Introduction
The landscape of medical devices is vast and intricate, encompassing a diverse range of instruments and technologies that play a critical role in healthcare. From simple tools like bandages to sophisticated systems such as robotic surgical devices, the classification of these devices into three distinct categories—Class I, Class II, and Class III—serves as a foundation for understanding their regulatory requirements and associated risks.
As the medical device market is poised for significant growth, anticipated to reach over $673 billion by 2028, the importance of these classifications becomes increasingly evident. This article delves into the complexities of medical device clinical trials, exploring the stages of research, the challenges faced by developers, the regulatory framework governing these trials, and the emerging trends that are shaping the future of healthcare.
By examining these elements, a clearer picture emerges of how innovation and regulatory compliance intersect in the quest to enhance patient outcomes through advanced medical technologies.
Understanding Medical Devices: Definitions and Classifications
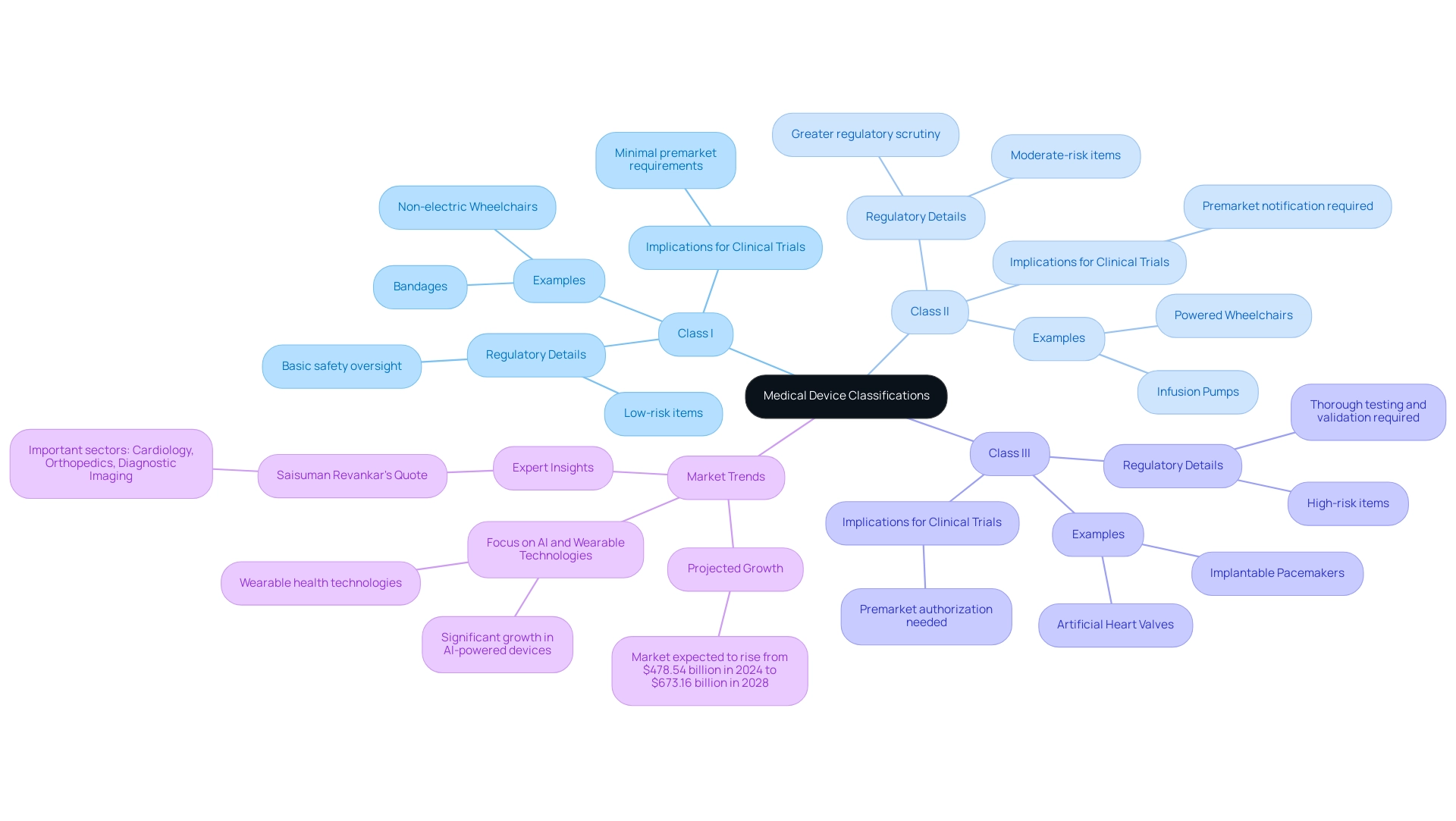
Medical instruments encompass a wide array of tools, apparatuses, machines, and implants designed for the diagnosis, prevention, monitoring, treatment, or alleviation of diseases. Their complexity varies significantly, ranging from simple implements such as tongue depressors to intricate technologies like pacemakers and robotic surgical systems. For regulatory clarity and safety, medical instruments are categorized into three distinct classes based on their intended use and associated risk levels:
- Class I: These are low-risk items, such as bandages and non-electric wheelchairs, which face the least regulatory control, ensuring basic safety oversight without extensive premarket requirements.
- Class II: Representing moderate-risk items, this category includes infusion pumps and powered wheelchairs. These instruments typically necessitate greater regulatory scrutiny, often requiring premarket notification to ensure their safety and efficacy.
- Class III: High-risk items, including implantable pacemakers and artificial heart valves, fall into this category. They require premarket authorization, which entails thorough testing and validation procedures to show their safety and effectiveness before they can enter the market.
Comprehending these classifications is essential for understanding the wider context of medical device clinical trials. In Latin America, companies like bioaccess® provide comprehensive management services for Medical Device Clinical Trials, including feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting. They specialize in various types of studies, including Medical Device Clinical Trials such as Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring that trials meet regulatory standards set forth by INVIMA (Colombia's National Food and Drug Surveillance Institute).
This oversight is essential as INVIMA is acknowledged as a Level 4 regional reference health authority by PAHO/WHO, highlighting its role in ensuring the safety and quality of healthcare products.
As the healthcare product market is projected to soar from $478.54 billion in 2024 to an impressive $673.16 billion by 2028, the importance of these classifications becomes even more pronounced. Notably, the advanced imaging systems market was valued at $50 billion in 2023 and is expected to rise, reflecting the growing emphasis on innovative technologies. The emphasis on AI-driven tools and wearable health technologies is expected to reshape the realm of healthcare instruments, with fields like cardiology and diagnostic imaging at the forefront of this change.
As noted by industry expert Saisuman Revankar, 'Important sectors like cardiology, orthopedics, and diagnostic imaging equipment will probably continue to rise due to continuous developments and increasing demand worldwide.' This insight highlights the ongoing advancements and regulatory updates in medical classifications, which are essential for ensuring that medical device clinical trials align with the latest standards and innovations. Moreover, the case study named 'Future Outlook and Trends' highlights the promising growth anticipated in AI-driven products and wearable health technologies, additionally demonstrating the significance of these classifications in the context of market evolution.
Navigating the Stages of Medical Device Clinical Trials
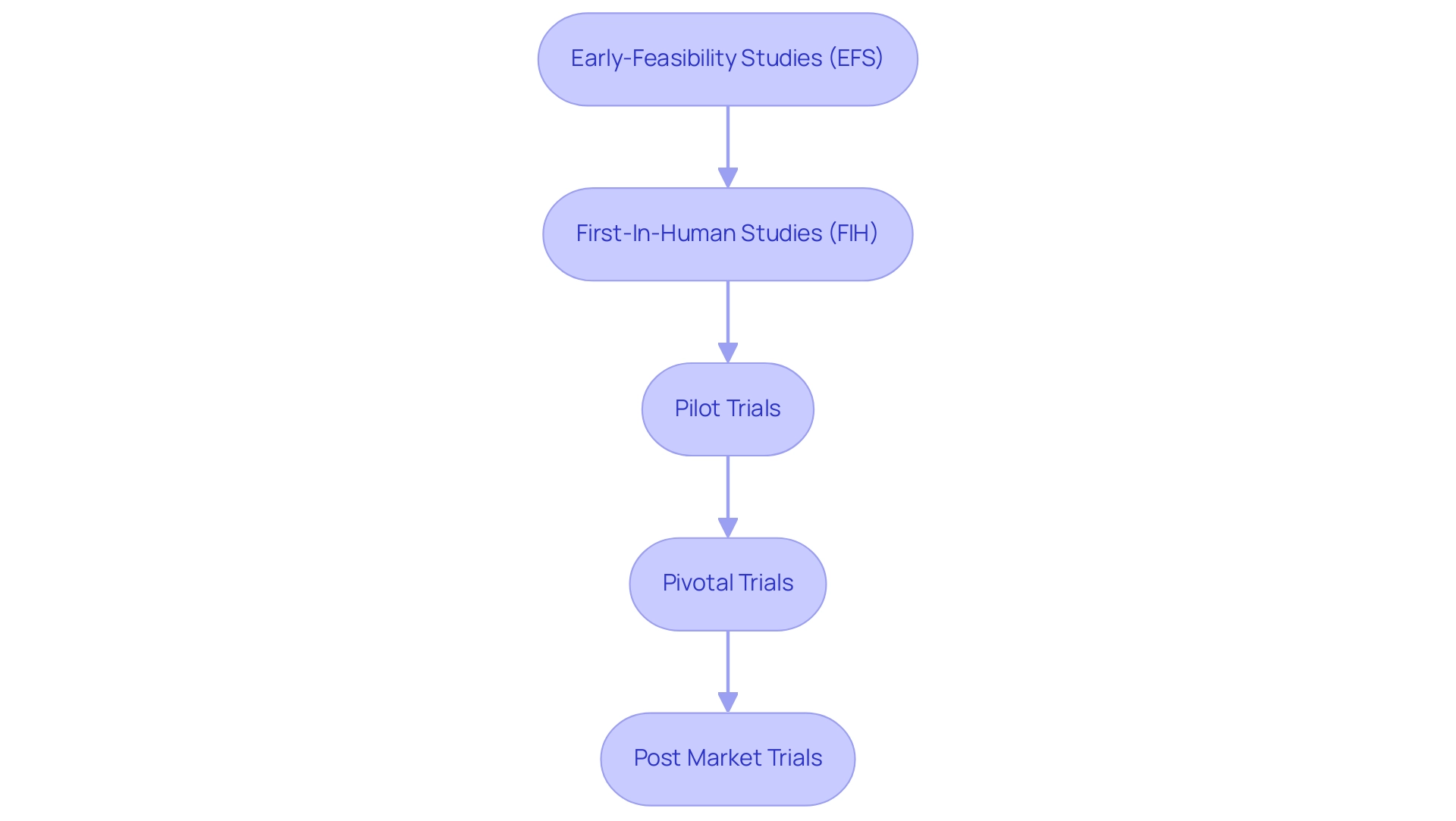
Clinical evaluations for medical instruments generally advance through several key stages, all of which are expertly managed by bioaccess® to ensure compliance and success:
- Early-Feasibility Studies (EFS): These initial studies evaluate the viability of the concept and are essential in the early phases of development. At bioaccess®, we assist in site selection and principal investigator review to enhance these studies.
- First-In-Human Studies (FIH): These studies are intended to assess the safety and initial effectiveness of the equipment in humans. Our team ensures rigorous project management and monitoring throughout this critical phase.
- Pilot Trials: Small-scale studies aimed at assessing the feasibility, time, cost, and adverse events involved in a clinical study. At bioaccess®, we assist in site selection and principal investigator review to enhance these studies.
- Pivotal Trials: Larger studies that evaluate the efficacy and safety of the product, often required for regulatory approval. These trials provide the primary data on equipment performance, and our team ensures rigorous project management and monitoring throughout.
- Post Market Trials: Conducted after a product has received approval, these studies monitor long-term safety and effectiveness in a broader patient population. At bioaccess®, we support the entire process, including reporting on study status and adverse events, to uphold the highest standards in clinical research.
Each phase plays an essential role in ensuring that medical device clinical trials confirm healthcare products are safe and effective before they reach the market. Our flexible approach allows us to adapt to the unique challenges of each test, ensuring optimal outcomes.
Challenges in Conducting Medical Device Clinical Trials
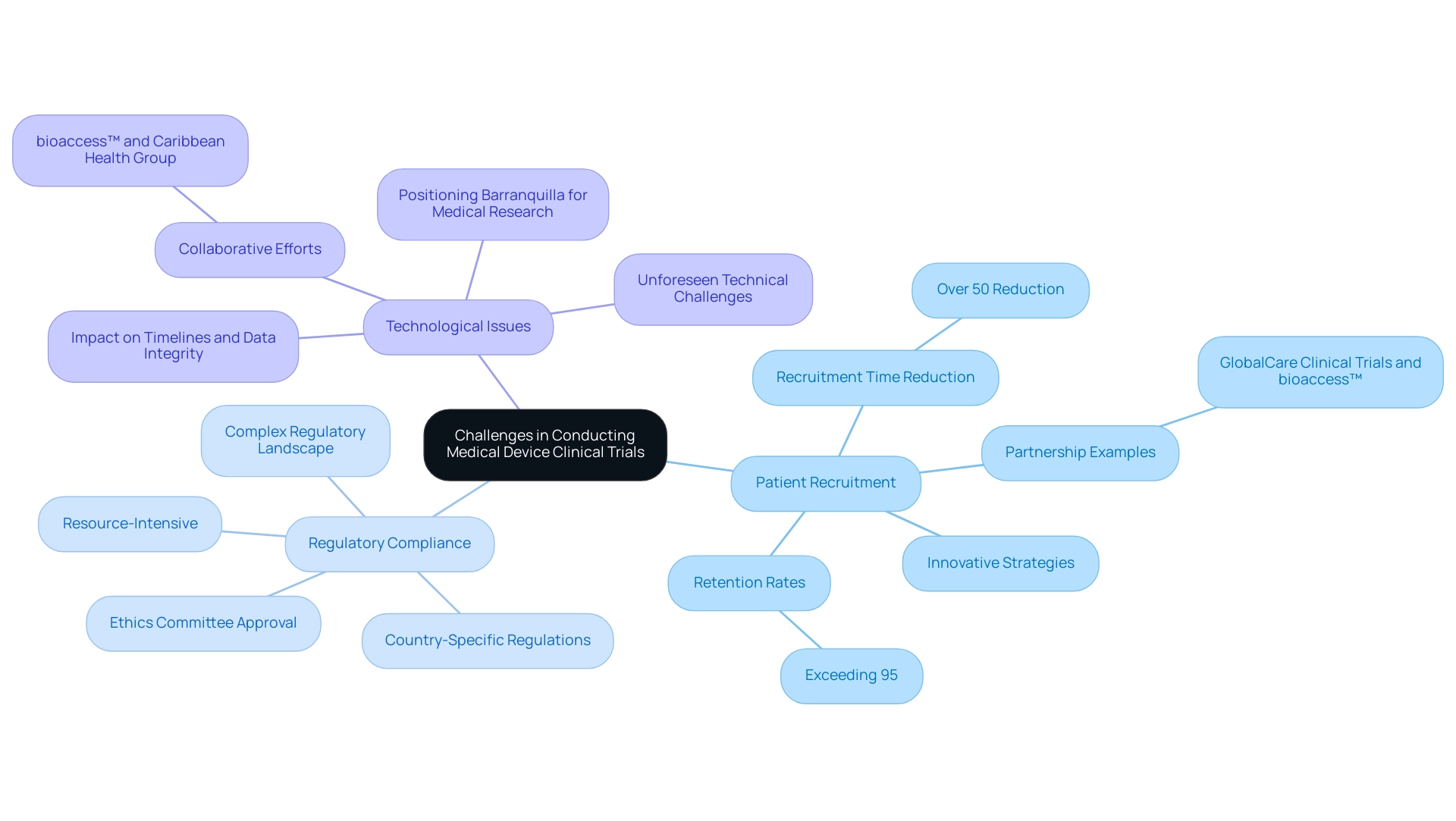
Carrying out medical equipment clinical studies presents several challenges, including:
- Patient Recruitment: Identifying appropriate participants who satisfy particular criteria can be challenging, especially for tools aimed at rare conditions. As evidenced by GlobalCare Clinical Trials partnering with bioaccess™ to enhance ambulatory services in Colombia, innovative strategies can lead to significant improvements in recruitment time—over 50% reduction—and retention rates exceeding 95%.
- Regulatory Compliance: Navigating the complex regulatory landscape requires meticulous attention to detail and adherence to strict guidelines, which can be resource-intensive. Medical equipment startups frequently encounter intricate regulatory demands that can hinder advancement and complicate the execution of Medical Device Clinical Trials. This includes obtaining ethics committee approval and ensuring compliance with country-specific regulations.
- Technological Issues: As numerous healthcare instruments involve advanced technology, unforeseen technical challenges can occur, affecting timelines and data integrity. Collaborative efforts, like those between bioaccess™ and Caribbean Health Group, demonstrate how pooling resources and expertise can help address these technological challenges effectively. This partnership also aims to position Barranquilla as a prominent location for medical research in Latin America.
Addressing these challenges requires strategic planning, innovative problem-solving, and strong project management skills, including effective reporting and monitoring of study status, inventory, and adverse events.
The Regulatory Framework for Medical Device Clinical Trials
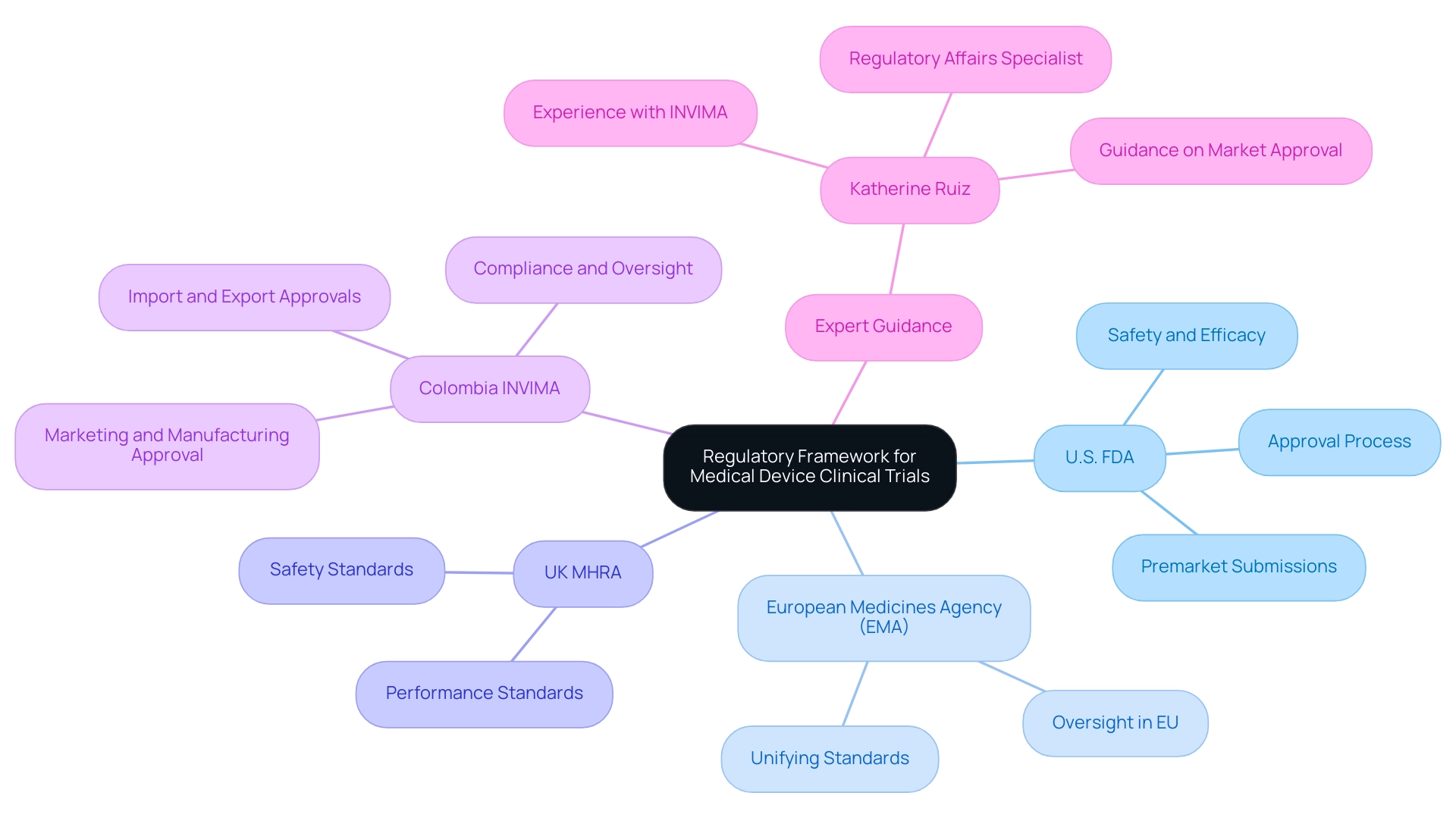
The regulatory framework overseeing clinical trials for health technologies differs by area, with key agencies including:
- U.S. Food and Drug Administration (FDA): Manages the approval process for healthcare products in the United States, necessitating premarket submissions that prove safety and efficacy.
- European Medicines Agency (EMA): Oversees health products in the European Union, concentrating on unifying standards across member states.
- UK Medicines and Healthcare products Regulatory Agency (MHRA): Regulates healthcare equipment in the United Kingdom, ensuring that products meet safety and performance standards.
In Colombia, the regulatory environment is mainly influenced by INVIMA (Colombia National Food and Drug Surveillance Institute), which plays a vital role in the approval and oversight of healthcare products. INVIMA is responsible for ensuring compliance with health standards, overseeing the marketing and manufacturing processes, and providing approvals for import and export activities. As a Level 4 health authority recognized by PAHO/WHO, INVIMA ensures that the safety, efficacy, and quality of medical devices are maintained.
For a comprehensive management approach to Medical Device Clinical Trials, it is essential for researchers to understand these regulations and engage with experienced professionals. Our service capabilities encompass feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
Katherine Ruiz, a Regulatory Affairs specialist focusing on healthcare products and in vitro diagnostics, has guided numerous foreign producers on securing market approval for their innovations in Colombia. Her experience with INVIMA provides her with the essential insights to navigate the complexities of research studies and regulatory compliance.
Emerging Trends and Therapeutic Areas in Medical Device Trials
Recent years have seen notable new patterns in medical equipment evaluations, influencing the future of healthcare:
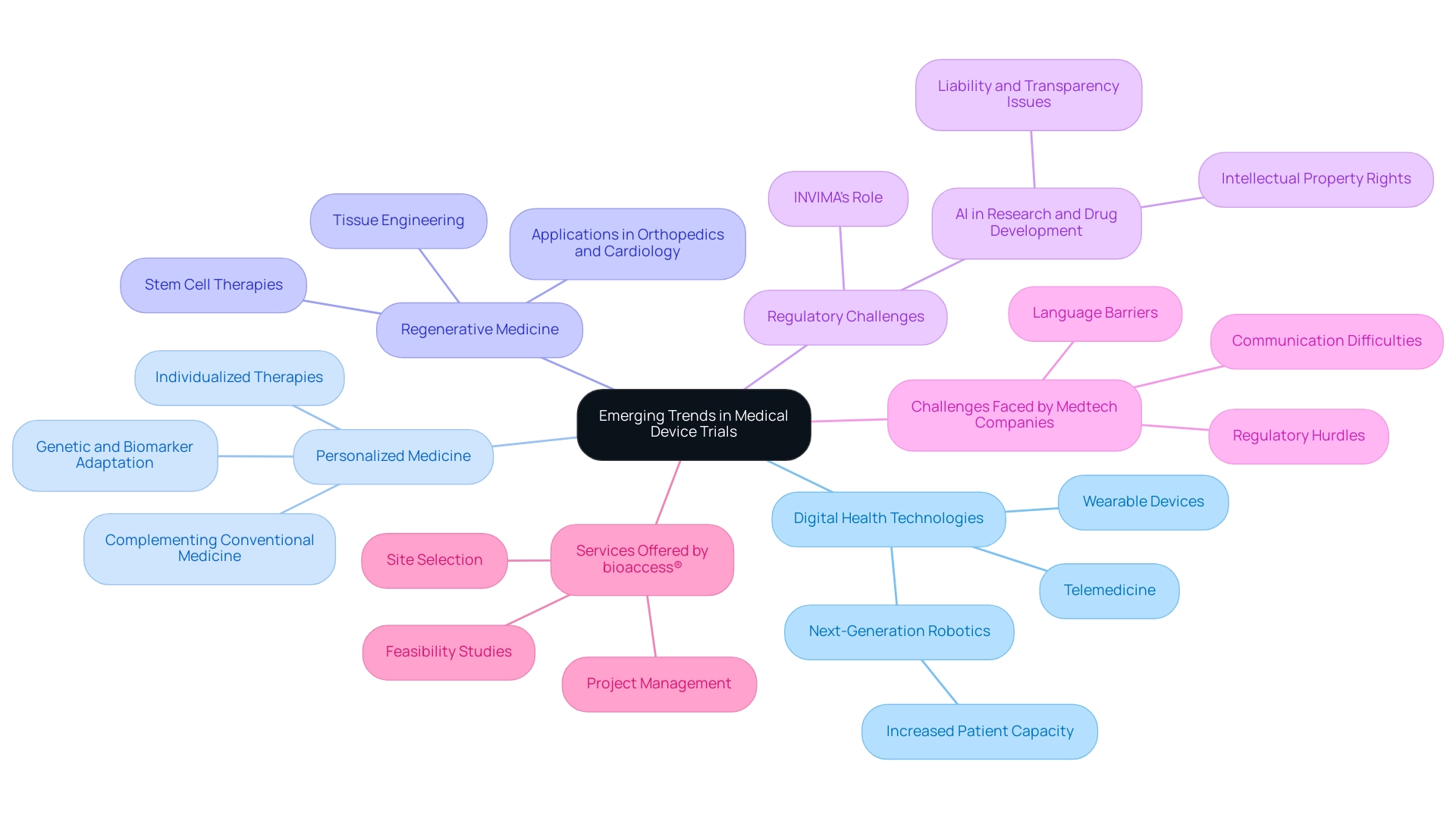
- Digital Health Technologies: The growth of telemedicine and wearable devices has fundamentally changed research methodologies, facilitating remote monitoring and enhancing data collection efficiency. This trend not only streamlines the testing process but also caters to the growing patient populations, particularly in countries with increasing healthcare demands. Next-generation robotics are poised to deliver more comprehensive care, positively impacting these regions. Significantly, partnerships, like the one between Greenlight Guru and bioaccess™, are further advancing these innovations by connecting Medtech manufacturers with Latin American markets for early-stage studies.
- Personalized Medicine: More and more, research studies are concentrating on individualized therapies adapted to genetic and biomarker characteristics, which greatly improves the significance of healthcare tools for particular patient groups. As noted by Deepthi Sathyajith, M.Pharm.,
Although conventional medicine continues to hold a prominent place in treatment and disease management, personalized medicines do show a promising future in providing 'right treatment to the right patient at the right time.'This approach not only complements traditional therapies by ensuring that patients receive the most effective treatment options but also paves the way for more effective and individualized healthcare solutions. - Regenerative Medicine: Innovations in tissue engineering and stem cell therapies are creating opportunities for the development of new healthcare tools, particularly in fields such as orthopedics and cardiology. These advancements signal a shift toward more sophisticated treatment options that leverage the body's own healing processes.
- Regulatory Challenges: The application of AI, especially GenAI, in research and drug development raises critical questions about liability, transparency, and intellectual property rights. Insights from the case study titled 'Regulatory Challenges for AI in Healthcare' highlight that regulatory developments, such as those from INVIMA—the Colombia National Food and Drug Surveillance Institute—will significantly impact the commercialization of AI-driven healthcare products and services. As a Level 4 health authority recognized by PAHO/WHO, INVIMA plays a crucial role in overseeing the safety and efficacy of medical devices, ensuring that innovations align with regulatory standards.
- Challenges Faced by Medtech Companies: Medtech companies in Latin America encounter various challenges, including regulatory hurdles, language barriers, and communication difficulties with local hospitals. These issues can postpone the research process and impact cooperation with American research clients. Tackling these challenges is crucial for promoting innovation and ensuring successful health outcomes.
- Services Offered by bioaccess®: To navigate these challenges, bioaccess® provides a range of services, including feasibility studies, site selection, and project management, ensuring that Medtech companies can effectively conduct research in Latin America while adhering to local regulations. These services are essential for improving the effectiveness and success of medical device clinical trials in the region. These trends reflect the ongoing evolution of the medical device landscape and the necessity for adaptive Medical Device Clinical Trials that can effectively accommodate these innovations while navigating the regulatory challenges associated with AI in healthcare.
Conclusion
The intricate landscape of medical devices is underscored by the critical role that classifications play in ensuring safety, efficacy, and regulatory compliance. By categorizing these devices into Class I, Class II, and Class III, stakeholders can better navigate the complexities of clinical trials and understand the varying levels of scrutiny each category demands. This foundational knowledge is essential as the medical device market continues to expand, projected to achieve remarkable growth in the coming years.
As outlined, the stages of medical device clinical trials—from early feasibility studies to post-market evaluations—are crucial for validating the safety and effectiveness of new technologies. However, the journey is fraught with challenges, including patient recruitment, regulatory compliance, and technological hurdles. Addressing these challenges through innovative strategies and robust project management is vital for the successful development of medical devices.
Emerging trends such as digital health technologies, personalized medicine, and regenerative medicine are reshaping clinical trial methodologies, emphasizing the need for adaptability in response to rapid advancements. The regulatory landscape, particularly in regions like Latin America, demands an understanding of local frameworks, such as those established by INVIMA, to ensure that clinical research aligns with the latest standards.
In summary, the intersection of innovation and regulatory compliance in medical device development is paramount for enhancing patient outcomes. As the industry evolves, stakeholders must remain vigilant in addressing challenges and embracing emerging trends to drive forward the future of healthcare technology.
Frequently Asked Questions
What are medical instruments?
Medical instruments are tools, apparatuses, machines, and implants designed for the diagnosis, prevention, monitoring, treatment, or alleviation of diseases. Their complexity ranges from simple items like tongue depressors to advanced technologies such as pacemakers and robotic surgical systems.
How are medical instruments classified?
Medical instruments are classified into three categories based on their intended use and associated risk levels: 1. Class I: Low-risk items (e.g., bandages, non-electric wheelchairs) with minimal regulatory control. 2. Class II: Moderate-risk items (e.g., infusion pumps, powered wheelchairs) requiring greater regulatory scrutiny and premarket notification. 3. Class III: High-risk items (e.g., implantable pacemakers, artificial heart valves) that require thorough testing and premarket authorization.
What role does bioaccess® play in medical device clinical trials?
Bioaccess® provides comprehensive management services for Medical Device Clinical Trials in Latin America, including feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting. They specialize in various types of studies, ensuring compliance with regulatory standards.
What is the significance of INVIMA in Colombia?
INVIMA (Colombia's National Food and Drug Surveillance Institute) is a key regulatory authority responsible for ensuring the safety, efficacy, and quality of healthcare products in Colombia. It is recognized as a Level 4 regional reference health authority by PAHO/WHO.
What are the stages of clinical evaluations for medical instruments?
The stages of clinical evaluations include: 1. Early-Feasibility Studies (EFS) 2. First-In-Human Studies (FIH) 3. Pilot Trials 4. Pivotal Trials 5. Post Market Trials. Bioaccess® manages these stages to ensure compliance and success in clinical trials.
What challenges are faced in conducting medical equipment clinical studies?
Key challenges include: 1. Patient Recruitment: Difficulty in finding suitable participants, especially for rare conditions. 2. Regulatory Compliance: Navigating complex regulations and obtaining necessary approvals. 3. Technological Issues: Addressing unforeseen technical challenges that may affect timelines and data integrity.
What are the key regulatory agencies involved in clinical trials?
Key regulatory agencies include: 1. U.S. Food and Drug Administration (FDA): Oversees healthcare product approvals in the U.S. 2. European Medicines Agency (EMA): Regulates health products in the EU. 3. UK Medicines and Healthcare products Regulatory Agency (MHRA): Ensures product safety in the UK. 4. INVIMA: Regulates healthcare products in Colombia.
What trends are influencing medical equipment evaluations?
Notable trends include: 1. Digital Health Technologies: Growth in telemedicine and wearable devices. 2. Personalized Medicine: Focus on individualized therapies. 3. Regenerative Medicine: Innovations in tissue engineering and stem cell therapies. 4. Regulatory Challenges: Concerns regarding AI in research and drug development. 5. Challenges Faced by Medtech Companies: Regulatory hurdles and communication difficulties.
How does bioaccess® support Medtech companies?
Bioaccess® offers services such as feasibility studies, site selection, and project management to help Medtech companies navigate challenges and successfully conduct research in Latin America while adhering to local regulations.

