Overview
To design trials for the Colombian market effectively, researchers must navigate the regulatory framework established by INVIMA. This includes strict compliance with ethical guidelines and the necessity of obtaining informed consent from participants. Understanding local dynamics—such as community engagement and the significance of ethical practices—alongside INVIMA's specific requirements, is crucial. This knowledge not only enhances participant recruitment but also ensures successful trial outcomes. By addressing these aspects, researchers can significantly improve their trial designs and foster trust within the community.
Introduction
Colombia is emerging as a promising hub for clinical trials, driven by a robust regulatory framework and a commitment to ethical research practices. Understanding the intricacies of the National Food and Drug Surveillance Institute (INVIMA) regulations is essential for researchers looking to navigate this landscape effectively.
With the pivotal Resolution 2378/2008 guiding trial design and execution, and ethical considerations outlined in Resolution 8430 of 1993, researchers must prioritize participant rights and safety. Furthermore, a significant portion of the population remains unaware of clinical trial opportunities, presenting a unique potential to enhance recruitment strategies.
As Colombia positions itself as a leading destination for clinical research, the interplay of regulatory compliance, ethical considerations, and innovative recruitment strategies will be critical in shaping the future of clinical trials in the region.
Understanding Colombia's Regulatory Framework for Clinical Trials
Creating medical studies in Colombia requires a comprehensive understanding of trial design tailored to the Colombian market, particularly in relation to the regulatory framework established by the National Food and Drug Surveillance Institute (INVIMA). Central to this framework is Resolution 2378/2008, which delineates the essential requirements for conducting medical studies. This regulation is vital for ensuring that experiments are designed and executed in compliance with national standards.
In addition to regulatory requirements, researchers must consider the ethical guidelines outlined in Resolution 8430 of 1993. This resolution underscores the importance of protecting individuals' rights and safety, which is paramount in research studies. A thorough understanding of the role of local ethics committees is essential, as these bodies are responsible for reviewing and approving research protocols to uphold ethical integrity.
Furthermore, obtaining informed consent from participants is a critical aspect of compliance. This process not only fulfills legal obligations but also fosters trust between researchers and participants, which is essential for effective recruitment and retention in research studies.
Statistics reveal that a significant portion of the population remains unaware of research opportunities; for instance, 85% of patients are not informed about their options at diagnosis. Notably, 75% of patients who were unaware of research studies indicated they would have been willing to participate had they known it was an option. This gap presents a unique opportunity for researchers to enhance trial designs for the Colombian market by refining patient recruitment strategies in the region.
Moreover, specialists note that dropout rates in Latin America are approximately one-third of those in the U.S. and EU, creating a favorable environment for understanding trial design within the Colombian context. The strong relationships between patients and doctors in the region further bolster recruitment efforts, providing valuable insights on how to effectively design trials, thereby positioning Colombia as an attractive locale for research. As Virginia Cozzi from Roche highlights, the robust connection between patients and doctors enhances recruitment and retention, which is crucial for the success of research studies.
Additionally, the Colombian government is actively promoting the nation as a premier research destination, with aspirations to enhance its status as a knowledge-based economy by 2031. This supportive environment, coupled with bioaccess's extensive management services for research initiatives—including feasibility studies, site selection, compliance reviews, project setup, import permits, project oversight, and reporting—underscores the potential for successful medical research in the country.
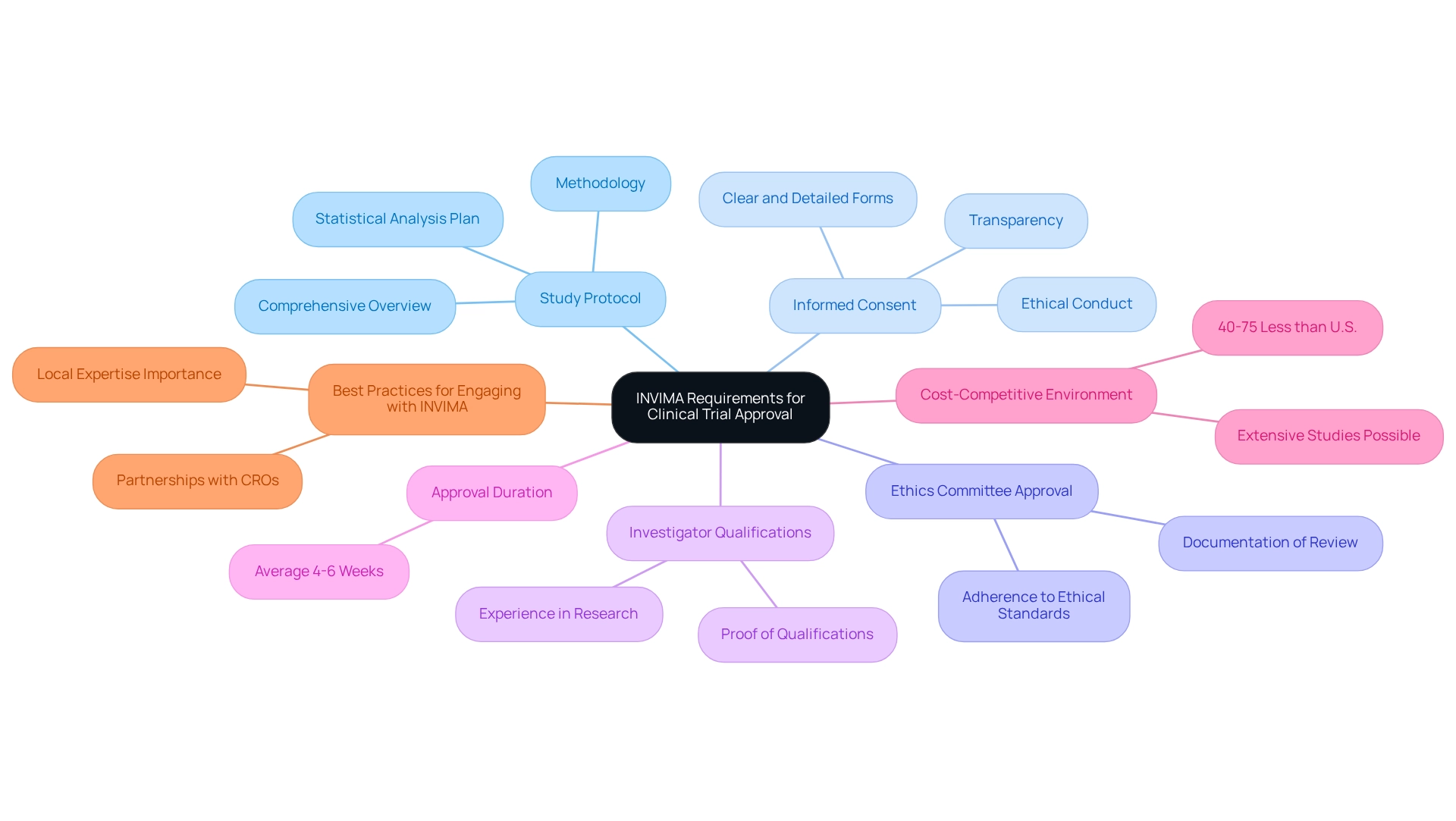
Key INVIMA Requirements for Clinical Trial Approval
To secure approval for clinical trials in Colombia, researchers must navigate a structured application process with INVIMA. This process involves submitting a thorough application that includes several critical components:
- Study Protocol: This document must provide a comprehensive overview of the trial's objectives, methodology, and statistical analysis plan, ensuring that all aspects of the study are clearly defined and scientifically sound.
- Informed Consent: Researchers are required to prepare clear and detailed consent forms that inform participants about the study's purpose, procedures, potential risks, and benefits, fostering transparency and ethical conduct.
- Ethics Committee Approval: It is essential to include documentation that verifies the local ethics committee has reviewed and approved the study, reflecting adherence to ethical standards in medical research.
- Investigator Qualifications: Proof showing the lead investigator's qualifications and experience in conducting research studies is essential to establish credibility and competence.
Furthermore, researchers should foresee possible evaluations by INVIMA to guarantee adherence to Good Clinical Practice (GCP) standards, which are vital for understanding how to design trials for the Colombian market. The average duration for clinical study approval in the country is usually about 4-6 weeks, demonstrating the effectiveness of the regulatory framework. INVIMA has specific requirements for first-in-human medical device studies, emphasizing safety and ethical conduct, which are paramount in the approval process.
Moreover, the cost-competitive environment in Colombia allows for medical procedures to be conducted at 40%–75% less than in the U.S., enabling more extensive studies without sacrificing quality.
Case studies emphasize best practices for interacting with INVIMA, particularly focusing on how to design trials for the Colombian market. This includes the significance of local knowledge and partnership with contract research organizations (CROs) like bioaccess, which is at the forefront of facilitating medical device studies in Latin America. Applying these strategies not only places companies advantageously in the competitive global market but also increases the chances of successful approvals. As Julio G. Martinez-Clark mentions, "Assisting pharmaceutical, medtech & biotech startups to effortlessly carry out research studies in the nation" highlights the encouraging atmosphere for medical investigations in the region.
Ethical Considerations in Clinical Trial Design
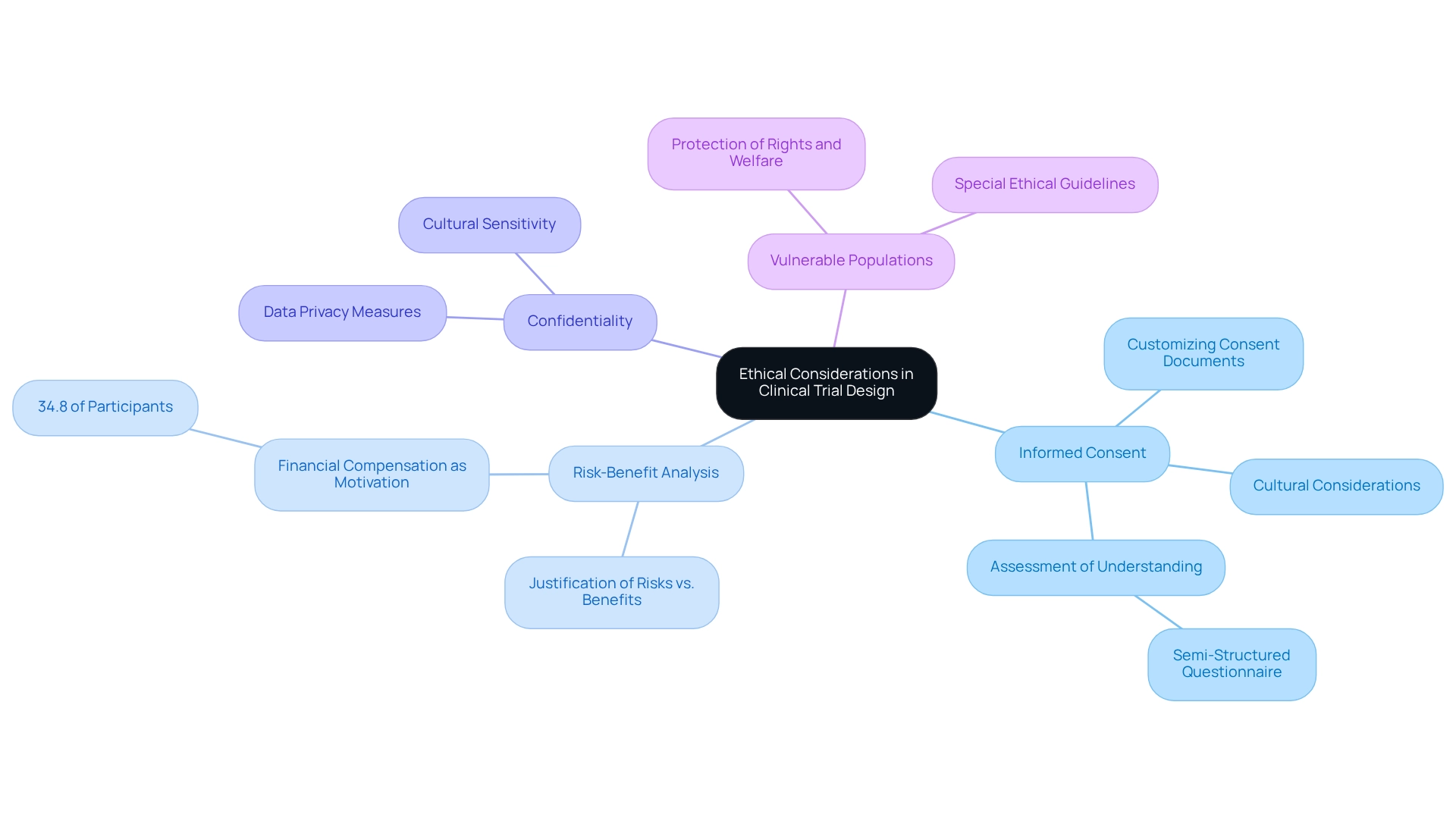
When designing trials for the Colombian market, researchers must prioritize ethical considerations to safeguard individuals and uphold the integrity of the research. This focus on ethics is not merely a regulatory requirement; it is a cornerstone of responsible research practice. The following key ethical principles are essential:
- Informed Consent: It is imperative that individuals are fully informed about the study's purpose, procedures, risks, and benefits before agreeing to participate. This process not only honors the independence of those involved but also nurtures confidence in the research process. A recent assessment of informed consent procedures emphasized the significance of customizing consent documents to align with country-specific regulations, ensuring that individuals comprehend the study's rationale and implications. This evaluation showed that informed consent forms (ICFs) endorsed by ethical review committees were essential in improving individuals' comprehension across various cultural and educational settings.
- Risk-Benefit Analysis: A comprehensive assessment must be carried out to ensure that the potential benefits of the research surpass the risks to subjects. This analysis is crucial in maintaining ethical standards and is particularly relevant in Colombia, where the regulatory framework, including INVIMA's specific requirements for first-in-human medical device clinical trials, emphasizes the need for a clear justification of risks versus benefits. Statistics indicate that a significant portion of individuals in the country, approximately 34.8%, cite financial compensation as a motivating factor for involvement, underscoring the need for careful consideration of how benefits are presented.
- Confidentiality: Safeguarding individuals' personal information is essential. Researchers must implement robust measures to protect data privacy, ensuring that sensitive information is handled with the utmost care. This is especially significant in a diverse cultural setting like Colombia, where different levels of education and awareness about data privacy can affect individuals' comfort levels. bioaccess® is dedicated to ensuring information security and client trust, demonstrating its commitment to data protection in medical device research trials.
- Vulnerable Populations: Special care should be taken when involving vulnerable populations, such as children or individuals with limited decision-making capacity. Researchers must ensure that their rights and welfare are prioritized throughout the study. Ethical guidelines require that further protections be established to safeguard these groups, demonstrating a dedication to ethical research methods.
Integrating these ethical standards into the framework of research studies not only improves participant safety but also adds to the scientific validity and trustworthiness of the research. As noted by David J Diemert from The George Washington University School of Medicine and Health Sciences, adherence to ethical standards is crucial in fostering trust and integrity in research involving patients. By following these guidelines, researchers can cultivate a more ethical and efficient research environment in Colombia, supported by bioaccess's comprehensive management services for studies, including feasibility assessments, site selection, compliance reviews, and project oversight.
Evaluating Scientific Validity and Feasibility for Trials
To ensure that clinical trials are both scientifically valid and feasible, researchers must consider several critical factors.
- Study Design: Selecting an appropriate study design is paramount. Options may consist of randomized controlled experiments, cohort studies, or observational studies, each designed to address specific research questions effectively. The selection of design directly affects the reliability of the results.
- Sample Size Calculation: A well-justified sample size is essential for achieving statistically significant results. Researchers should provide a clear rationale for their proposed sample size, ensuring it is adequate to detect meaningful differences or effects. This calculation should be based on preliminary data or established benchmarks relevant to the Colombian population.
- Endpoints: Clearly defined primary and secondary endpoints are crucial for evaluating the study's success. These endpoints should align with the study's objectives and be measurable, allowing for a robust evaluation of the intervention's efficacy.
- Feasibility Assessment: Conducting a thorough feasibility assessment is vital to evaluate the availability of the target population, necessary resources, and realistic timelines for recruitment and data collection. Insights from recent case studies in a South American nation, such as ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain, indicate that understanding local demographics and healthcare infrastructure can significantly enhance recruitment strategies. Leveraging community health networks has proven effective in reaching diverse patient populations. Additionally, the performance of various regression models, such as the CatBoost regressor, has shown promise in predicting accrual percentages, providing valuable insights into optimizing recruitment efforts.
As Michael J. Welsh aptly states, "stop being invisible," highlighting the importance of visibility in recruitment strategies. In 2025, as the environment of medical studies in Colombia progresses, preserving scientific integrity will necessitate continual adjustments to regulatory modifications and patient requirements. Future studies should concentrate on longitudinal data gathering and enhanced techniques to better comprehend the elements affecting patient enrollment in medical studies.
By addressing these elements, researchers can enhance the likelihood of successful study outcomes and contribute to the advancement of medical technologies in the region. Moreover, collaborating with entities such as bioaccess, which provides extensive research management services—including setup, compliance evaluations, project oversight, and reporting—can enable more efficient operations, from feasibility assessments to compliance evaluations, ultimately enhancing recruitment effectiveness and study success. Furthermore, comprehending the regulatory roles of INVIMA, the National Food and Drug Surveillance Institute, is essential for navigating the research landscape efficiently.
Strategies for Participant Recruitment in Colombian Clinical Trials
Recruiting participants for clinical trials in Colombia presents distinct challenges; however, implementing effective strategies can significantly enhance recruitment efforts.
- Community Engagement is paramount. Establishing strong relationships with local communities and healthcare providers fosters trust and encourages participation. Individuals are more likely to engage in trials when they feel connected to the research and its potential benefits.
- Targeted Advertising through social media and local media sources effectively connects with potential contributors. By highlighting the benefits of participation—such as access to cutting-edge treatments and contributing to medical advancements—recruitment campaigns resonate more deeply with the community.
- Incentives play a crucial role. Providing incentives like transportation reimbursement or complimentary health check-ups motivates individuals to participate. These incentives not only reduce possible obstacles to involvement but also demonstrate gratitude for the individual's time and dedication.
- Patient Registries can simplify the recruitment process by efficiently identifying eligible individuals. This approach improves recruitment speed and ensures that selected individuals meet the study's required standards.
- Expert Insights from Julio Martinez-Clark, CEO of bioaccess™, emphasize that "The region is an untapped market for Medtech innovation." This underscores the importance of tailored recruitment strategies that resonate with the unique demographics and cultural contexts of Colombian communities.
- Support from the Health Minister of Colombia has significantly endorsed the initiative to improve clinical trials in Barranquilla, highlighting the government's dedication to positioning the region as a premier location for clinical research.
- A notable case study on data protection and consent illustrates the complexities of local data protection laws, emphasizing the need for clear communication regarding data handling procedures. Simplifying consent processes is essential for building trust and encouraging participation in clinical trials.
- Statistical Evidence reveals the recruitment challenges faced in Colombia. The research team transcribed 49 of the first-contact CATI survey recordings from individuals who refused to partake in the strategy, illustrating the significance of comprehending attendee concerns and responding to them effectively.
- Creative Approaches, including decentralized research studies, position Latin America as a strong competitor in the global medical investigation field. These strategies enhance recruitment efforts by making participation more accessible and convenient for potential participants.
By concentrating on these strategies, study sponsors can better understand how to design trials for the Colombian market. This allows them to navigate the recruitment environment in Colombia more efficiently, ultimately resulting in positive study results. Furthermore, bioaccess™ offers extensive research management services, encompassing feasibility studies, site selection, compliance reviews, setup for studies, import permits, project management, and reporting, which improve compliance and efficiency during the recruitment process.
Post-Market Surveillance and Continuous Monitoring
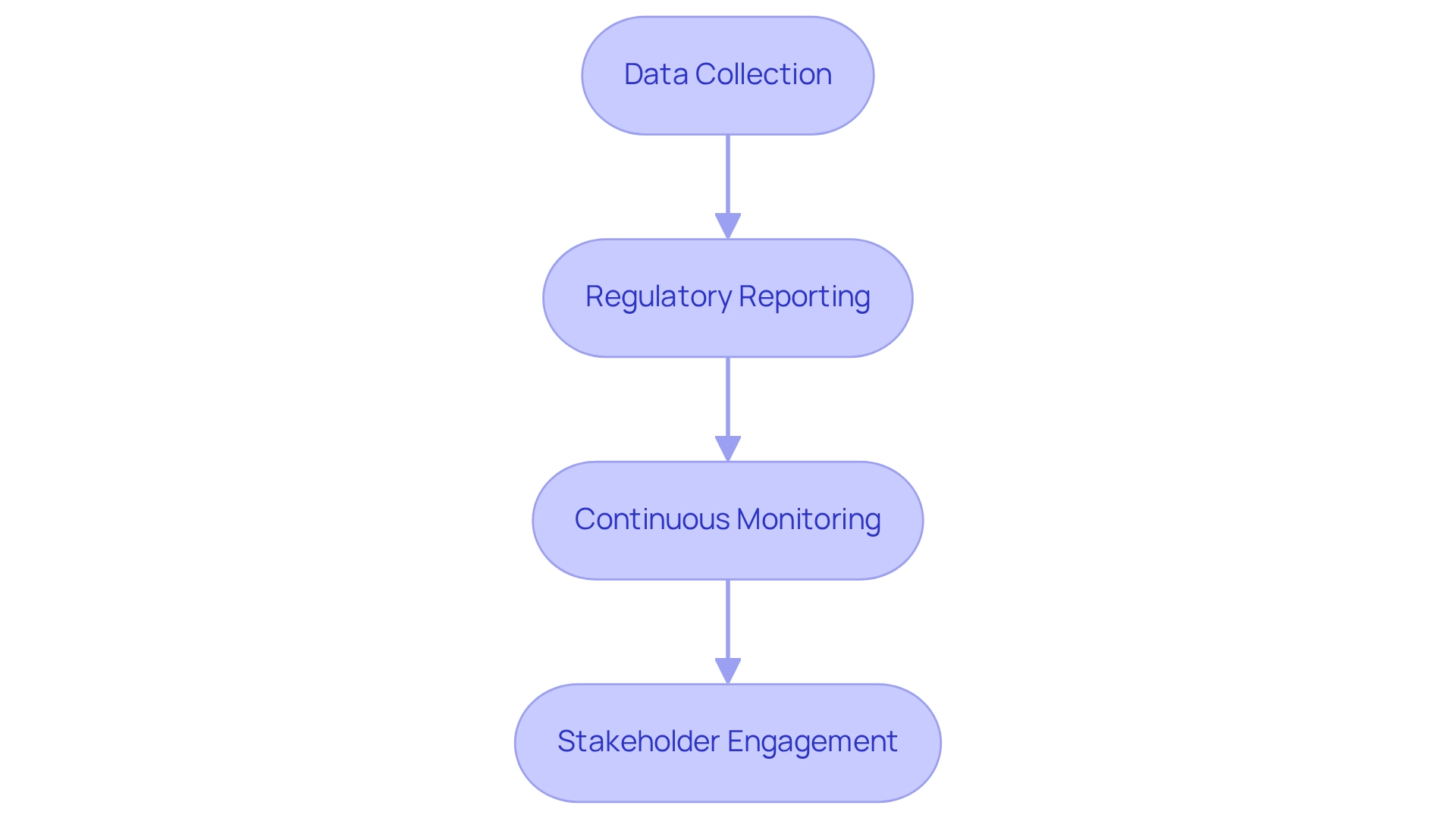
Post-market surveillance is a critical component in ensuring the ongoing safety and effectiveness of medical devices following clinical trials. This process involves several key strategies:
- Data Collection: Establish robust systems for gathering data on adverse events and device performance from both users and healthcare providers. This data is vital for understanding the real-world impact of medical devices and for making informed decisions regarding their use.
- Regulatory Reporting: Adherence to INVIMA's stringent reporting requirements is essential. INVIMA (National Food and Drug Surveillance Institute) oversees the marketing and manufacturing of health products, ensuring compliance with health standards. Timely submission of safety reports to INVIMA helps maintain compliance and ensures that any potential issues are addressed promptly. As highlighted in a case study on the registration process for medical devices in that country, U.S. companies must register their products with INVIMA under their own name to avoid complications with distributor changes.
Notably, INVIMA is classified as a Level 4 health authority by the Pan American Health Organization/World Health Organization, underscoring its competence in health regulation.
- Continuous Monitoring: Implementing continuous monitoring protocols is crucial for assessing the long-term effects of medical devices. This proactive approach allows for the identification of emerging safety concerns, ensuring that any risks are managed effectively.
- Stakeholder Engagement: Maintaining open lines of communication with stakeholders, including healthcare providers and patients, is vital. In the country, the regulatory framework for medical devices is designed to align with international standards, which aids in the efficient review of lower-risk devices.
However, the registration process can present challenges for newcomers, making it imperative for companies to navigate these regulations carefully. With over 15 years of experience in the Medtech sector, bioaccess™ understands these complexities and can provide valuable support as a vetted CRO and consulting partner for U.S. medical device companies. Bioaccess™ employs tailored methodologies to assist U.S. companies in navigating the regulatory landscape, ensuring compliance and facilitating market access.
As Alvaro Enrique Rincon Mautner from SPI Americas notes, effective post-market surveillance is essential in this growing market, especially given the significant increase in import quantities for consumables, diagnostic imaging, and orthopedics & prosthetics. By focusing on these post-market surveillance strategies, organizations can enhance the safety and efficacy of their medical devices, ultimately benefiting patients and healthcare systems alike.
Timeline Considerations for Clinical Trials in Colombia
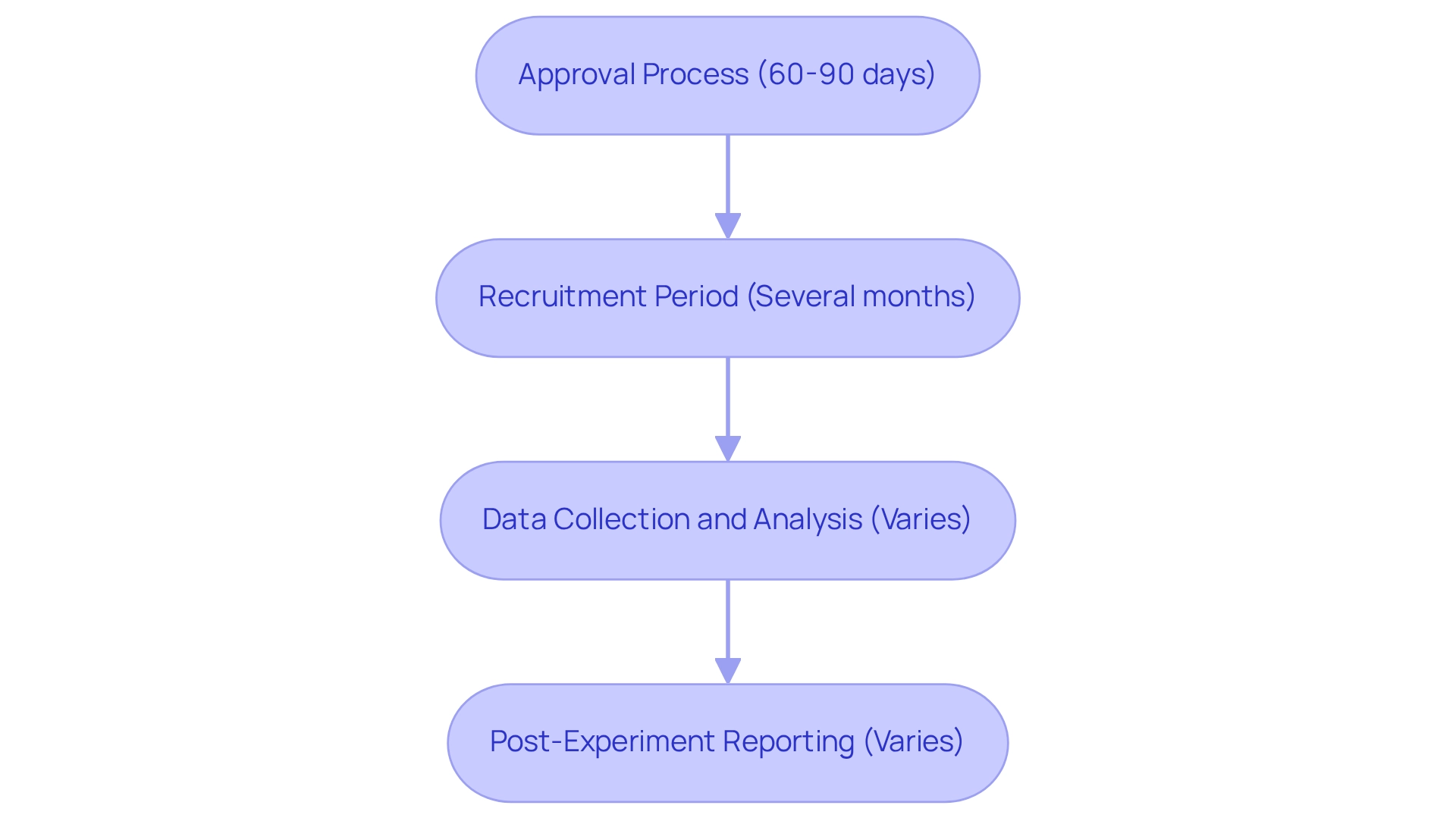
When planning clinical trials in Colombia, researchers must navigate several critical timeline factors to ensure a smooth process:
-
Approval Process: The average duration for INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) to review and approve clinical trial applications is approximately 60 to 90 days. This timeline is crucial for researchers to include in their project schedules, as it establishes the foundation for later stages of the experiment.
-
Recruitment Period: The recruitment phase can vary significantly based on the study's design and the target population. On average, recruitment can take several months, emphasizing the importance of strategic community engagement and outreach efforts. Effective recruitment strategies are essential to ensure a varied group of individuals, which can enhance the study's validity and applicability. Partnerships, like the one between bioaccess™ and Caribbean Health Group, have shown success in this field, establishing Barranquilla as a prominent location for clinical studies in Latin America, backed by Colombia's Minister of Health. Statistics indicate that 266 partners can utilize special purposes to save and communicate privacy choices, which can be beneficial in recruitment efforts.
-
Data Collection and Analysis: Adequate time must be designated for data gathering, which can vary depending on the study's complexity and the number of participants involved. Following data collection, researchers should also plan for an adequate analysis period, as this step is crucial for interpreting results and drawing meaningful conclusions.
-
Post-Experiment Reporting: Once the study is completed, researchers are required to prepare and submit final reports to INVIMA. The time needed for this process can vary depending on the study's findings and the complexity of the data. Timely and thorough reporting is essential for compliance with regulatory requirements and for informing future research initiatives.
In addition to these factors, understanding the local context is vital. For example, honoring local culture while safeguarding patient rights is essential for foreign sponsors undertaking research studies. As Julio G. Martinez-Clark, CEO of bioaccess, states, "The nation’s combination of a large and diverse population, experienced research sites, efficient regulatory processes, a cost-competitive environment, and a history of successful medtech studies since 2010 make it an appealing option for U.S. medical device companies."
Furthermore, recent advancements, including the Clinical Study Readiness Score, emphasize the nation's potential as an appealing location for research studies, especially for U.S. medical device firms aiming to utilize the country's varied demographics and effective regulatory frameworks. Tackling language barriers and cultural differences is crucial, as demonstrated by the case study on language barriers and translation in medical research, which highlights the significance of precise translation for ethical treatment and informed consent. By considering these timeline factors and local nuances, researchers can enhance their understanding of how to design trials for the Colombian market, thereby improving their chances of successful execution in Colombia.
Additionally, bioaccess provides extensive research study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting. These abilities are essential for guaranteeing that medical studies are carried out efficiently and effectively. Notably, bioaccess has achieved over a 50% reduction in recruitment time and maintained a 95% retention rate in its clinical trials, demonstrating its commitment to excellence in patient recruitment and trial management.
Conclusion
Colombia is establishing itself as a pivotal player in the clinical trials arena, bolstered by a robust regulatory framework and a commitment to ethical research practices. The National Food and Drug Surveillance Institute (INVIMA) plays a crucial role in steering researchers through essential regulations, including Resolution 2378/2008 and Resolution 8430 of 1993, which safeguard participant rights and ensure their safety.
A notable challenge persists in participant recruitment, as a significant number of individuals remain unaware of available clinical trial opportunities. By implementing targeted strategies such as community engagement, social media outreach, and partnerships with patient registries, researchers can effectively broaden their reach. The strong existing relationships between patients and healthcare providers further enhance these recruitment efforts.
Moreover, upholding ethical standards and scientific validity in trial design is vital for achieving successful outcomes. Organizations like bioaccess provide invaluable support in navigating regulatory processes and managing clinical trials, thereby streamlining research initiatives.
In summary, Colombia’s combination of a solid regulatory environment, ethical dedication, and innovative recruitment strategies positions it as an appealing destination for clinical trials in Latin America. By addressing recruitment challenges and adhering to established guidelines, researchers can significantly contribute to medical advancement while prioritizing participant welfare. The future of clinical trials in Colombia holds great promise for both the research community and the patients involved.
Frequently Asked Questions
What is required to create medical studies in Colombia?
Creating medical studies in Colombia requires a comprehensive understanding of trial design tailored to the Colombian market, particularly regarding the regulatory framework established by the National Food and Drug Surveillance Institute (INVIMA), specifically Resolution 2378/2008.
What does Resolution 2378/2008 entail?
Resolution 2378/2008 outlines the essential requirements for conducting medical studies in Colombia, ensuring that experiments are designed and executed in compliance with national standards.
What ethical guidelines must researchers in Colombia follow?
Researchers must adhere to the ethical guidelines outlined in Resolution 8430 of 1993, which emphasizes the protection of individuals' rights and safety in research studies.
Why is obtaining informed consent important in medical studies?
Obtaining informed consent is critical as it fulfills legal obligations and fosters trust between researchers and participants, which is essential for effective recruitment and retention in research studies.
What statistics highlight the awareness of research opportunities among patients in Colombia?
Statistics indicate that 85% of patients are not informed about research opportunities at diagnosis, and 75% of those unaware would have been willing to participate if they had known.
What challenges do researchers face regarding patient recruitment in Colombia?
There is a significant gap in patient awareness of research opportunities, presenting challenges in recruitment strategies, despite the favorable environment for understanding trial design due to strong patient-doctor relationships.
How does the Colombian government support medical research?
The Colombian government actively promotes the country as a premier research destination, aiming to enhance its status as a knowledge-based economy by 2031.
What is the application process for clinical trials in Colombia?
Researchers must submit a thorough application to INVIMA that includes a study protocol, informed consent forms, ethics committee approval, and proof of investigator qualifications.
How long does it typically take to get clinical study approval in Colombia?
The average duration for clinical study approval in Colombia is about 4-6 weeks.
What advantages does Colombia offer for conducting medical studies?
Colombia offers a cost-competitive environment, allowing medical procedures to be conducted at 40%–75% less than in the U.S., enabling more extensive studies without sacrificing quality.




