Introduction
The medical device landscape in Latin America is rapidly evolving, influenced by a multitude of factors ranging from regulatory complexities to the integration of cutting-edge technologies. In Colombia, the classification of medical devices into distinct risk categories lays the groundwork for a robust regulatory framework, ensuring patient safety and product efficacy.
As researchers and industry professionals embark on the journey of clinical trials, understanding the intricate stages—from pilot to post-market studies—becomes paramount. However, this path is fraught with challenges, including:
- Patient recruitment
- Regulatory compliance
- Data management
All of which demand innovative strategies and meticulous planning.
With emerging trends and therapeutic areas gaining traction, the collaboration between stakeholders is more critical than ever, paving the way for advancements that promise to enhance healthcare outcomes.
This article delves into the essential aspects of medical device trials in Colombia, offering insights into the regulatory landscape, trial management, and the future of Medtech innovations in the region.
Fundamentals of Medical Devices: Definitions and Classifications
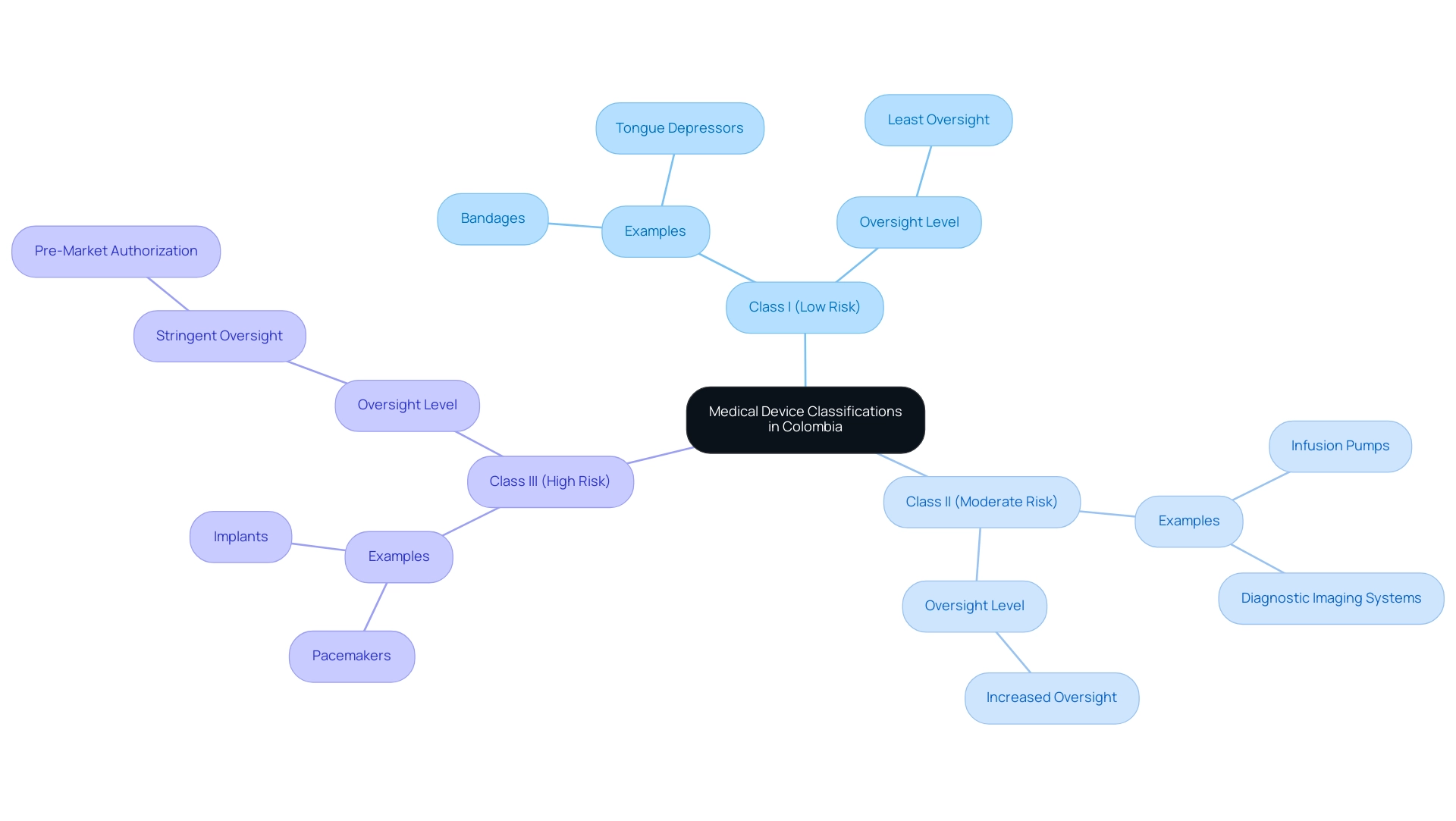
In Colombia, medical instruments are classified into three categories according to their risk level, as established by authorities such as INVIMA (Colombia National Food and Drug Surveillance Institute).
- Class I products, such as bandages and tongue depressors, pose the lowest risk and are subject to the least oversight.
- Class II products, including infusion pumps and diagnostic imaging systems, need increased oversight because of their moderate risk.
- Class III instruments, like pacemakers and implants, are considered the highest risk and require stringent oversight, including pre-market authorization due to their potential effect on patient health.
Comprehending these classifications is essential for researchers maneuvering through the intricacies of Medical Device Trials and equipment evaluations efficiently, especially in the framework of INVIMA's supervision, which guarantees the safety, efficacy, and quality of medical products in line with PAHO/WHO standards. INVIMA's Directorate for Medical Devices and other Technologies plays a vital role in monitoring compliance, suggesting technical standards, and overseeing both pre- and post-market evaluations.
Katherine Ruiz, a Regulatory Affairs expert with extensive experience at INVIMA, emphasizes the importance of adhering to these classifications and her contributions in facilitating the import licenses for diagnostic reagents and medical products, underlining her depth of expertise in the regulatory process.
Navigating the Stages of Medical Device Clinical Trials
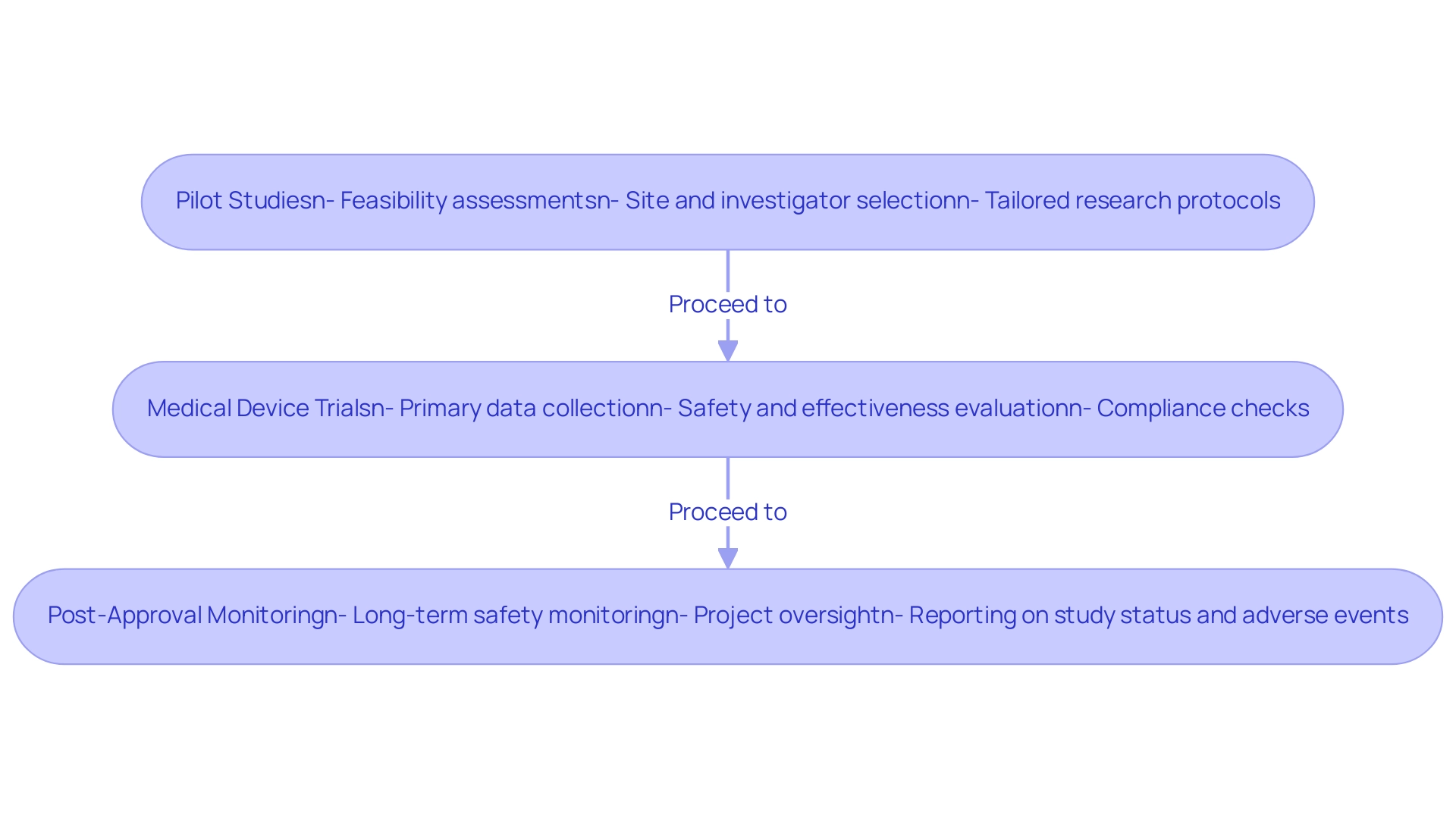
Medical device clinical studies typically advance through several key stages, each of which is supported by comprehensive management services from bioaccess®:
-
Medical Device Trials include pilot studies that assess feasibility, safety, and design before larger research. bioaccess® assists with feasibility and selection of research sites and principal investigators, helping to refine research hypotheses and protocols. Their customized approach ensures that each test is tailored to meet specific needs.
Medical Device Trials: These larger studies provide the primary data for regulatory approval, concentrating on the safety and effectiveness of the tool in a specific patient population. With over 20 years of experience in Medtech, bioaccess® ensures compliance through thorough reviews of study documents and experiment setup, enhancing the reliability of the data collected. Medical Device Trials are conducted after a product is approved to monitor long-term safety and effectiveness in a real-world setting. bioaccess® manages project oversight and provides detailed reporting on study status, inventory, and adverse events. Comprehending these stages, along with the knowledge and tailored services offered by bioaccess®, is essential for researchers to design and oversee studies effectively.
Challenges in Conducting Medical Device Clinical Trials
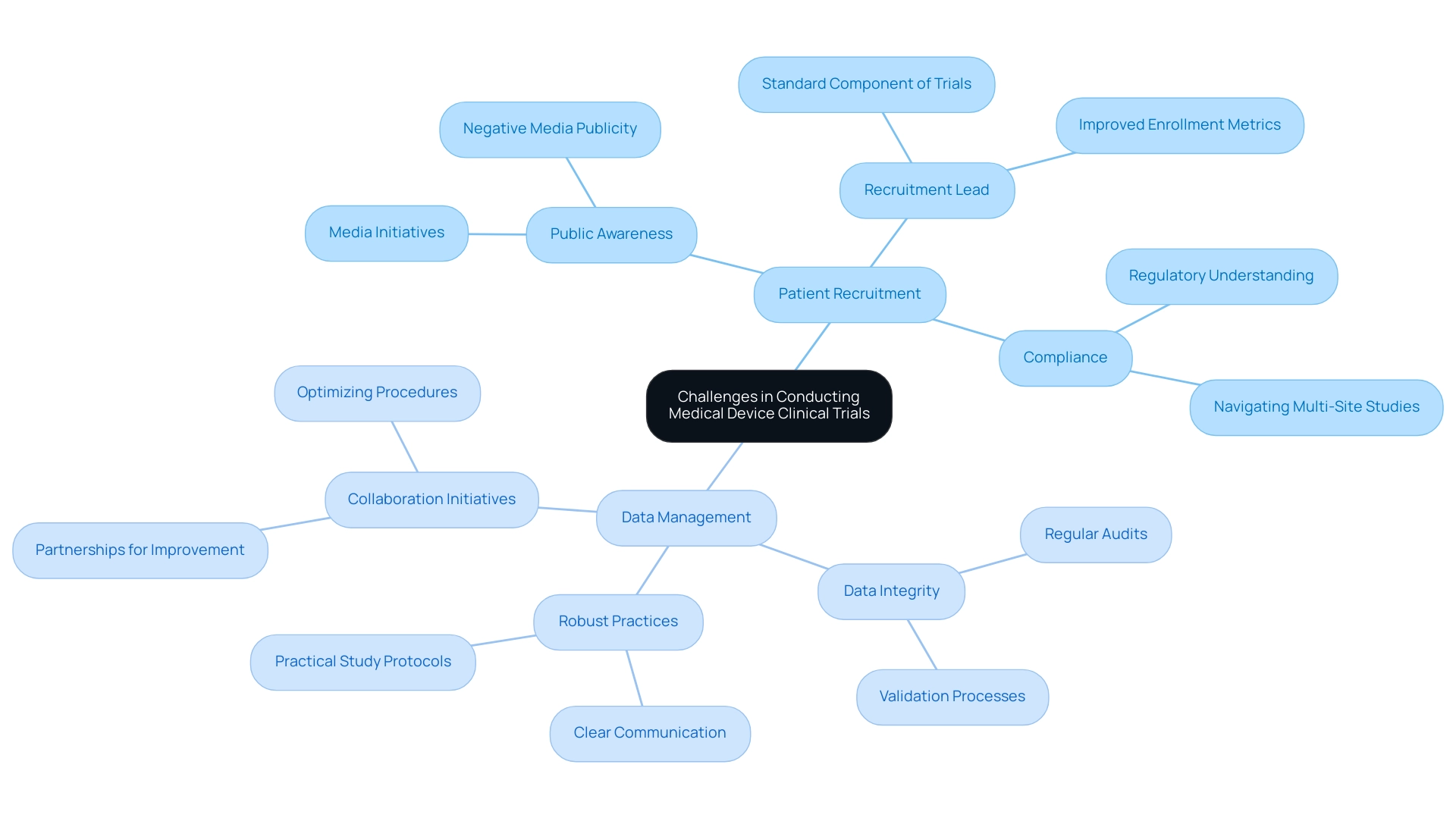
Carrying out medical equipment research in Colombia entails managing a variety of obstacles that can greatly influence the success of the study:
-
Patient Recruitment: One of the most pressing challenges is the recruitment of eligible participants, particularly for niche devices or specific populations. A survey suggests that 63% of participants strongly believe that increasing public awareness about clinical studies through media initiatives, such as those reported by Clinical Leader, could enhance recruitment efforts. This emphasizes the necessity for innovative and effective recruitment strategies that not only attract participants but also inform them about the process. As highlighted by Premier Research,
When experiments are make-or-break for the sponsor, or provide critical hope for patients in need, the necessity to speed up the timeline is even stronger.
Moreover, having a Recruitment Lead as a standard element of experiments could significantly enhance enrollment metrics, ensuring that recruitment strategies are effectively implemented. Compliance with regulations is crucial, as the oversight framework for medical device trials is complex and constantly changing. Researchers must cultivate a thorough understanding of the regulations to ensure adherence and avoid potential delays or penalties. Common compliance challenges include navigating varying regulations across regions, which can complicate multi-site studies. Staying informed about the latest compliance issues for 2024 is essential for sponsors of Medical Device Trials to mitigate risks effectively. This encompasses grasping the extensive management services offered for research studies, including compliance assessments and setup procedures, which are essential for upholding standards.
-
Data Management: Ensuring the integrity and precision of study data is paramount, as any errors can lead to significant setbacks in compliance submissions and compromise the validity of the research outcomes. Researchers must implement robust data management practices that include regular audits and validation processes. The research on patient recruitment strategies emphasized that successful recruitment depends on clear communication and practical study protocols, further highlighting the significance of careful data management in the context of regulatory oversight.
Moreover, initiatives such as the partnership between bioaccess™ and Caribbean Health Group seek to establish Barranquilla as a prominent location for Medical Device Trials in Latin America, backed by Colombia's Minister of Health. This effort, along with collaborations such as GlobalCare Clinical Trials with bioaccess™, is anticipated to improve ambulatory services for studies, achieving significant reductions in recruitment duration and high retention rates. The partnership's influence on optimizing procedures and enhancing participant involvement is essential for progressing medical technologies through research studies, ultimately aiding patient safety and innovation in healthcare.
Understanding the Regulatory Landscape for Medical Device Trials
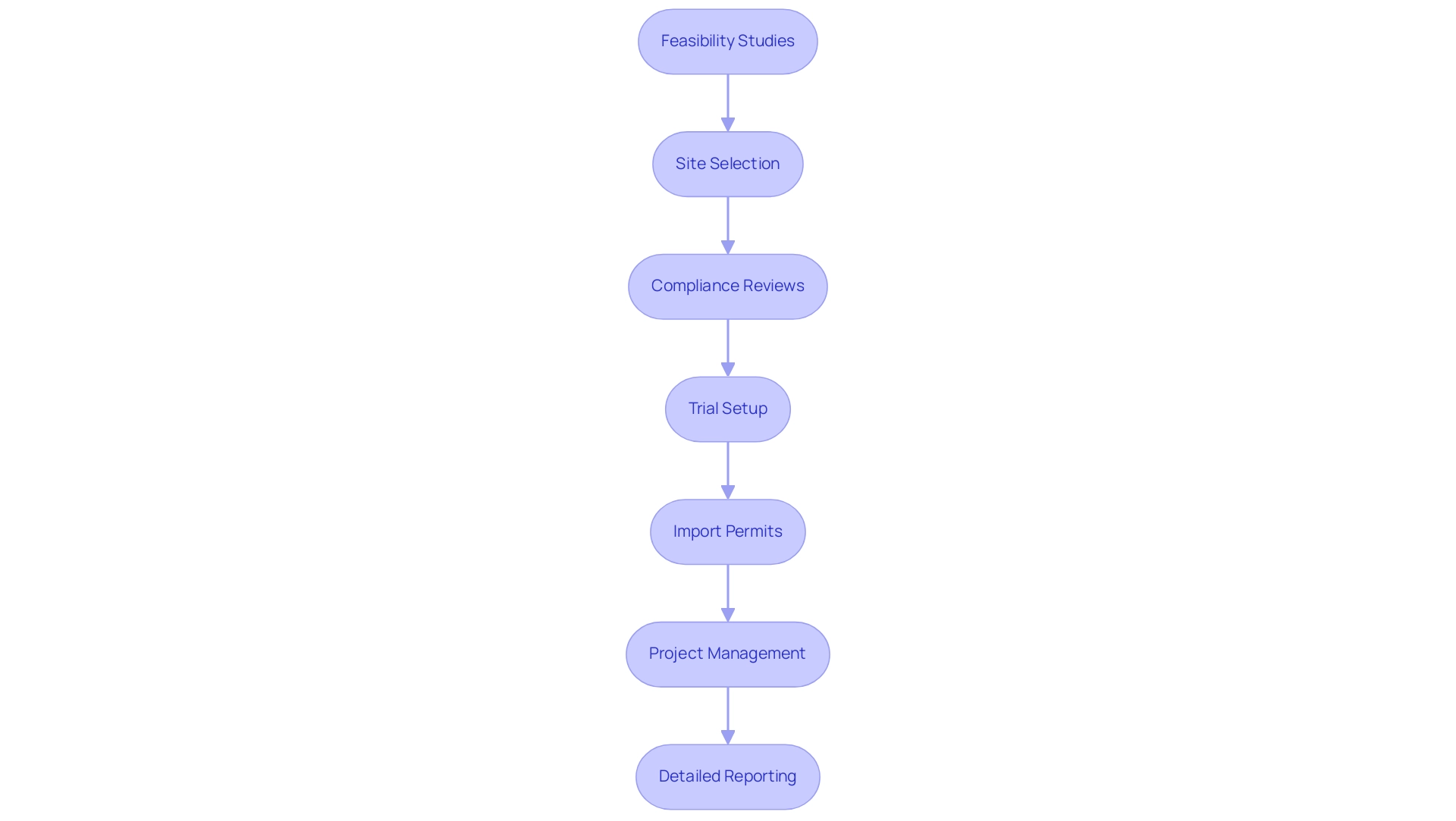
Medical equipment evaluations function within a complicated oversight framework, requiring strict compliance with set protocols to guarantee patient safety and product effectiveness. Our extensive clinical trial management services for Medical Device Trials include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Detailed reporting
This ensures that each trial adheres to standards. Specifically, our compliance reviews involve a systematic evaluation of study documents to ensure they meet country-specific requirements, along with feedback processes that facilitate necessary adjustments before submission.
In the United States, the FDA regulates medical instruments under the Federal Food, Drug, and Cosmetic Act, mandating different pre-market submissions tailored to the specific category. This regulatory framework is designed to evaluate safety and effectiveness before a product can enter the market. Meanwhile, in Europe, the Medical Device Regulation (MDR) specifies the criteria for CE marking and clinical evaluation, emphasizing a comprehensive approach to patient safety.
The UK's MHRA similarly monitors compliance, ensuring that products meet stringent standards. Katherine Ruiz, our Regulatory Affairs Expert, brings invaluable expertise in navigating these landscapes, having advised numerous foreign manufacturers on obtaining market clearance for their innovations in Colombia. As Ram Kannan, Quality Manager in Design & Development, noted,
Introduced on the back of the Poly Implant Prostheses (PIP) breast implant scandal, where substandard implants were given to about 300,000 women in Europe and South America, the new regulations aim to improve patient safety.
This historical context emphasizes the critical nature of compliance in safeguarding public health and directly impacts our medical device trials management processes by necessitating rigorous adherence to evolving standards. Notably, in November 2019, the European Union published a corrigendum extending compliance deadlines for certain Class I products by four years, pending adoption by the European Parliament. This corrigendum, along with a second corrigendum published around the same time, reflects the ongoing adjustments within the regulatory framework, allowing manufacturers additional time to meet the evolving requirements.
Additionally, the guidance document outlines essential criteria for software qualification and classification under the new medical equipment regulations. For medical researchers, a comprehensive grasp of these regulations is crucial for ensuring that medical device trials align with legal requirements while prioritizing the safety and well-being of patients.
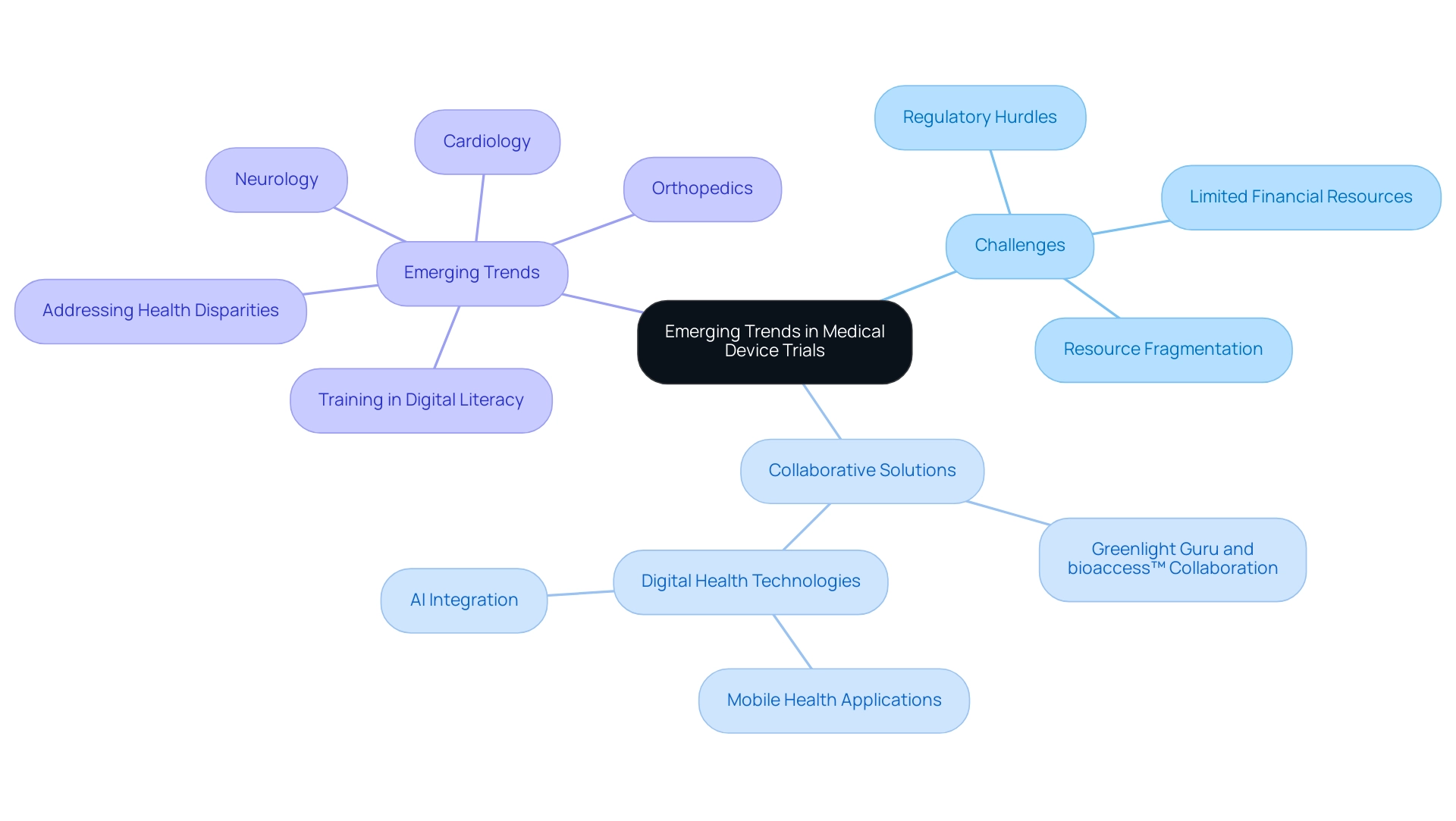
Emerging Trends and Therapeutic Areas in Medical Device Trials
The landscape of medical device trials in Latin America is increasingly influenced by the challenges encountered by Medtech companies, including:
- Regulatory hurdles
- Limited financial resources
- Resource fragmentation
These challenges hinder effective communication and cooperation between Latin American hospitals and American research clients. However, collaboration is emerging as a vital solution in Medical Device Trials.
The collaboration between Greenlight Guru and bioaccess™ seeks to expedite Medtech innovations and simplify studies in the region, enabling quicker market access and improving patient outcomes. This is demonstrated by PAVmed's recent success with the first in-human study of its Portion medical device in Colombia, highlighting the potential for effective execution in medical device trials. Furthermore, the integration of digital health technologies, such as mobile health applications and AI, is transforming research methodologies in Medical Device Trials, enabling efficient data handling and real-time insights that enhance decision-making.
As therapeutic areas like cardiology, orthopedics, and neurology experience growth, it is crucial to train future healthcare professionals in both digital literacy and effective communication to prepare them for Medical Device Trials. Addressing health disparities, particularly in low SES communities, remains a key focus as digital health technologies aim to penetrate these areas first. As Eleni Linos of Stanford University emphasizes, collaboration across disciplines is essential for navigating the complexities of modern clinical trials, particularly Medical Device Trials, as exemplified by initiatives like the Center for Digital Health at Stanford, which seeks to bridge technology and health challenges through innovative partnerships.
Conclusion
The evolving landscape of medical device trials in Colombia underscores the intricate interplay of regulatory frameworks, clinical methodologies, and emerging technologies. With medical devices categorized into distinct risk classes by INVIMA, researchers are equipped with a structured approach that promotes patient safety and product efficacy. Understanding the stages of clinical trials—from pilot to post-market—is essential for navigating the complexities inherent in the research process.
However, challenges such as:
- Patient recruitment
- Regulatory compliance
- Data management
persist, necessitating innovative strategies and robust planning. The importance of collaboration among stakeholders cannot be overstated, as partnerships are crucial for addressing these challenges and fostering advancements in Medtech. Efforts to improve public awareness about clinical trials and to streamline the recruitment process will be vital in enhancing participation and ensuring that trials are conducted efficiently.
Looking ahead, the integration of digital health technologies promises to revolutionize the way clinical trials are conducted, offering new avenues for data management and real-time insights. As therapeutic areas continue to expand, particularly in cardiology and neurology, the focus on training healthcare professionals in digital literacy will be paramount. Ultimately, the future of medical device trials in Colombia hinges on the ability to adapt to regulatory changes, leverage technological advancements, and foster collaborative environments that prioritize patient safety and innovative healthcare solutions.
Frequently Asked Questions
How are medical instruments classified in Colombia?
Medical instruments in Colombia are classified into three categories based on their risk level: Class I (low risk, e.g., bandages), Class II (moderate risk, e.g., infusion pumps), and Class III (high risk, e.g., pacemakers). Each class has different levels of oversight, with Class III requiring the most stringent regulations.
What role does INVIMA play in the regulation of medical devices in Colombia?
INVIMA (Colombia National Food and Drug Surveillance Institute) is responsible for ensuring the safety, efficacy, and quality of medical products in Colombia. It oversees compliance, suggests technical standards, and manages both pre- and post-market evaluations of medical devices.
What are the key stages in medical device clinical studies?
Medical device clinical studies typically progress through several stages, including pilot studies to assess feasibility, safety, and design, followed by larger Medical Device Trials that focus on safety and effectiveness in specific patient populations. Post-Approval Monitoring is also conducted to ensure long-term safety and effectiveness.
What challenges do researchers face in conducting medical equipment research in Colombia?
Researchers face challenges such as patient recruitment, data management, and compliance with complex regulations. Effective recruitment strategies and robust data management practices are essential to overcome these obstacles and ensure successful studies.
What services are included in clinical trial management for medical device trials?
Clinical trial management services for medical device trials include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and detailed reporting to ensure adherence to standards and regulations.
How do regulatory frameworks differ between Colombia and other regions?
In Colombia, medical devices are regulated by INVIMA, while in the United States, the FDA oversees medical instruments under the Federal Food, Drug, and Cosmetic Act. Europe follows the Medical Device Regulation (MDR), which specifies criteria for CE marking and clinical evaluation. Each region has its own specific requirements for pre-market submissions.
What is the significance of collaboration in medical device trials in Latin America?
Collaboration is essential in overcoming challenges faced by Medtech companies in Latin America, such as regulatory hurdles and limited resources. Partnerships, like those between Greenlight Guru and bioaccess™, aim to expedite Medtech innovations and improve patient outcomes by simplifying studies and facilitating quicker market access.
How is digital health technology impacting medical device trials?
Digital health technologies, including mobile health applications and AI, are transforming research methodologies in medical device trials by enabling efficient data handling and real-time insights, which enhance decision-making processes.
Why is training in digital literacy important for healthcare professionals?
Training future healthcare professionals in digital literacy is crucial to prepare them for the evolving landscape of medical device trials, which increasingly relies on digital health technologies and effective communication to address health disparities and improve patient care.

