Overview
To develop effective trial protocols for COFEPRIS approval, researchers must adhere to a structured process that encompasses:
- Defining study objectives
- Conducting literature reviews
- Drafting detailed protocols
- Engaging with ethics committees
This article delineates these essential steps and underscores the significance of regulatory compliance and adaptability. It illustrates how meticulous preparation can significantly enhance the likelihood of obtaining approval for medical device trials in Mexico.
Introduction
Navigating the complex landscape of medical device regulation in Mexico necessitates a profound understanding of COFEPRIS, the Federal Commission for Protection against Sanitary Risk. This authority, tasked with ensuring the safety and efficacy of medical devices, plays a pivotal role in the approval process, significantly affecting clinical trials and market entry for innovative technologies.
For researchers and companies aiming to conduct trials in Mexico, comprehending COFEPRIS regulations is not merely advantageous—it's imperative. This article explores the intricacies of COFEPRIS, providing insights into the:
- Development of compliant trial protocols
- Engagement with ethics committees
- Obligations that follow approval
By leveraging expert knowledge and strategic planning, stakeholders can adeptly navigate this regulatory environment, ultimately fostering advancements in healthcare and medical technology.
Understanding COFEPRIS: The Regulatory Authority for Medical Devices in Mexico
The Federal Commission for Protection against Sanitary Risk serves as the Mexican regulatory authority tasked with ensuring the safety and efficacy of medical devices and pharmaceuticals. Established to protect public health, this agency guarantees that all medical devices comply with stringent safety standards before they reach the market. For researchers and businesses conducting studies in Mexico, comprehending the agency's role is essential, particularly in relation to the trial protocols required for COFEPRIS approval, which directly influences both the approval process and compliance standards.
As the leading contract research organization (CRO) in Latin America, bioaccess® excels in navigating these complex regulatory landscapes, delivering tailored solutions specifically designed for Medtech startups. Our expertise ensures that trial protocols for COFEPRIS approval are effectively evaluated, while ongoing studies are meticulously monitored in accordance with established regulations. bioaccess® offers vital services, including:
- Assurance of trial protocol compliance for COFEPRIS approval
- Research site activation
- Subject recruitment
All aimed at facilitating a seamless experience for our clients.
Moreover, bioaccess® is dedicated to maintaining information security and fostering client trust, proactively addressing concerns through our comprehensive grievance and data protection procedures. This commitment to transparency and adherence is fundamental in cultivating trust throughout the clinical study process.
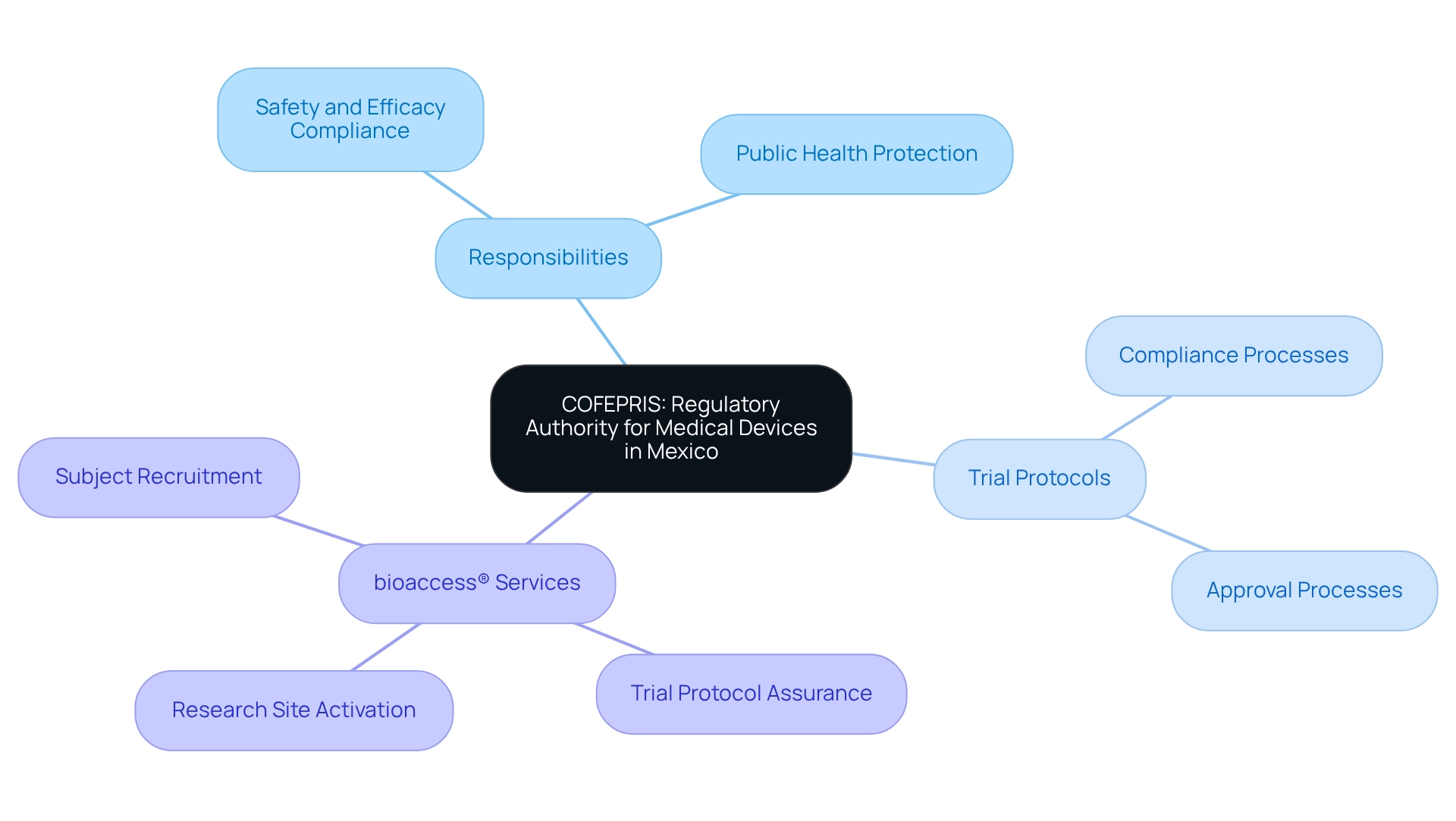
Step-by-Step Process for Developing COFEPRIS-Compliant Trial Protocols
To develop COFEPRIS-compliant research protocols effectively, follow these structured steps:
- Define the Study Objectives: Clearly articulate both primary and secondary objectives of the research, ensuring alignment with COFEPRIS requirements. This foundational step is crucial as it sets the direction for the entire protocol.
- Conduct a Literature Review: Perform a comprehensive review of existing research and data pertinent to your study. This not only supports the rationale behind your protocol but also helps identify gaps in current knowledge that your trial aims to address.
- Draft the Protocol: Create a detailed draft that includes essential sections such as methodology, participant selection criteria, data collection methods, and statistical analysis plans. A well-structured protocol is vital for clarity and compliance in the context of trial protocols for COFEPRIS approval. When drafting, ensure to include mechanisms for continuity of medical treatment and indemnity for research participants in the event of trial-related injuries, as this is a regulatory requirement.
- Incorporate Trial Protocols for COFEPRIS Approval: Ensure that your protocol strictly adheres to the applicable guidelines. This includes implementing safety monitoring and reporting procedures, which are critical for participant protection and regulatory approval. Additionally, be aware of the need to obtain consent from legal guardians when processing personal data of minors, particularly in the context of participant selection criteria.
- Engage with Ethics Committees: Early engagement with ethics committees is essential. Seek their feedback to proactively address any ethical concerns, which can streamline the approval process later on.
- Utilize the Comprehensive Service Center (CIS): Leverage the resources provided by the CIS, a public service system established to streamline the agency's procedures and services, to facilitate your application process.
- Submit for Approval: Prepare and submit the Clinical Trial Application (CTA) to the regulatory authority, ensuring that all necessary documentation and supporting materials are included. It is important to note that about 70% of medical study protocols satisfy regulatory requirements on first submission, emphasizing the significance of thorough preparation.
- Revise as Necessary: Be prepared to make adjustments based on feedback from regulatory bodies or ethics committees. Adaptability at this phase is essential to guarantee adherence and improve the chances of approval.
By adhering to these steps, researchers can navigate the intricacies of regulatory requirements more efficiently, ultimately promoting the prompt progression of studies in Mexico, particularly concerning trial protocols for COFEPRIS approval. As emphasized by industry experts, including Katherine Ruiz, the importance of local regulatory knowledge cannot be overstated. Furthermore, reflecting on the lessons learned from the COVID-19 pandemic, it is essential to adapt to regulatory challenges and harmonize technical requirements across regions to ensure timely access to life-saving medicines.
At bioaccess, we specialize in offering extensive clinical research management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting, to support your clinical research efforts in Latin America.
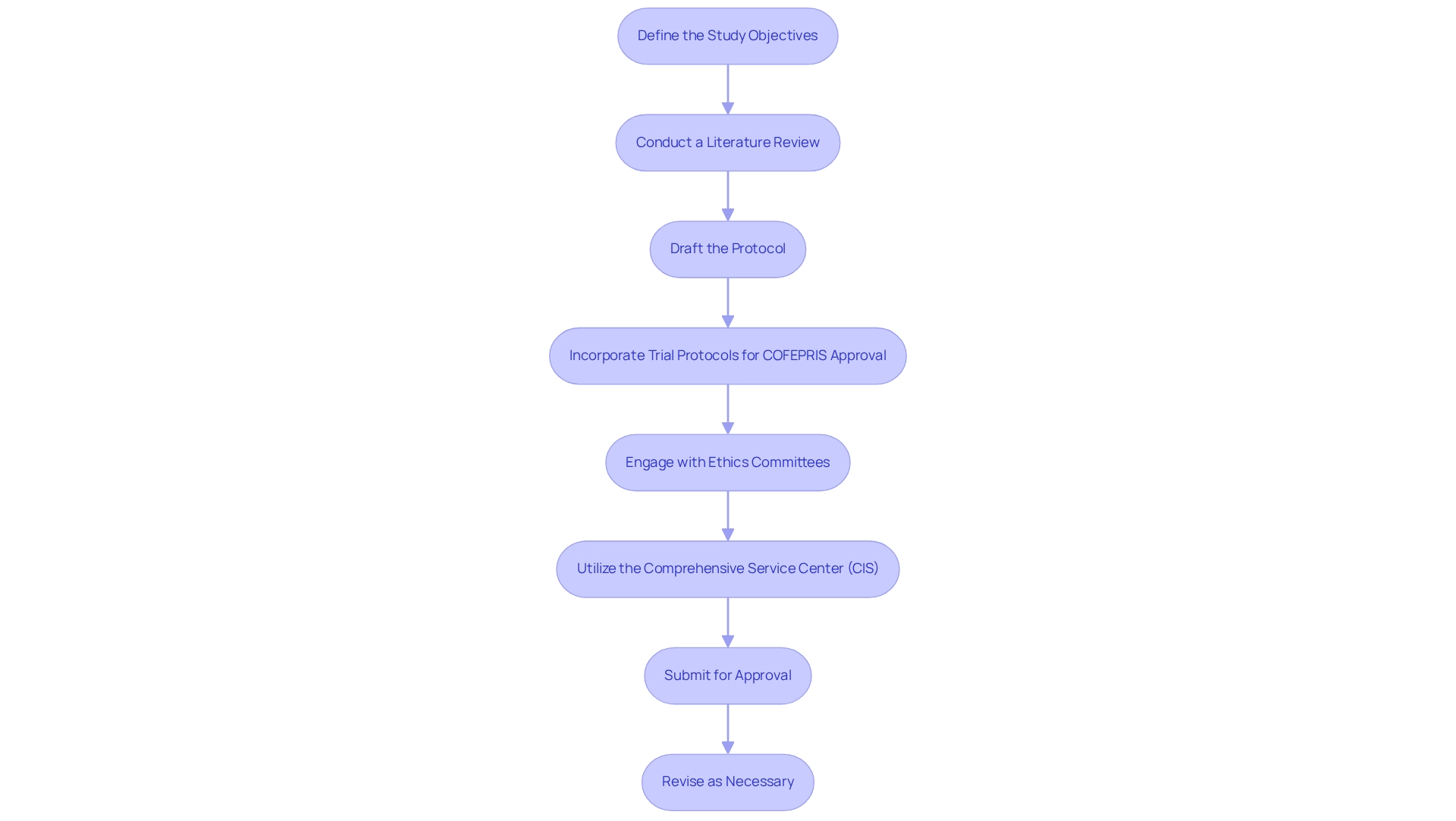
Key Components of a COFEPRIS Trial Protocol: What to Include
To ensure compliance and facilitate successful approval, trial protocols for COFEPRIS approval must encompass several critical components. These components include:
-
Title and Research Design: The protocol should begin with a clear and concise title that reflects the research's focus, followed by a detailed description of the research design, such as whether it is a randomized controlled trial or another format.
As indicated by Gordis et al., matching controls is crucial to guarantee that the research design is robust and reduces bias.
-
Objectives: Clearly define both primary and secondary goals, outlining what the research aims to achieve. This clarity assists in aligning the objectives of the research with regulatory expectations.
Understanding the objectives is crucial as Phase I trials focus on determining safe dosages, while Phase II evaluates safety and preliminary efficacy.
-
Study Population: Provide a comprehensive description of the target population, including specific inclusion and exclusion criteria. This guarantees that the research is structured to produce pertinent and useful outcomes.
Thorough evaluation of the research population is essential to prevent selection bias, as emphasized in the case analysis on bias in research.
-
Intervention Details: Include thorough information about the medical device under investigation, detailing its intended use, mechanism of action, and any relevant background information that supports its clinical relevance. Utilizing bioaccess's expertise in managing Early-Feasibility and First-In-Human trials, along with Pilot Trials and Post-Market Clinical Follow-Up Trials, can enhance the depth of this section.
-
Outcome Measures: Specify the primary and secondary outcome measures that will be assessed throughout the research. This section should clarify how these measures correspond with the research objectives and regulatory requirements. The outcomes should be clearly defined to facilitate the transition from Phase III, which confirms efficacy, to Phase IV, which monitors long-term effects post-approval.
-
Statistical Analysis Plan: Outline the statistical methods that will be employed to analyze the data collected during the trial. This plan should detail how the data will be interpreted to ensure robust and valid conclusions.
-
Ethical Considerations: Address ethical aspects, including informed consent procedures and measures taken to ensure participant safety throughout the research. This is crucial for maintaining ethical standards and regulatory compliance. Ensuring participant safety aligns with the ethical obligations of researchers and the regulatory framework.
-
Timeline: Provide a detailed schedule for the research, highlighting key milestones and expected completion dates. This assists in handling expectations and guaranteeing that the study advances as intended.
By carefully addressing these elements and incorporating insights from research phases, scientists can improve the quality and adherence of trial protocols for COFEPRIS approval. This ultimately enables more efficient approval procedures and promotes the advancement of cutting-edge medical technologies, backed by bioaccess's extensive research management services and over 20 years of experience in the Medtech sector.
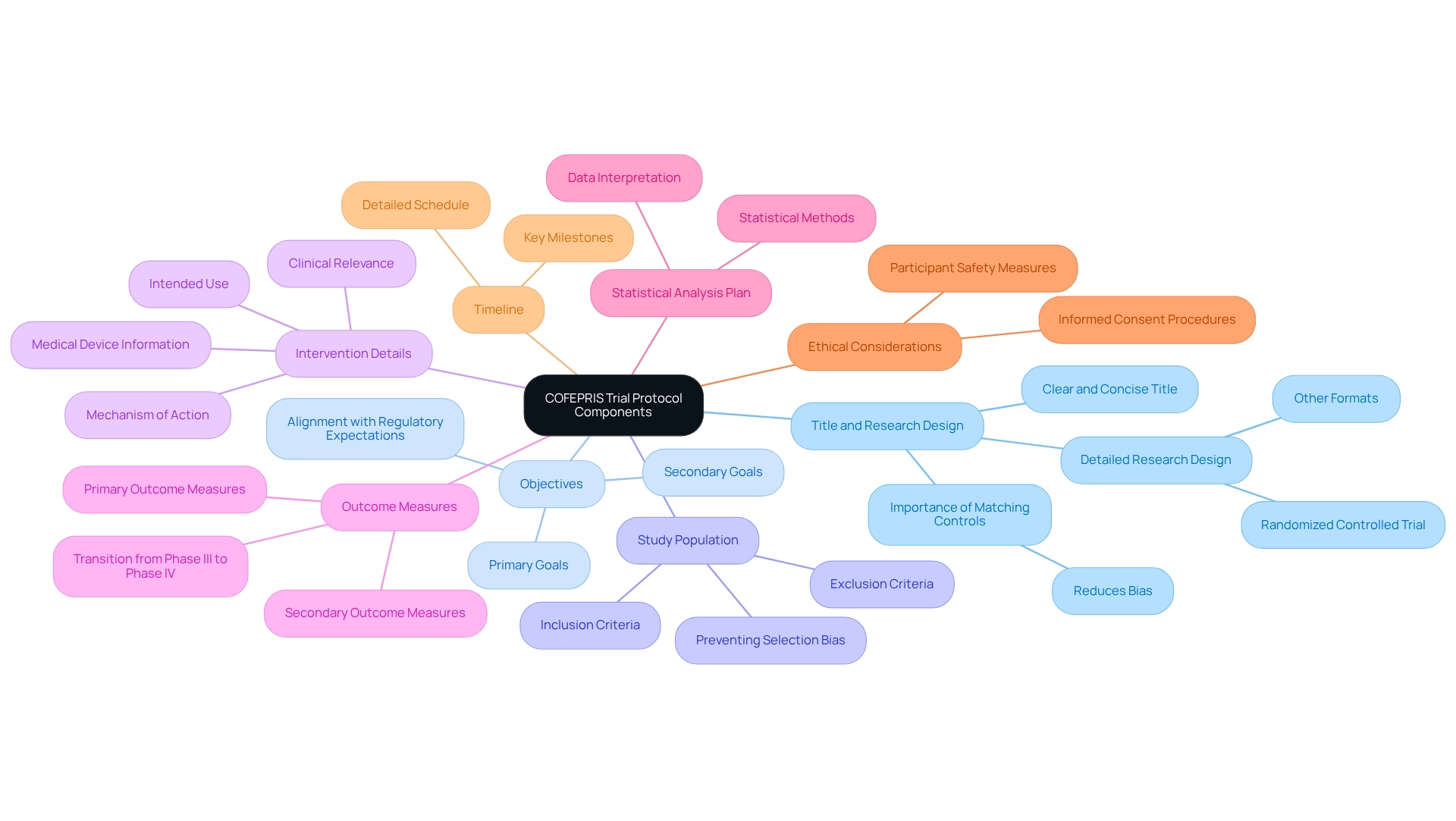
The Role of Clinical Trials in COFEPRIS Approval: Requirements and Expectations
Research studies are integral to the regulatory approval process, providing essential medical data necessary for trial protocols required by COFEPRIS. These studies demonstrate the safety and effectiveness of medical devices. Notably, bioaccess™ has supported Avantec Vascular in their pioneering first-in-human research of an innovative vascular device in Latin America. This support includes the selection of a principal investigator, the submission of trial protocols for COFEPRIS approval, and various tasks essential for conducting the study. Such collaboration underscores the critical role of partnerships in advancing medical technology.
COFEPRIS mandates that:
- Preclinical Data: Sponsors must submit preclinical data validating the device's safety before initiating trials on human subjects. All trial data submissions must comply with the trial protocols for COFEPRIS approval, encompassing comprehensive information and results from all trial phases.
- Compliance with GCP: Trials must adhere to Good Clinical Practice (GCP) guidelines to ensure the safety of participants and the integrity of data.
- Reporting Obligations: Researchers are required to promptly report any adverse events or safety concerns to COFEPRIS.
- Post-Market Surveillance: After authorization, continuous monitoring of the device's market performance is essential to ensure ongoing safety and effectiveness.
In addition to these requirements, bioaccess™ is committed to protecting information security and fostering client confidence throughout the research process. Their grievance and data protection procedures are designed to address client concerns regarding compliance and transparency, reinforcing their dedication to ethical practices in medical device testing.
Ethical Considerations: Engaging Ethics Committees for COFEPRIS Approval
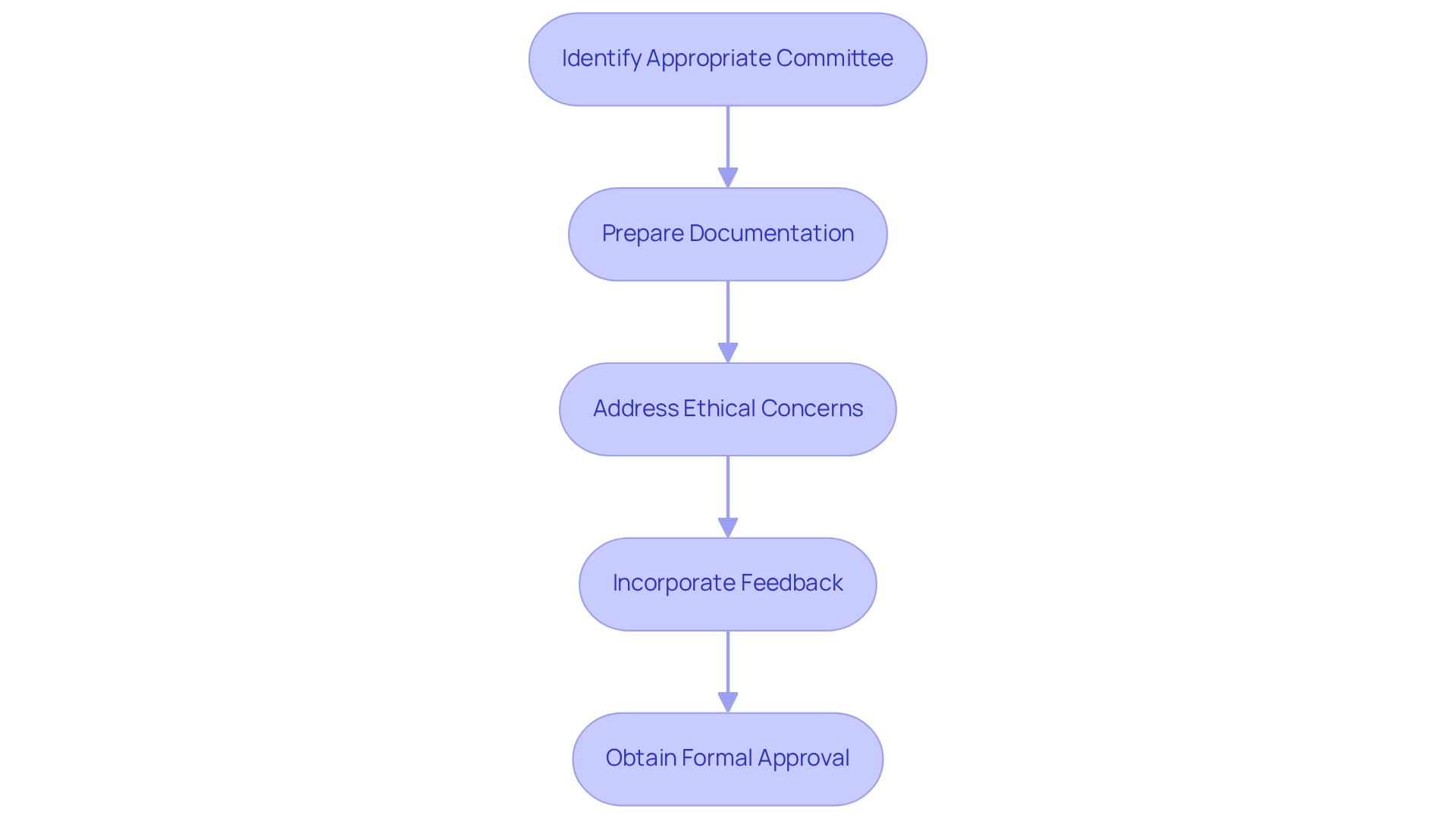
Engaging ethics committees is a critical step in the trial protocols for COFEPRIS approval process for clinical trials in Mexico. Researchers must first identify the appropriate committee, determining its relevance based on location and focus, ensuring alignment with local regulations and cultural considerations.
Next, it is essential to prepare documentation. Submit all necessary documents, including the research protocol, informed consent forms, and any other relevant materials, to facilitate a smooth review process. Addressing ethical concerns is paramount; be prepared to discuss issues raised by the committee, such as participant safety and informed consent processes, which are crucial for maintaining trust and compliance.
Incorporate feedback by revising the protocol based on the committee's input to ensure compliance with ethical standards. This demonstrates responsiveness to local expectations and enhances the project's credibility. Finally, obtain formal approval from the ethics committee before submitting the trial protocols for COFEPRIS approval to the regulatory authority. This step is essential for advancing your research project in the Latin American market.
By following these best practices, researchers can navigate the complexities of the Latin American Medtech landscape effectively. Utilizing bioaccess's extensive clinical trial management services—including feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project management, and reporting—ensures successful market access and adherence. Insights from industry leaders, such as those shared in the LATAM Medtech Leaders Podcast, further emphasize the importance of understanding local dynamics and adapting strategies accordingly.
Navigating Challenges in Protocol Development: Tips for Success
Navigating the trial protocols for COFEPRIS approval presents several challenges during the regulatory approval process. To enhance your success, consider these essential strategies:
- Start Early: Initiating the protocol development process well in advance is crucial. This proactive approach allows sufficient time for revisions and approvals, significantly impacting the overall timeline. For instance, the average duration from ethics committee (EC) or institutional review board (IRB) approval to the first patient randomized can vary, with research like APHINITY demonstrating a median of 118 days, compared to 169 days in ALTTO.
- Stay Informed: Regularly updating your knowledge of trial protocols for COFEPRIS approval and related guidelines is vital for ensuring compliance. This includes understanding the latest requirements and best practices that can affect trial protocols. Engaging with local experts, such as Ana Criado, who has extensive experience in regulatory affairs and biomedical engineering, can provide valuable insights into navigating the complexities of trial protocols.
- Engage Stakeholders: Involving key stakeholders—such as healthcare personnel, regulatory specialists, and potential research locations—early in the protocol development process provides diverse insights and enhances the quality of the protocol. This collaborative approach helps identify potential challenges and streamlines the trial protocols for COFEPRIS approval process. Furthermore, locating a nearby collaborator with research experience, such as Bioaccess, is essential for the successful conclusion of studies in Mexico, greatly influencing site selection and participant enrollment. Bioaccess offers extensive clinical trial management services, including feasibility assessments, compliance evaluations, trial organization, and project oversight, which are crucial for developing trial protocols and navigating the complexities of regulatory approval.
- Be Flexible: Adaptability is essential when developing protocols. Be ready to adjust your protocol according to input from ethics committees and regulatory authorities. This flexibility facilitates smoother interactions and quicker approvals. As highlighted by Sergio A. Sanchez, sponsors must comprehensively evaluate their partner’s performance in choosing research locations and investigators, recruiting participants, and adhering to project deadlines.
- Document Everything: Maintaining meticulous records of all communications and revisions is critical. This practice guarantees transparency and accountability, aiding in resolving any inquiries from regulatory bodies during the review process. Along with these strategies, it is essential to acknowledge frequent challenges encountered in protocol development for the relevant authority. These may include navigating complex regulatory requirements and ensuring that all necessary documentation for trial protocols is properly in place.
By learning from case studies, such as the Mayo Clinic's implementation of parallel processing to reduce activation time by 70%, organizations can adopt innovative strategies to overcome these hurdles. This method, which involves conducting activation steps in parallel rather than in series, significantly minimizes non-value-added time and streamlines the overall process. Ultimately, comprehensive preparation and strategic planning are essential for effective protocol development to gain regulatory approval.
Post-Approval Compliance: Understanding COFEPRIS Obligations
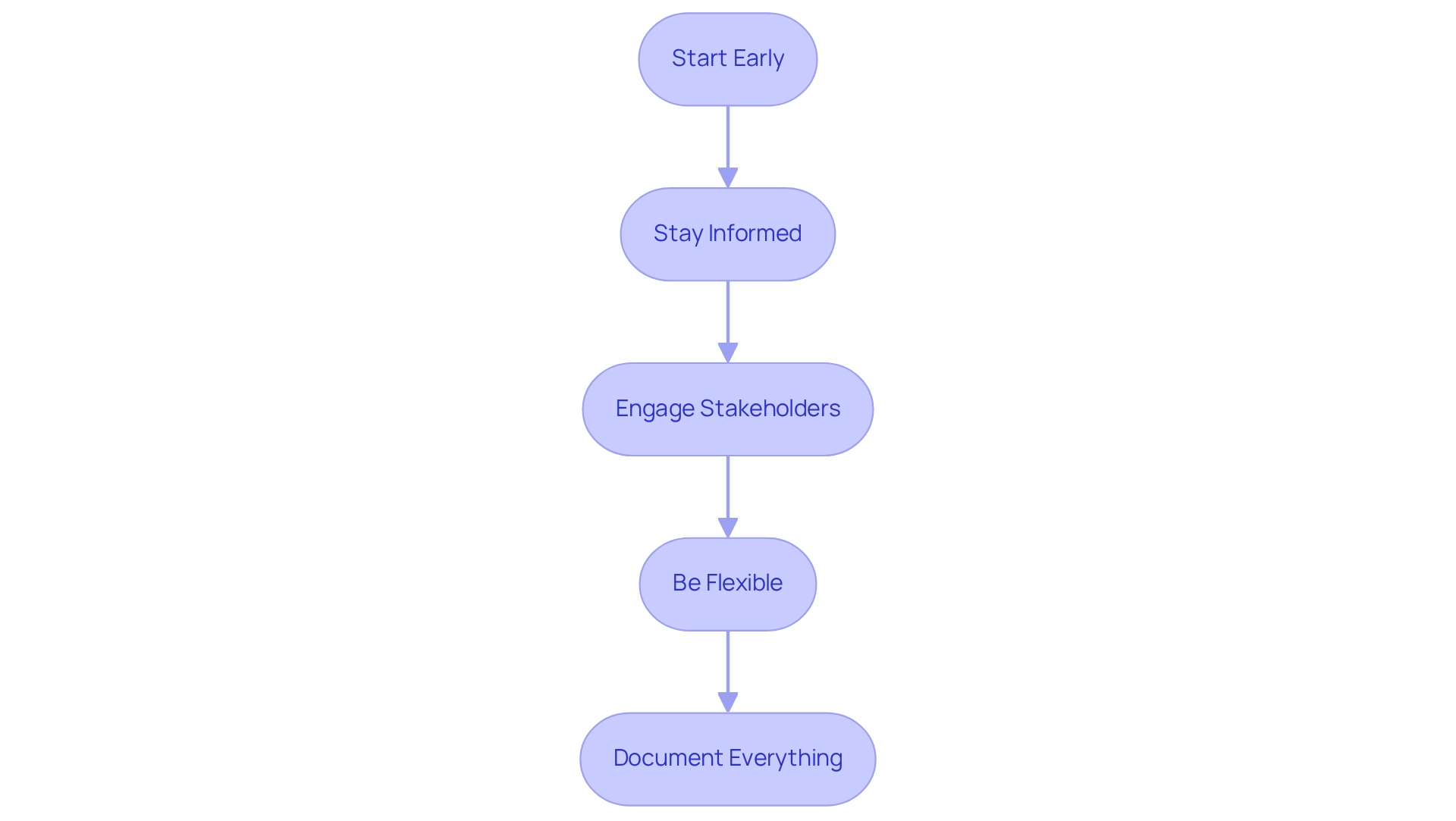
After obtaining COFEPRIS approval, researchers must adhere to several post-approval obligations to ensure compliance and maintain client trust:
- Ongoing Monitoring: Continuously monitor the safety and efficacy of the medical device throughout its lifecycle, ensuring that any potential issues are promptly addressed.
- Reporting Requirements: Submit periodic reports to the regulatory agency detailing the trial's progress and any adverse events, fostering transparency and accountability in the research process.
- Compliance with Changes: Inform the regulatory authority of any major alterations to the study protocol or device specifications, showcasing a commitment to regulatory adherence and ethical standards.
- Post-Market Surveillance: Implement a post-market surveillance plan to track the device's performance in real-world settings, which is crucial for ongoing safety assessments and client confidence.
- Maintain Documentation: Keep thorough records of all trial-related activities and communications with the regulatory authority for future reference, ensuring that all processes are transparent and compliant with applicable laws.
- Grievance Procedures: bioaccess® is committed to addressing client concerns promptly. For any queries or grievances regarding the processing of information, clients may contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com.
By following these responsibilities, bioaccess® not only guarantees the integrity of medical studies but also strengthens its dedication to data protection and client trust in the Latin American Medtech environment.
Looking Ahead: The Future of Clinical Trials and COFEPRIS Regulations in Mexico
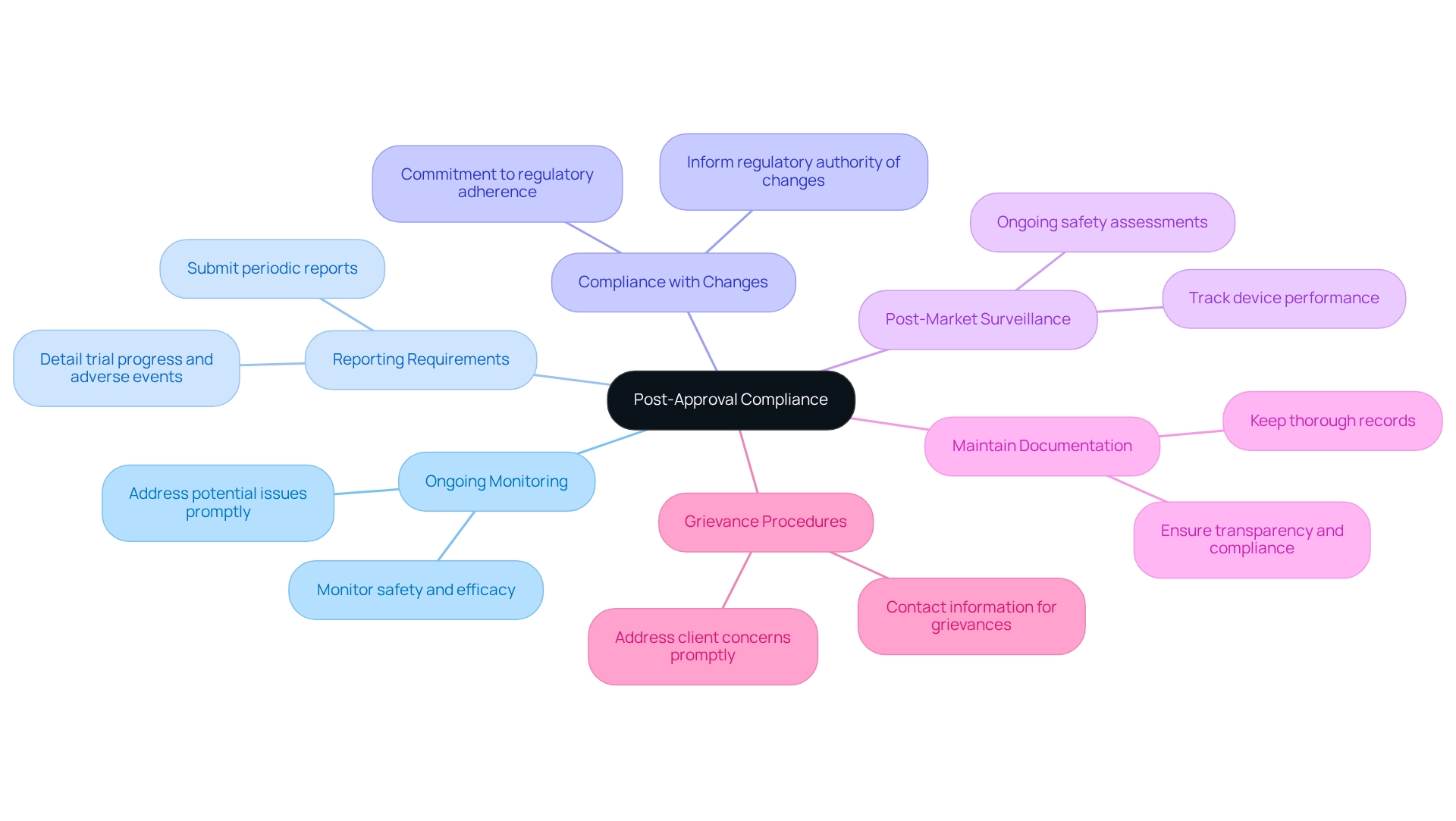
The future of medical studies in Mexico is poised for significant transformations as the regulatory authority enhances its framework. Key trends to monitor include:
- Enhanced Digitalization: COFEPRIS is anticipated to improve its digital platforms, considerably simplifying the submission and approval processes for research studies. This shift towards digitalization is expected to support the development of trial protocols for COFEPRIS approval, thereby reducing turnaround times and improving overall efficiency in regulatory interactions. As Scott Mussbacher noted, "You can make it seamless for all of the stakeholders within your organization. The workflows and rollout processes are critical to gaining user adoption and maximizing digital transformation investments."
- Regulatory Harmonization: There is a growing initiative to align Mexican regulations with international standards, which may simplify the approval process for foreign sponsors. This harmonization is essential for promoting collaboration and attracting global investment in medical research through the implementation of trial protocols for COFEPRIS approval.
- Focus on Patient Safety: A sustained emphasis on ethical considerations and patient safety will continue to influence future regulations and study designs. This focus ensures that trial protocols for COFEPRIS approval prioritize patient welfare at the forefront of medical research practices.
- Emerging Technologies: The integration of innovative technologies, such as telemedicine and remote monitoring, is set to influence regulatory approaches. These advancements enhance data collection and improve patient involvement and adherence during studies adhering to trial protocols for COFEPRIS approval.
- Adjustment to Worldwide Trends: COFEPRIS will need to adjust to global trends in medical research, including the increase of decentralized studies and the use of real-world evidence. This adaptability will be essential for maintaining relevance in an increasingly interconnected research environment.
As these trends develop, they will greatly influence the environment of trials in Mexico, highlighting the need for trial protocols for COFEPRIS approval, which presents both challenges and opportunities for stakeholders in the Medtech sector. With over 20 years of experience in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), bioaccess® exemplifies how organizations can successfully bridge the gap between innovative Medtech companies and the potential for conducting clinical research in Latin America. Our customized approach not only accelerates the development and market entry of medical devices but also navigates companies towards acquisition, contributing to job creation, economic growth, and healthcare improvement in local economies.
Conclusion
Navigating the regulatory landscape of medical devices in Mexico necessitates a comprehensive understanding of COFEPRIS and its pivotal role in ensuring the safety and efficacy of clinical trials. By adopting a structured approach to developing compliant trial protocols, engaging with ethics committees, and fulfilling post-approval obligations, researchers and companies can significantly elevate their prospects for successful market entry.
The essential components of a COFEPRIS trial protocol—spanning from the definition of study objectives to the maintenance of thorough documentation—are critical for meeting regulatory standards and cultivating trust among stakeholders. Moreover, the focus on ethical considerations and ongoing monitoring post-approval highlights the unwavering commitment to participant safety and data integrity, which are fundamental in clinical research.
Looking ahead, the future of clinical trials in Mexico is on the brink of transformation, fueled by digitalization, regulatory harmonization, and the integration of emerging technologies. These advancements offer new avenues for innovation and collaboration within the Medtech sector, underscoring the necessity of strategic planning and local expertise in navigating the intricacies of COFEPRIS regulations.
Ultimately, by harnessing the insights and services provided by organizations like bioaccess®, stakeholders can effectively bridge the gap between regulatory compliance and the progression of medical technologies, paving the way for enhanced healthcare outcomes in Mexico and beyond.
Frequently Asked Questions
What is the role of the Federal Commission for Protection against Sanitary Risk (COFEPRIS)?
COFEPRIS is the Mexican regulatory authority responsible for ensuring the safety and efficacy of medical devices and pharmaceuticals. It protects public health by ensuring that all medical devices meet stringent safety standards before they are marketed.
Why is it important for researchers and businesses to understand COFEPRIS's role?
Understanding COFEPRIS's role is essential for researchers and businesses conducting studies in Mexico, particularly regarding the trial protocols required for COFEPRIS approval, which directly impacts the approval process and compliance standards.
What services does bioaccess® provide to support Medtech startups?
bioaccess® offers services including assurance of trial protocol compliance for COFEPRIS approval, research site activation, and subject recruitment, all aimed at facilitating a seamless experience for clients.
How does bioaccess® maintain client trust and information security?
bioaccess® is dedicated to maintaining information security and fostering client trust by proactively addressing concerns through comprehensive grievance and data protection procedures, emphasizing transparency and adherence throughout the clinical study process.
What are the initial steps to develop COFEPRIS-compliant research protocols?
The initial steps include defining study objectives, conducting a literature review, and drafting the protocol, which should include methodology, participant selection criteria, data collection methods, and statistical analysis plans.
What should be included in the trial protocol for COFEPRIS approval?
The protocol must adhere to applicable guidelines, implement safety monitoring and reporting procedures, and ensure consent from legal guardians when processing personal data of minors.
Why is early engagement with ethics committees important?
Early engagement with ethics committees is crucial to seek feedback and address ethical concerns proactively, which can streamline the approval process later on.
What is the Comprehensive Service Center (CIS) and how can it help?
The CIS is a public service system established to streamline COFEPRIS procedures and services, providing resources to facilitate the application process for researchers.
What should researchers do after submitting their Clinical Trial Application (CTA)?
Researchers should be prepared to revise their application based on feedback from regulatory bodies or ethics committees, ensuring adaptability to guarantee adherence and improve approval chances.
What is emphasized about local regulatory knowledge in clinical research?
Local regulatory knowledge is crucial for navigating regulatory requirements efficiently, promoting the timely progression of studies in Mexico, especially concerning trial protocols for COFEPRIS approval.




