Overview
To ensure compliance with international and local regulations, organizations should implement a structured approach that includes conducting conformity audits, creating adherence plans, establishing standard operating procedures, and engaging stakeholders throughout the regulatory process. The article outlines these strategies as essential steps for enhancing regulatory adherence, emphasizing the importance of continuous monitoring, training, and leveraging technology to navigate the complexities of compliance effectively.
Introduction
Navigating the intricate landscape of regulatory compliance in clinical research presents a formidable challenge for Medtech companies, particularly in a diverse region like Latin America. With varying local regulations and stringent international guidelines, organizations must equip themselves with comprehensive knowledge and strategic approaches to ensure adherence.
This article delves into essential compliance strategies, the importance of staying informed about regulatory changes, and the role of technology in streamlining compliance management. By exploring these critical aspects, Medtech companies can not only enhance their compliance posture but also drive innovation and improve clinical trial outcomes in a rapidly evolving environment.
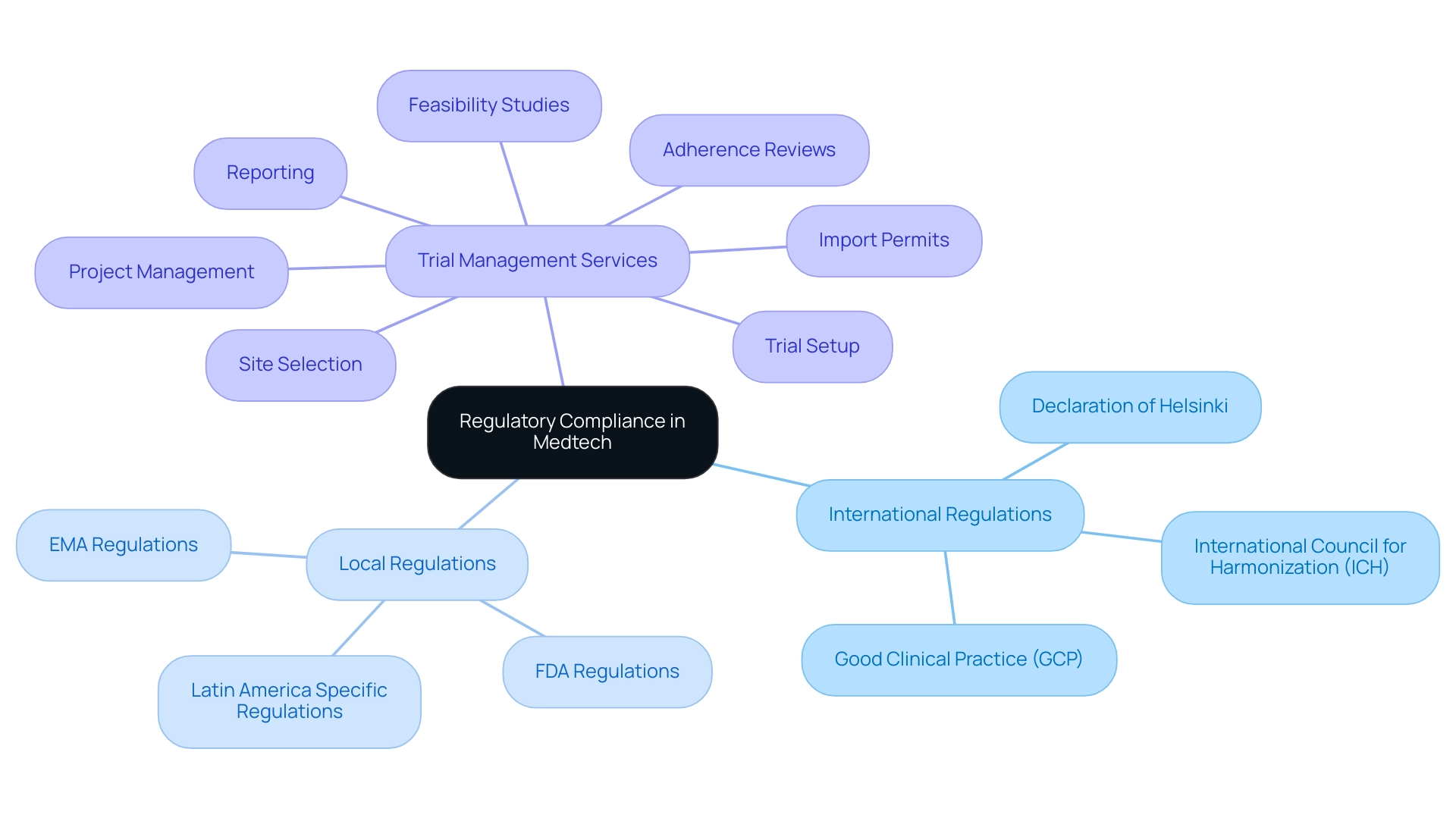
Understanding the Landscape of International and Local Regulations
To attain adherence to standards in medical research, it is crucial for Medtech firms to understand how to ensure compliance with international and local regulations, including the Good Clinical Practice (GCP) guidelines, the Declaration of Helsinki, and the International Council for Harmonization (ICH) guidelines. Understanding how to ensure compliance with international and local regulations in Latin America is equally important, as these regulations may differ significantly by country, particularly those established by the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. Significantly, medical studies must not be initiated until 30 days after the FDA receives the IND, unless earlier notification is provided, emphasizing the importance of adhering to regulatory timelines.
Thorough trial management services—covering:
- Feasibility studies
- Site selection
- Adherence reviews
- Trial setup
- Import permits
- Project management
- Reporting
are essential for closing the gaps in research and innovation in Latin America. These services directly address challenges such as language barriers and fragmentation of resources, which can impede seamless communication between US Medtech companies and Latin American hospitals. Conducting a thorough literature review will enhance your awareness of evolving regulations, while consulting with legal experts will help you understand how to ensure compliance with international and local regulations relevant to your research activities.
This foundational knowledge is essential in directing your adherence efforts and reducing the risks linked to non-adherence, which can have significant implications for the integrity of your study and the safety of participants. Moreover, cooperation between entities such as Greenlight Guru and bioaccess® seeks to expedite Medtech innovations and trials in Latin America, as emphasized by PAVmed's first-in-human study in Colombia. By following established protocols and utilizing extensive services, organizations can not only improve their regulatory stance but also aid in the overall progress of research in the area.
The successful execution of these services exemplifies how overcoming compliance challenges can lead to significant advancements in clinical trial outcomes.
Step-by-Step Strategies for Achieving Compliance
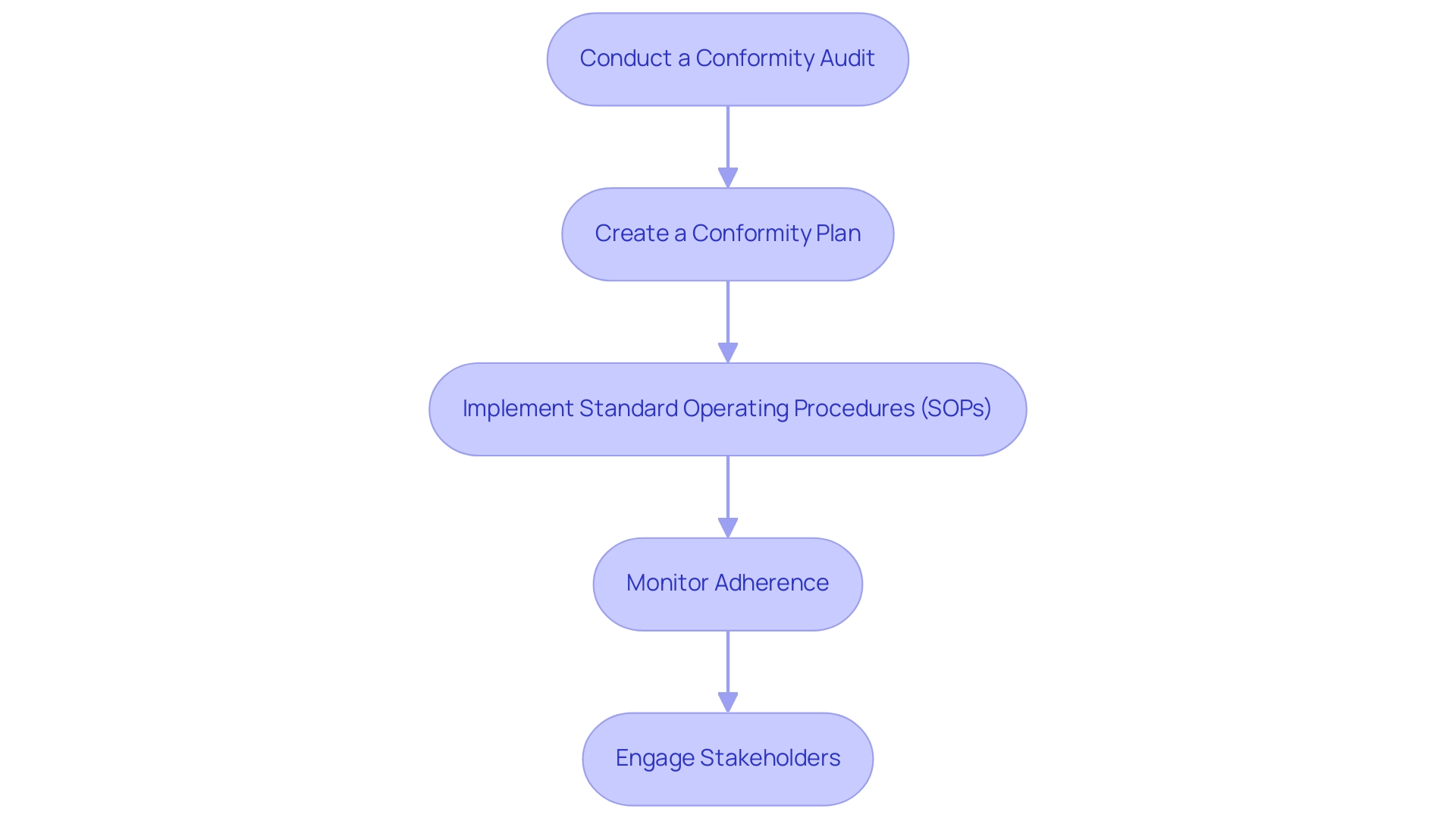
- Conduct a Conformity Audit: Start your process with a comprehensive conformity audit aimed at evaluating how to ensure compliance with international and local regulations in relation to your current practices. This evaluation should focus on identifying gaps and areas that require improvement. By conducting regular audits, organizations can significantly enhance their adherence effectiveness and achieve notable cost savings—averaging $520,000—by establishing a formal charter, directly linking the audit process to financial benefits.
- Create a Conformity Plan: After the audit, develop an extensive adherence plan that outlines your strategies for how to ensure compliance with international and local regulations. This plan should include specific timelines, responsible parties, and clear objectives to ensure accountability and efficient execution. Incorporating expert insights, such as those from Ayush Saxena, emphasizes that this demonstrates the growing significance of adherence in modern times, underlining the necessity of a well-structured approach.
- Implement Standard Operating Procedures (SOPs): Establish Standard Operating Procedures as a key strategy for how to ensure compliance with international and local regulations. These SOPs are crucial for standardizing processes throughout your organization, thereby reducing variability and improving overall adherence. Organizations that have adopted best practices in regulatory adherence have reported substantial cost reductions, with an average savings of $3.01 million realized through centralized data governance initiatives.
- Monitor Adherence: It is crucial to regularly review adherence metrics and reports on how to ensure compliance with international and local regulations. This proactive monitoring is crucial not only for identifying potential issues early but also for addressing the evolving landscape of regulations, particularly with 77% of corporate risk and regulatory professionals prioritizing staying informed on the latest developments related to ESG. This reflects the need for continuous improvement in regulatory practices to mitigate risks effectively.
- Engage Stakeholders: Foster transparency and collaboration by involving all relevant stakeholders throughout the regulatory process to understand how to ensure compliance with international and local regulations. This encompasses governing bodies, ethics committees, and internal teams. Involving these parties not only fosters trust but also improves the efficacy of your adherence strategies, ensuring that they are well-aligned with both legal expectations and organizational objectives. Furthermore, acknowledging that 35% of executives view third-party breaches as a significant cyber threat emphasizes the necessity of adhering to regulations in reducing risks linked to third-party relationships.
The Importance of Staying Informed on Regulatory Changes
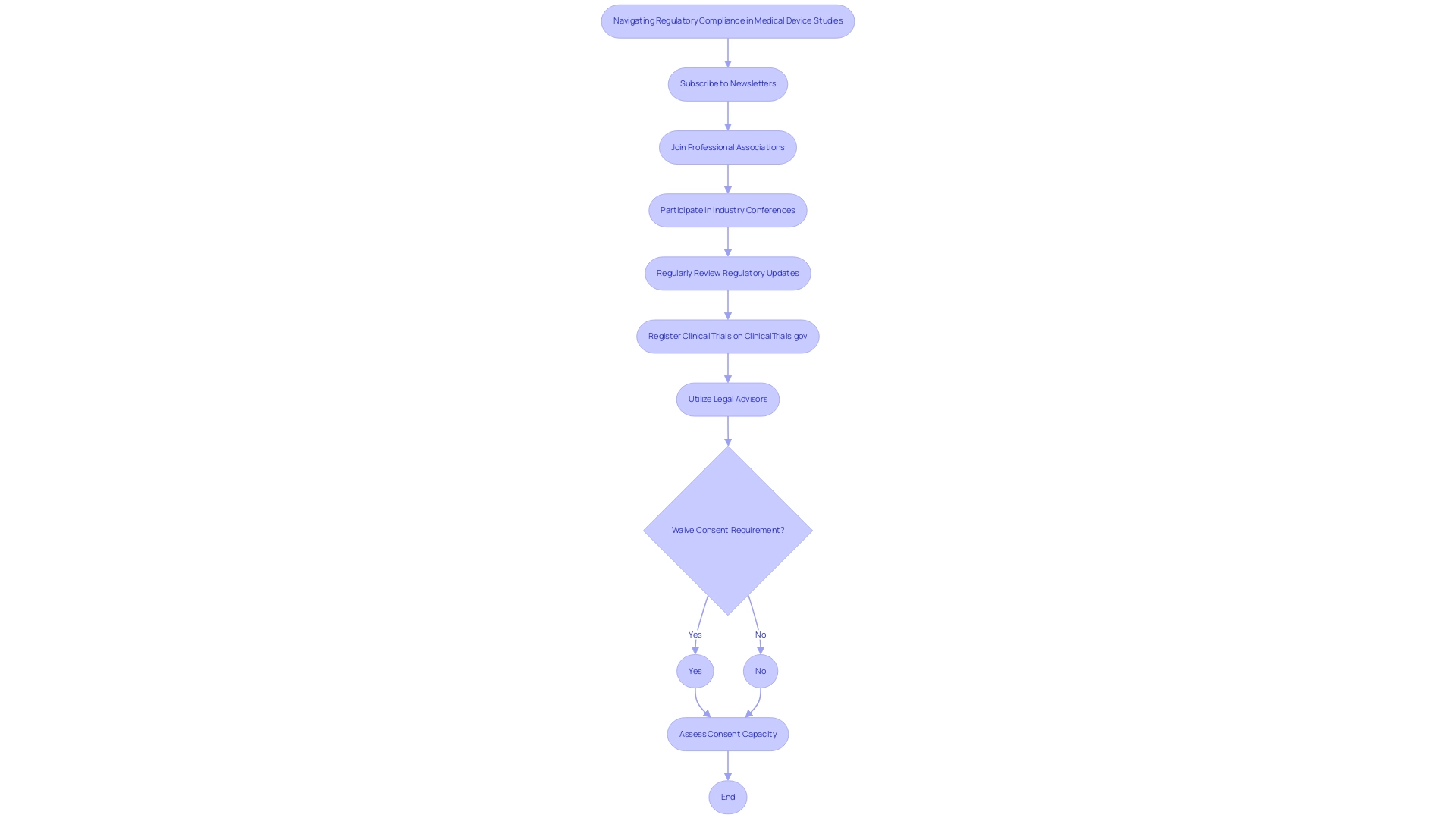
To effectively navigate the complexities of legal adherence in medical device studies, understanding how to ensure compliance with international and local regulations is essential for establishing a comprehensive system for tracking changes in regulations. This encompasses feasibility and selection of research locations and lead investigators, along with understanding how to ensure compliance with international and local regulations through thorough review and feedback on study documents. Key processes such as trial set-up, obtaining import permits, and nationalization of investigational devices are critical components of this system.
Subscribing to pertinent newsletters, joining professional associations, and actively participating in industry conferences can further aid in staying informed. Regularly reviewing updates from regulatory agencies is crucial, especially given the pace of innovation demonstrated by 24 first-in-class launches in the U.S. in 2023. Clinical trials must be registered electronically with ClinicalTrials.gov no later than 21 days after the first participant is enrolled, highlighting a critical requirement that must not be overlooked. Utilizing resources like legal advisors or regulatory consultants can provide expert interpretation of new regulations, which is crucial for understanding how to ensure compliance with international and local regulations. Furthermore, developing a schedule that highlights important dates and deadlines for updates can assist your entity in proactively adjusting its practices.
This approach not only mitigates risks associated with non-compliance but also provides insights on how to ensure compliance with international and local regulations, thereby strengthening your organization’s credibility within the industry. As Noah Aleshire, chief compliance officer at the CDC, notes, 'A collaborative and informed process is invaluable in understanding how to ensure compliance with international and local regulations.' This perspective underscores the importance of collaboration in navigating regulatory complexities and understanding how to ensure compliance with international and local regulations.
Furthermore, institutional ethics committees play a critical role in assessing consent capacity, particularly for individuals with impaired consent capacity, highlighting the need for ethical considerations in research. A relevant case study on the waiver of consent illustrates that ethics committees may waive the requirement for a signed informed consent form under specific conditions, such as minimal risk research or cultural considerations, allowing for ethical engagement while preserving participant rights. To discuss how we can support your clinical trial needs, book a meeting with us today.
Leveraging Technology for Effective Compliance Management
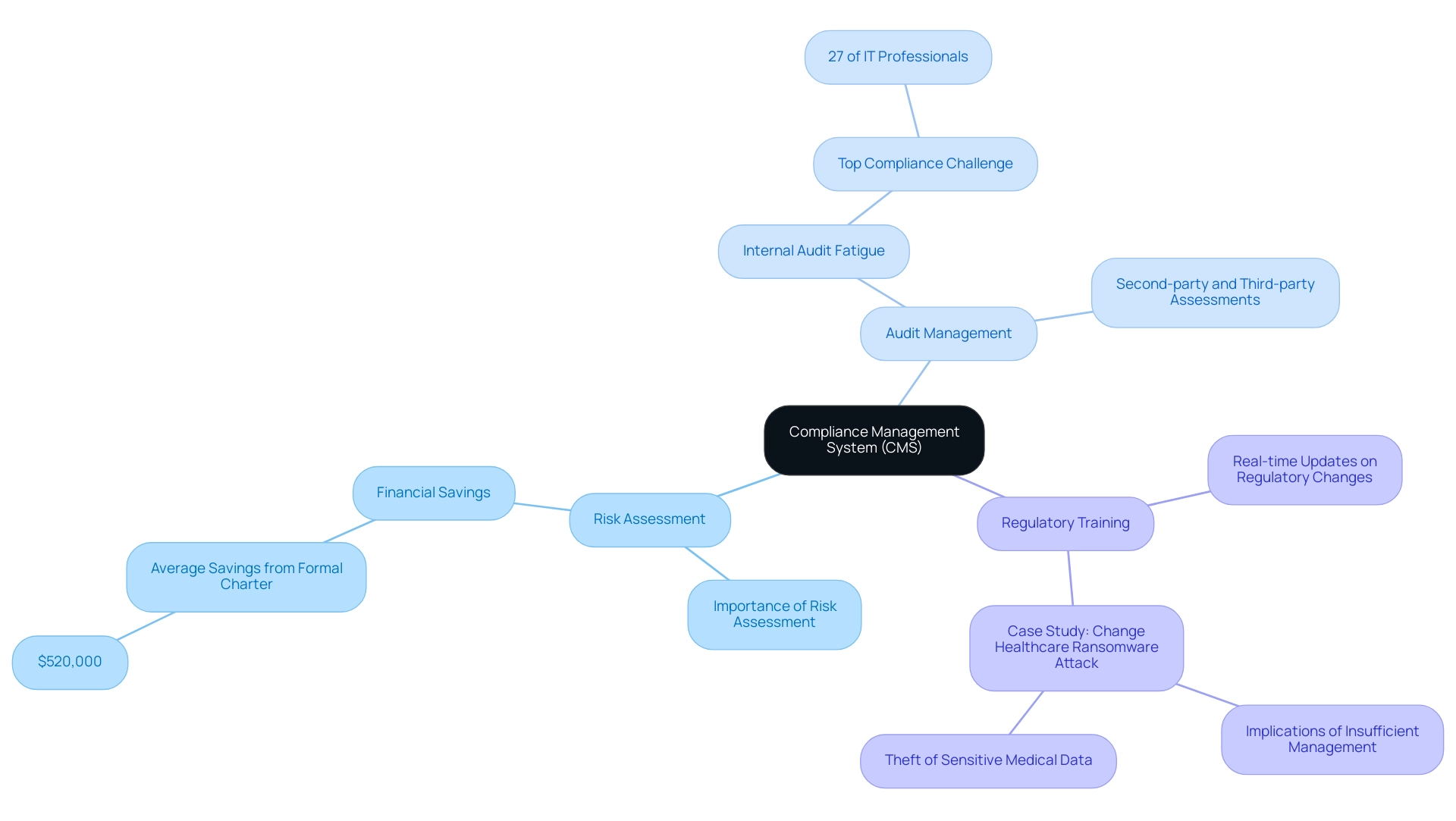
Establishing a strong management system (CMS) is crucial for automating documentation, tracking, and reporting processes efficiently. By adopting advanced software tools, companies can significantly enhance their capabilities in:
- Risk assessment
- Audit management
- Regulatory training
This is essential for understanding how to ensure compliance with international and local regulations. Significantly, organizations that create a formal charter for regulations can save an average of $520,000, emphasizing the financial advantages of CMS.
Platforms that provide real-time updates on regulatory changes enhance smooth communication among team members, which is vital for understanding how to ensure compliance with international and local regulations. The recent ransomware attack on Change Healthcare highlights the real-world implications of insufficient regulatory management, illustrating the necessity of robust systems to safeguard sensitive information. Furthermore, 27% of security and IT professionals have ranked alleviating internal audit fatigue from recurring second-party and third-party assessments as a top program challenge.
Utilizing technology in this way not only reduces manual errors and strengthens data integrity but also promotes a culture of continuous improvement in regulatory efforts. With the growing intricacy of compliance landscapes, knowing how to ensure compliance with international and local regulations has become a strategic necessity for firms aiming to maintain their competitive advantage.
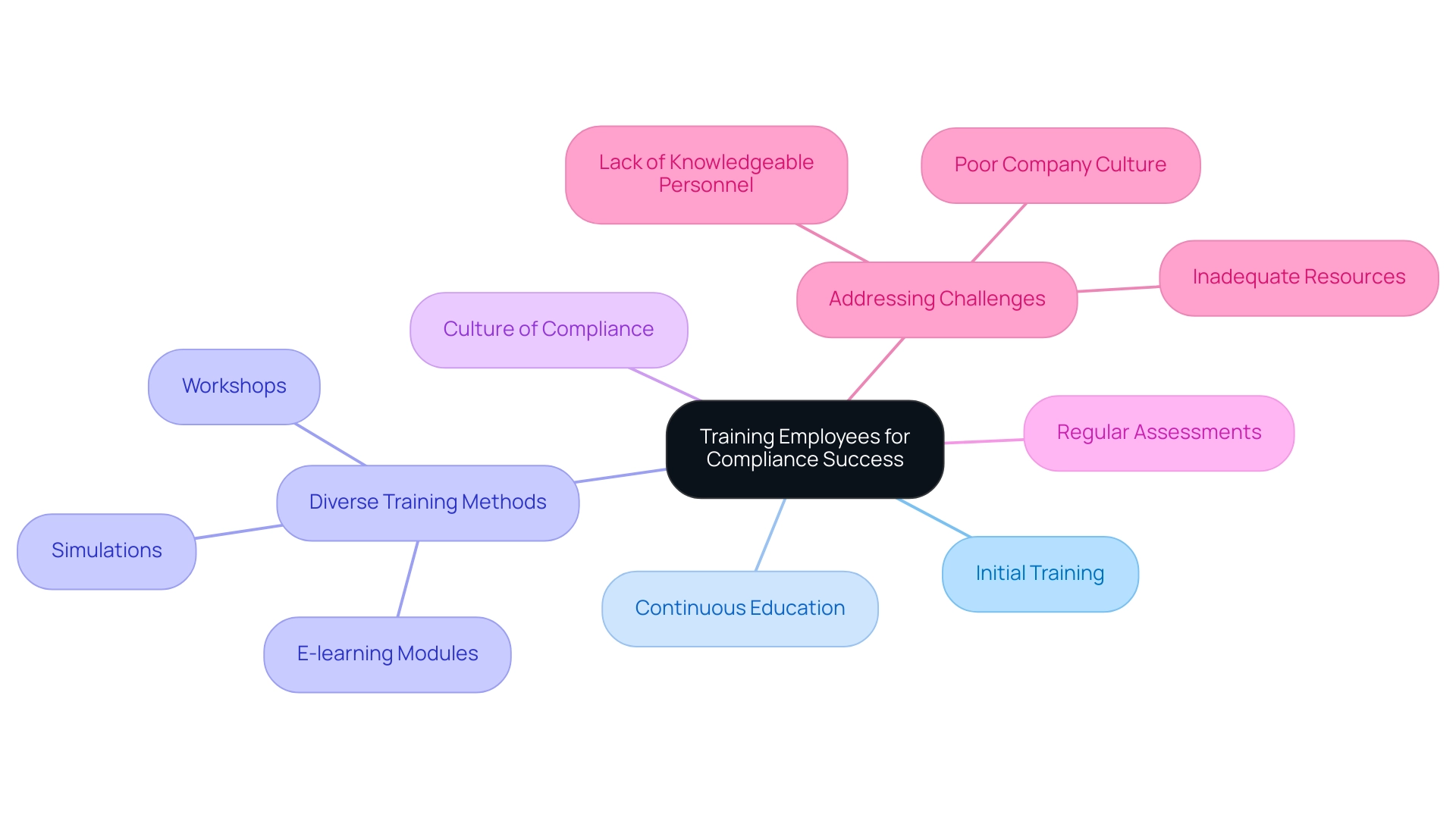
Training Employees for Compliance Success
To create a thorough training program, it is essential to address all aspects relevant to your organization. This program should encompass both initial training for new employees and continuous education for existing staff on how to ensure compliance with international and local regulations, ensuring that everyone remains informed about the latest compliance developments. A multifaceted approach is recommended, incorporating diverse training methods such as:
- Workshops
- E-learning modules
- Simulations
This variety caters to different learning styles, enhancing overall engagement and retention of the material. Furthermore, fostering a culture of adherence is crucial for understanding how to ensure compliance with international and local regulations; encouraging open discussions about regulatory requirements and their implications promotes understanding and accountability among staff. Regular assessments of training effectiveness should be conducted through feedback and performance evaluations, allowing for necessary adjustments to the program.
This continuous improvement process not only enhances regulatory capabilities but also demonstrates how to ensure compliance with international and local regulations, aligning with the insights that 77% of corporate risk and regulatory professionals value updates on ESG-related developments. As Jane Smith, CFO, remarked, 'We anticipate substantial growth next quarter,' highlighting the necessity for strong training to support this growth. Furthermore, addressing obstacles to adherence confidence, such as a lack of knowledgeable personnel and inadequate resources, is essential for enhancing training initiatives.
By tackling these challenges, organizations can significantly strengthen their compliance training programs and learn how to ensure compliance with international and local regulations.
Conclusion
Successfully navigating the complex regulatory landscape in clinical research is imperative for Medtech companies operating in Latin America. This article has highlighted the necessity of understanding both international and local regulations, emphasizing that compliance is not merely a legal obligation but a strategic advantage. By conducting compliance audits, developing comprehensive plans, and implementing standardized operating procedures, organizations can enhance their operational efficiency and mitigate risks associated with non-compliance.
Staying informed about regulatory changes is equally vital. Establishing a robust system for tracking updates, engaging with legal experts, and fostering collaboration with stakeholders ensures that organizations remain agile and responsive to the evolving regulatory environment. The importance of leveraging technology cannot be overstated; adopting advanced compliance management systems streamlines processes and strengthens data integrity, ultimately safeguarding sensitive information.
Furthermore, investing in continuous training for employees fosters a culture of compliance, equipping teams with the knowledge needed to navigate regulatory challenges effectively. By prioritizing these strategies, Medtech companies can not only enhance their compliance posture but also drive innovation and improve clinical trial outcomes. In a region characterized by rapid change, a proactive and informed approach to regulatory compliance will be the cornerstone of success in advancing clinical research and development.
Frequently Asked Questions
Why is compliance with international and local regulations important for Medtech firms?
Compliance is crucial for ensuring adherence to standards in medical research, which includes following guidelines like Good Clinical Practice (GCP), the Declaration of Helsinki, and the International Council for Harmonization (ICH). It helps maintain the integrity of studies and the safety of participants.
What are some key international regulations that Medtech firms must understand?
Key international regulations include the Good Clinical Practice (GCP) guidelines, the Declaration of Helsinki, and the International Council for Harmonization (ICH) guidelines.
How does regulatory compliance differ in Latin America compared to other regions?
Regulatory compliance in Latin America can differ significantly by country, particularly in relation to regulations established by the FDA in the United States and the EMA in Europe.
What is the significance of the 30-day rule regarding the FDA and medical studies?
Medical studies must not be initiated until 30 days after the FDA receives the Investigational New Drug (IND) application, unless earlier notification is provided, highlighting the importance of adhering to regulatory timelines.
What trial management services are essential for research in Latin America?
Essential trial management services include feasibility studies, site selection, adherence reviews, trial setup, import permits, project management, and reporting.
What challenges do US Medtech companies face when communicating with Latin American hospitals?
Challenges include language barriers and fragmentation of resources, which can impede seamless communication.
How can conducting a thorough literature review help Medtech firms?
Conducting a literature review enhances awareness of evolving regulations, which is crucial for ensuring compliance with international and local regulations.
Why is cooperation between entities like Greenlight Guru and bioaccess® important?
Cooperation aims to expedite Medtech innovations and trials in Latin America, facilitating advancements in clinical research.
What steps can organizations take to improve compliance with regulations?
Organizations can conduct conformity audits, create a conformity plan, implement Standard Operating Procedures (SOPs), monitor adherence, and engage stakeholders throughout the regulatory process.
What are the financial benefits of conducting regular compliance audits?
Regular audits can lead to significant cost savings, averaging $520,000, by enhancing adherence effectiveness and establishing a formal charter.
How can monitoring adherence metrics help organizations?
Regularly reviewing adherence metrics allows organizations to identify potential issues early and adapt to the evolving regulatory landscape, thereby mitigating risks effectively.
Why is stakeholder engagement important in the compliance process?
Engaging stakeholders fosters transparency, collaboration, and trust, which improves the efficacy of adherence strategies and aligns them with legal expectations and organizational objectives.




