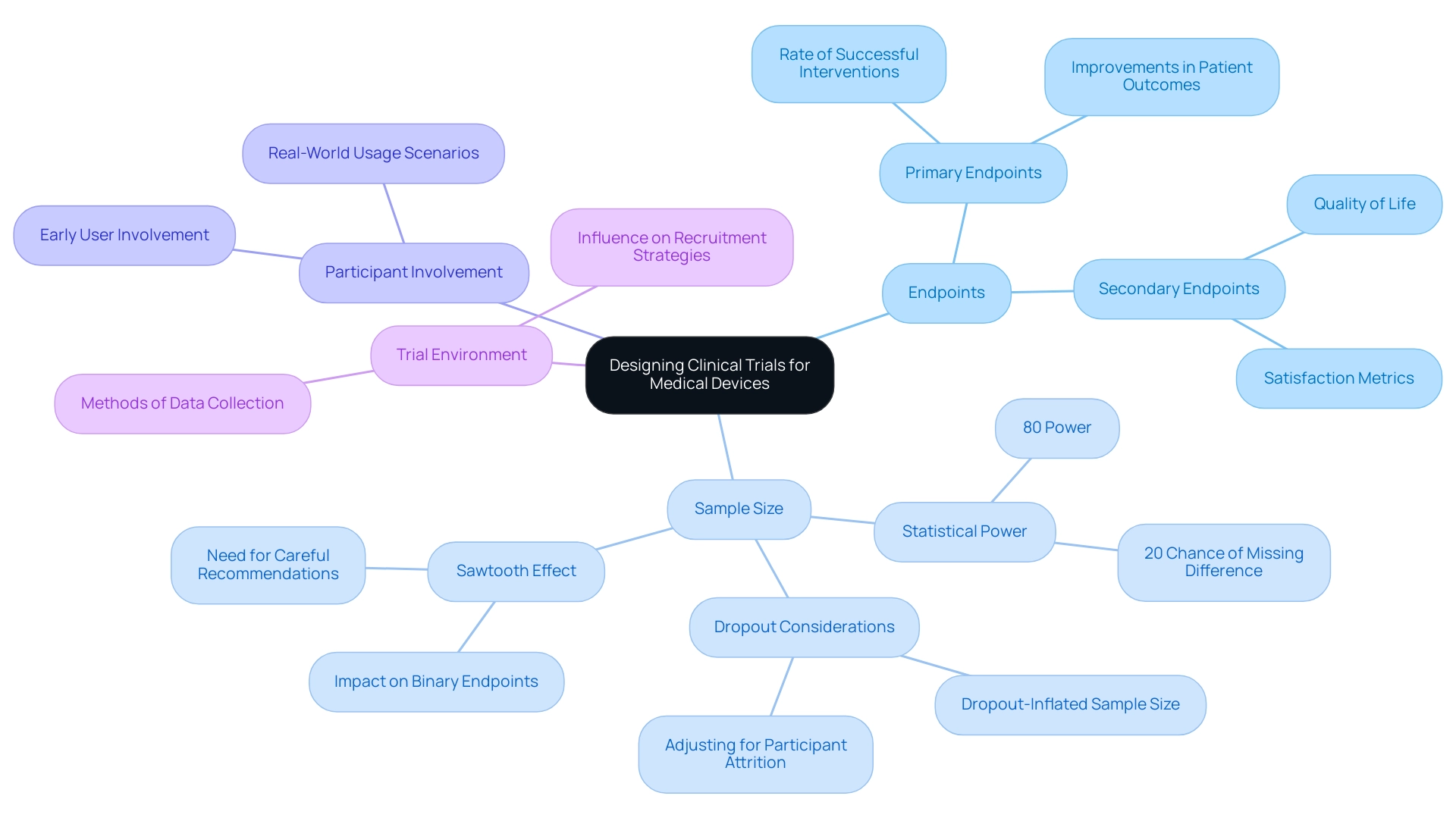
Designing effective clinical trials for medical devices requires a thorough understanding of several critical considerations, especially when leveraging comprehensive clinical trial management services like those offered by bioaccess®, which boasts over 20 years of experience in the Medtech field. First and foremost, it's essential to define clear primary and secondary endpoints that accurately reflect the intended use of the apparatus. These endpoints serve as the foundation for evaluating the device's effectiveness and safety.
For instance, common primary endpoints might include:
- The rate of successful interventions
- Improvements in patient outcomes
While secondary endpoints could measure:
- Quality of life
- Satisfaction metrics
Determining an appropriate sample size is another crucial step to ensure statistical power. Most clinical trials operate with a power of 80%, indicating a 20% chance of failing to detect a real difference. This statistic highlights the significance of careful sample size calculations, as an insufficient sample can result in underpowered research, undermining the reliability of results.
Furthermore, the 'sawtooth effect' can occur for binary endpoints, where a small increase in sample size may inadvertently decrease research power, necessitating careful sample size recommendations.
Incorporating the expertise of a vetted CRO like bioaccess® can also help account for potential participant dropouts, which significantly impact research power. For instance, addressing dropout considerations through suitably increased sample sizes can result in more dependable outcomes, as demonstrated in case studies concentrating on dropout rates in research trials.
Involving users early in the design process is crucial for designing effective clinical trials for medical devices to align the study with real-world usage scenarios and enhance the product's applicability. This method is especially pertinent for bioaccess®'s emphasis on Designing Effective Clinical Trials for Medical Devices, which includes overseeing Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), ensuring that potential pitfalls are recognized and enhancing the process for effective recruitment and data collection. Furthermore, the medical environment where the device will be utilized plays a significant role in influencing both recruitment strategies and the methods of data collection.
Comprehending these dynamics is crucial for optimizing the experiment's success and ensuring that the results are relevant to everyday medical environments.
Lastly, it is important to mention that among randomized protocol guidelines that outline a sample size computation, only 4-40% sufficiently detail all elements of the calculation, including the outcome metrics and assumed values for each participant group. A well-organized protocol should clarify these elements to enhance transparency and reproducibility in research, emphasizing the necessity of designing effective clinical trials for medical devices to ensure rigorous experimental designs. bioaccess®'s adaptable and personalized strategy enables bespoke solutions that tackle the distinct challenges encountered in study design, further improving the success of your research.
Navigating Regulatory Requirements in Clinical Trial Design
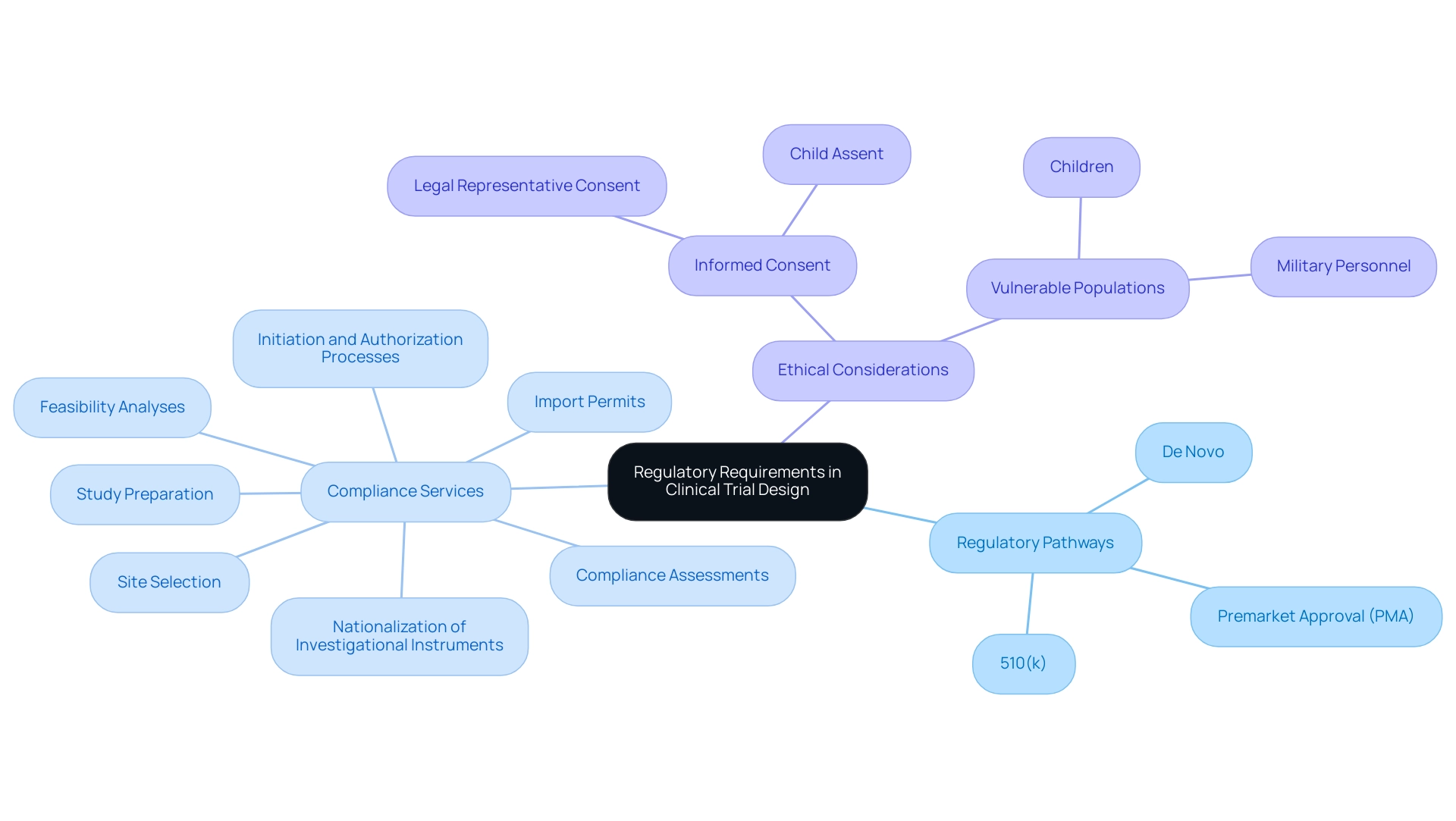
Navigating the regulatory environment for medical products requires a thorough understanding of the requirements set by the FDA and other regulatory bodies. A critical first step is to determine the appropriate regulatory pathway for the medical product, which may involve classifications such as:
- 510(k)
- Premarket Approval (PMA)
- De Novo
Each pathway has unique criteria and implications for the study process.
To ensure compliance and increase the chances of approval, it is vital to prepare a comprehensive research protocol that follows Good Clinical Practice (GCP) standards. This involves thorough planning of study goals, methodology, and participant safety protocols.
Our extensive management services for research encompass:
- Feasibility analyses
- Site selection
- Compliance assessments
- Study preparation
- Initiation and authorization processes
- Import permits
- Nationalization of investigational instruments to ensure a seamless study setup
If the examination includes an experimental instrument, submitting an Investigational Instrument Exemption (IDE) application may be necessary. This application permits the medical examination of products that fall under the FDA's jurisdiction and is essential for studies that require FDA supervision.
Interacting with regulatory authorities at the beginning of designing effective clinical trials for medical devices can provide invaluable insights. By encouraging open dialogue with these organizations, researchers can clarify expectations, address potential concerns, and streamline the approval process, ultimately leading to designing effective clinical trials for medical devices and improved outcomes in medical device development.
Katherine Ruiz, an expert in regulatory affairs for medical devices and in vitro diagnostics in Colombia, highlights the importance of understanding local regulatory nuances that can impact study progression. It is also important to mention that in certain military operations, the US President can waive consent requirements for military personnel, which may have consequences for studies involving this population. Furthermore, if a New Drug Application (NDA) is not approved, records must be retained for two years after the shipment and delivery of the investigational product is discontinued, underscoring the importance of regulatory compliance.
As Lauren K. Roth, Associate Commissioner for Policy, stated, 'This guidance represents the current thinking of FDA on Conducting Trials With Decentralized Elements,' highlighting the evolving landscape of trials.
Moreover, ethical considerations are paramount in research, particularly when involving vulnerable populations such as children. The case analysis on informed consent for children highlights that further safeguards must be established to protect their rights and welfare, necessitating informed consent from a legal representative and assent from the child when suitable.
For more information on how we can assist with your research needs, please BOOK A MEETING.
Leveraging Technology for Enhanced Clinical Trial Design
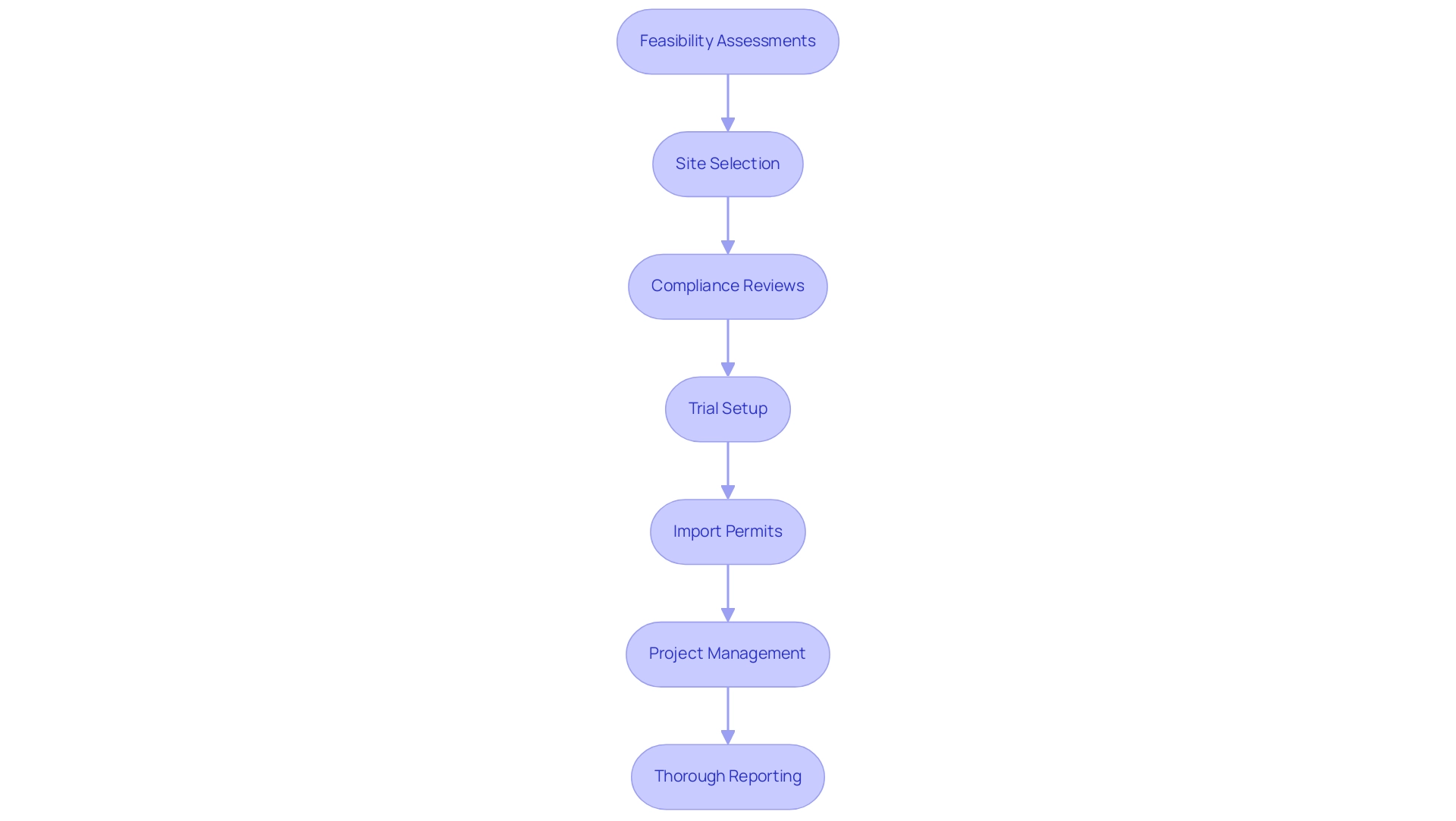
Incorporating technology into research design is crucial for designing effective clinical trials for medical devices, enhancing efficiency and guaranteeing high data quality. Our extensive trial management services encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Thorough reporting
All intended to comply with regulatory standards and national requirements. The FDA's 2017 guidance on electronic records in research investigations underscores the importance of implementing electronic data capture (EDC) systems, which can streamline data collection processes, enhance accuracy, and reduce the likelihood of errors.
In 2024, the increasing reliance on mobile health (mHealth) applications stands out, facilitating remote patient monitoring and significantly bolstering participant engagement. For instance, the ResearchKit software exemplifies this trend; it successfully recruited 11,000 participants for a cardiovascular study within just 24 hours. However, challenges persist in ensuring data quality and participant privacy, particularly when designing effective clinical trials for medical devices that involve recruiting diverse populations.
Utilizing data analytics tools is essential in designing effective clinical trials for medical devices, as it enables real-time monitoring of study progress and outcomes while fostering adaptive designs that can quickly respond to emerging data. As emphasized by Reuter, many social media users do not view the monitoring of their accounts for randomized controlled study recruitment as a breach of privacy, indicating a potential avenue for increasing participant diversity and suggesting that social media could be a valuable resource for reaching a broader participant base. Furthermore, our services highlight the essential function of ethics committees and the choice of a qualified principal investigator (PI) to ensure compliance and integrity throughout the research process.
Our services are poised to contribute to local economies through job creation, economic growth, and healthcare improvements, driven by international collaboration and innovation in MedTech. Therefore, adopting these technological advancements is not just advantageous but essential for designing effective clinical trials for medical devices in the future of medical studies.
The Role of Collaboration in Successful Clinical Trials
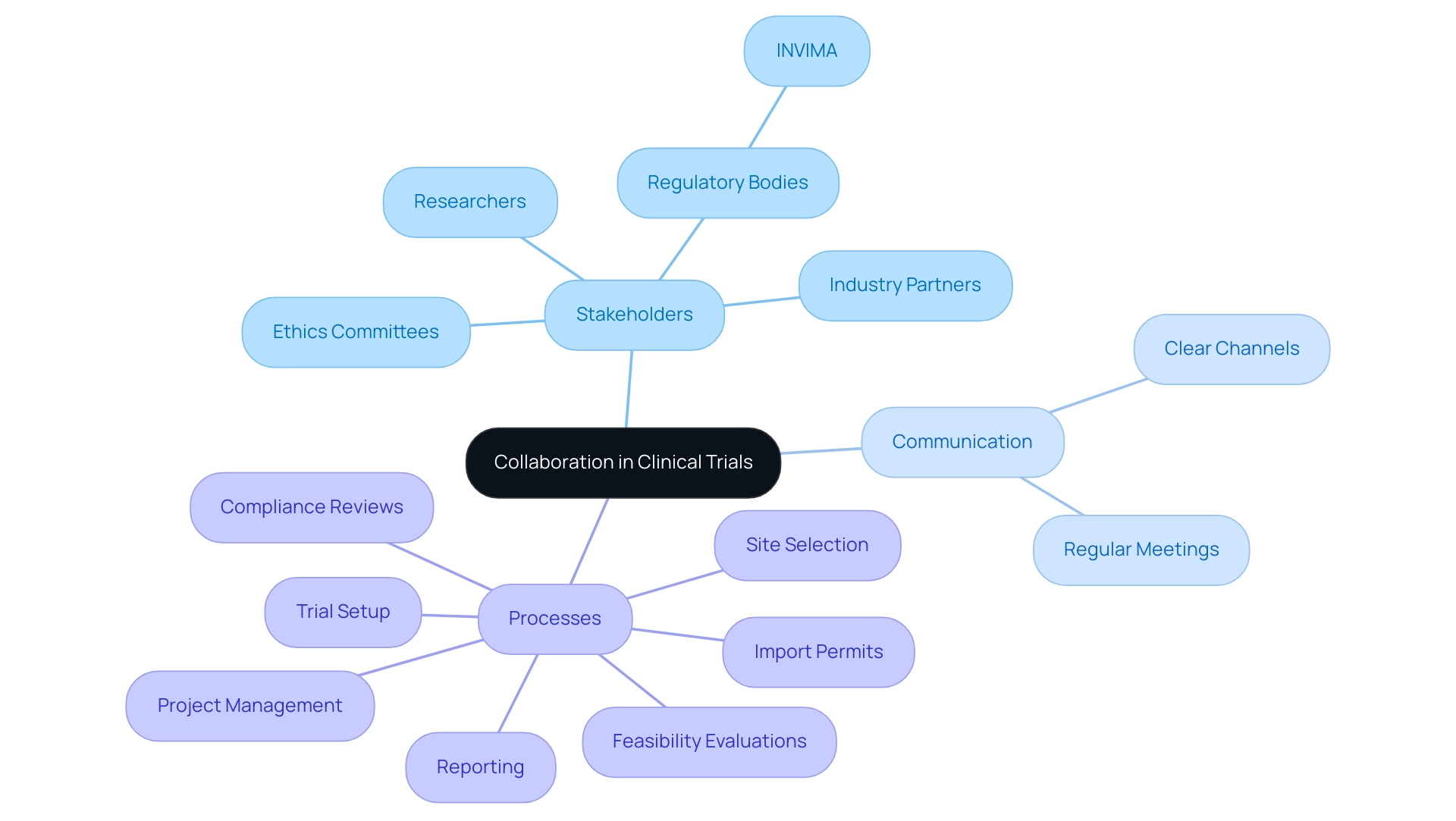
Successful execution of clinical studies is essential for designing effective clinical trials for medical devices and hinges on effective collaboration among researchers, regulatory bodies like INVIMA, industry partners, and ethics committees. Dushyanth Surakanti, the Founder and CEO of Sparta Biomedical, emphasizes the role of bioaccess® during its initial human study in Colombia, illustrating the importance of clear communication channels to define roles and responsibilities. This approach facilitates smoother interactions throughout the testing process.
Regular meetings and progress updates are crucial in ensuring all stakeholders stay aligned with the project's objectives and timelines. Evidence suggests that strong relationships among all parties enhance compliance and foster a culture of transparency and trust, crucial for navigating potential challenges. An investigation examining information from ClinicalTrials.gov highlights the importance of teamwork, showing that efficient communication significantly affects research success rates. This finding is further supported by Dr. John B. Simpson’s work on Avinger's OCT-guided atherectomy research in Cali, Colombia, showcasing the benefits of collaboration with LATAM CRO experts. Additionally, designing effective clinical trials for medical devices, along with thorough trial management services—including:
- Feasibility evaluations
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
are crucial for ensuring successful outcomes.
The economic influence of Medtech research studies on local economies—such as job creation, economic growth, and healthcare enhancement—highlights the significance of international collaboration. In the words of co-first authors Eungdo Kim and Jaehoon Yang, who contributed equally to the research, "Our joint efforts underscore the collective responsibility required for successful clinical research." Building these collaborative networks is not just beneficial; it is imperative for designing effective clinical trials for medical devices, advancing medical knowledge, and creating innovative solutions in the digital health and MedTech landscape.
Conclusion
Understanding the complexities involved in clinical trials is essential for advancing medical device development. The systematic categorization of trials into distinct phases—ranging from safety assessments in Phase I to post-market surveillance in Phase IV—provides a structured approach to evaluating both efficacy and safety. The evolving landscape, particularly influenced by the COVID-19 pandemic, has underscored the importance of adapting trial designs to meet contemporary challenges, especially in regions like Latin America where regulatory hurdles can impede progress.
Key considerations in trial design, such as:
- defining endpoints
- determining sample sizes
- engaging users early in the process
are critical for ensuring that studies yield reliable and applicable results. Incorporating technology, such as electronic data capture and mobile health applications, enhances data quality and participant engagement, which are vital for the success of clinical trials. Furthermore, effective collaboration among researchers, regulatory bodies, and industry partners fosters transparency and trust, which are crucial for navigating the complexities of clinical research.
In conclusion, the journey of medical device development through clinical trials is multifaceted, requiring careful planning, strategic collaboration, and innovative approaches. By understanding the various phases and types of trials, addressing regulatory requirements, leveraging technology, and fostering collaboration, Medtech companies can navigate the landscape more effectively. This comprehensive understanding not only aids in the successful execution of trials but also ultimately contributes to the advancement of healthcare solutions that can significantly improve patient outcomes.
Frequently Asked Questions
What are the four primary phases of clinical studies?
The four primary phases of clinical studies are: 1. Phase I - Focuses on assessing safety and determining dosage levels. 2. Phase II - Assesses the effectiveness of the apparatus. 3. Phase III - Evaluates the new device against established standard treatments. 4. Phase IV - Involves post-market surveillance to monitor ongoing safety and effectiveness.
What is the significance of Phase III studies?
Phase III studies adopt a comparative approach, and recent data indicates that their success rates have climbed to 66%, surpassing the 56% average observed over the past decade prior to the pandemic. This trend highlights an encouraging improvement in research outcomes.
What are the different types of clinical evaluations?
Clinical evaluations can be classified into three types: Interventional studies (actively test new treatments), Observational studies (monitor participants without intervention), and Registry studies (collect data on patients with specific conditions).
Why is understanding the phases and types of clinical studies important?
Understanding these categories is essential for designing effective clinical trials for medical devices and selecting the most appropriate design and methodology for specific studies, ensuring thorough data gathering for regulatory submissions and market approval.
How has the COVID-19 pandemic influenced clinical studies?
The pandemic has heightened interest in decentralized and hybrid models of study, particularly in the context of rare disease studies, making knowledge of the various phases and types crucial for successful research outcomes.
What challenges do Medtech companies face in Latin America?
Medtech companies in Latin America encounter regulatory hurdles and communication barriers. Customized services like Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH) offered by bioaccess® help navigate these challenges.
What are primary and secondary endpoints in clinical trials?
Primary endpoints are the main outcomes used to evaluate the device's effectiveness and safety, such as the rate of successful interventions. Secondary endpoints measure additional factors like quality of life and satisfaction metrics.
Why is determining an appropriate sample size important in clinical trials?
An appropriate sample size ensures statistical power, which is typically set at 80%, indicating a 20% chance of failing to detect a real difference. Insufficient sample sizes can lead to underpowered research and unreliable results.
What is the 'sawtooth effect' in clinical trials?
The 'sawtooth effect' can occur for binary endpoints, where a small increase in sample size may inadvertently decrease research power, necessitating careful sample size recommendations.
How does involving users early in the design process benefit clinical trials?
Involving users early helps align the study with real-world usage scenarios, enhancing the product's applicability and improving recruitment and data collection processes.
What should a well-organized protocol for clinical trials include?
A well-organized protocol should detail all elements of sample size calculation, including outcome metrics and assumed values for each participant group, enhancing transparency and reproducibility in research.




