Overview
To ensure regulatory compliance in pivotal studies in Latin America, researchers must thoroughly understand local regulations, obtain necessary ethics committee approvals, and maintain comprehensive documentation throughout the research process. The article emphasizes that engaging with regulatory authorities, adhering to international guidelines, and implementing quality management systems are critical for navigating the complex landscape of clinical trials in the region.
Introduction
In the dynamic landscape of clinical research, particularly within Latin America, understanding the intricate regulatory requirements is paramount for the success of pivotal studies. As researchers aim to navigate the complexities of compliance, they must familiarize themselves with the specific mandates set forth by each country's regulatory authorities, such as INVIMA in Colombia and ANVISA in Brazil.
This article delves into essential components of regulatory adherence, including:
- Securing ethics committee approval
- Developing informed consent documents
All while highlighting the collaborative efforts that position Latin America as a burgeoning hub for clinical trials. By comprehensively addressing these regulatory frameworks, researchers can not only ensure compliance but also enhance the integrity and effectiveness of their studies, paving the way for significant advancements in global healthcare innovation.
Understanding Regulatory Requirements for Pivotal Studies in Latin America
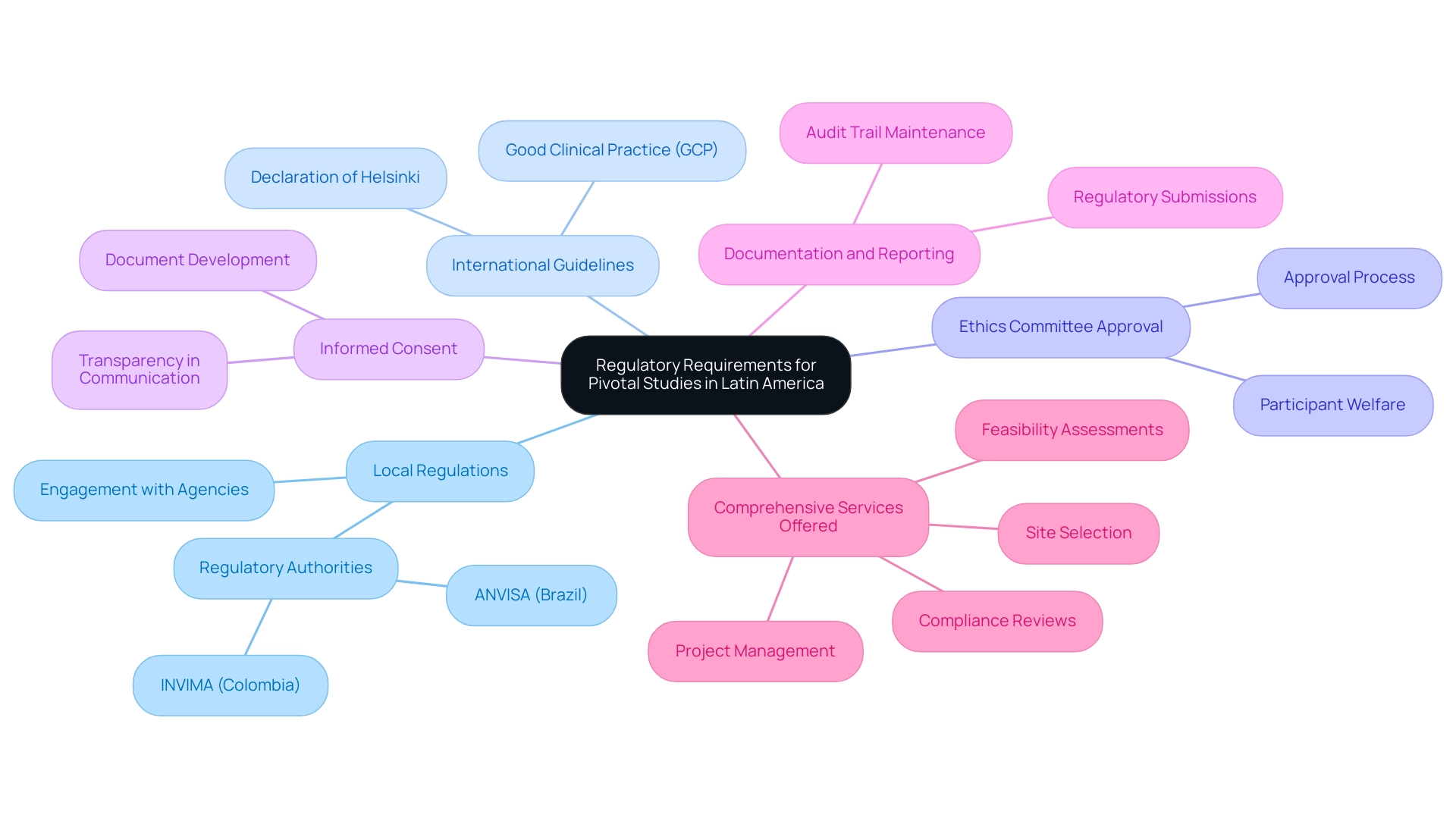
Ensuring regulatory compliance in pivotal studies in Latin America requires researchers to thoroughly understand the specific requirements established by each country's governing bodies. Key components include:
-
Understanding Local Regulations: Each nation has its regulatory authority, such as INVIMA in Colombia and ANVISA in Brazil, which outline the rules overseeing research studies.
Researchers should engage directly with these agencies to grasp their distinct requirements and processes, particularly in light of the collaboration between bioaccess™ and Caribbean Health Group aimed at positioning Barranquilla as a leading destination for clinical trials in Latin America, with the support of Colombia's Minister of Health.
-
International Guidelines: Familiarity with international standards, including Good Clinical Practice (GCP) and the Declaration of Helsinki, is essential. These frameworks guide researchers in maintaining ethical integrity throughout their studies.
-
Ethics Committee Approval: Before starting a research project, it is mandatory to secure approval from a local ethics committee. This body evaluates the research protocol to ensure it aligns with ethical standards, safeguarding participant welfare.
Informed Consent: The development of informed consent documents is critical. These documents must comply with local regulations and effectively communicate the project's objectives, processes, potential risks, and benefits to participants, fostering an environment of transparency.
Documentation and Reporting: Diligently maintaining records of all regulatory submissions and communications with regulatory bodies is vital. This practice not only demonstrates compliance throughout the research but also establishes a robust audit trail.
Notably, sponsors must notify ANVISA within a maximum of 15 consecutive days of any decision to suspend or cancel a clinical study, ensuring that all involved parties are informed immediately of such actions.
Comprehensive Services Offered: bioaccess™ and Caribbean Health Group provide a range of services including feasibility assessments, site selection, compliance reviews, setup for testing, import permits, project management, and reporting. These capabilities are essential for navigating the regulatory landscape effectively.
Case Example: A pertinent case reference is the guidance issued by ANVISA related to COVID-19 medical evaluations. This guidance provided researchers with updated technical notes, ensuring they have the latest information for conducting trials in this rapidly evolving landscape.
Additionally, as highlighted by Tim Fitzpatrick, the demand for clinical trials continues to grow, making investment in the Latin American market a transformative step in global healthcare innovation. The partnership efforts, like those between bioaccess™ and CHG, combined with the Southern hemisphere's seasonal benefits, establish an optimal setting for year-round research and provide significant opportunities for researchers to interact with a varied patient demographic.
By understanding these compliance necessities and the distinctive benefits of the region, researchers can effectively position themselves for ensuring regulatory compliance in pivotal studies in Latin America.
Navigating the Approval Process for Clinical Trials in Colombia
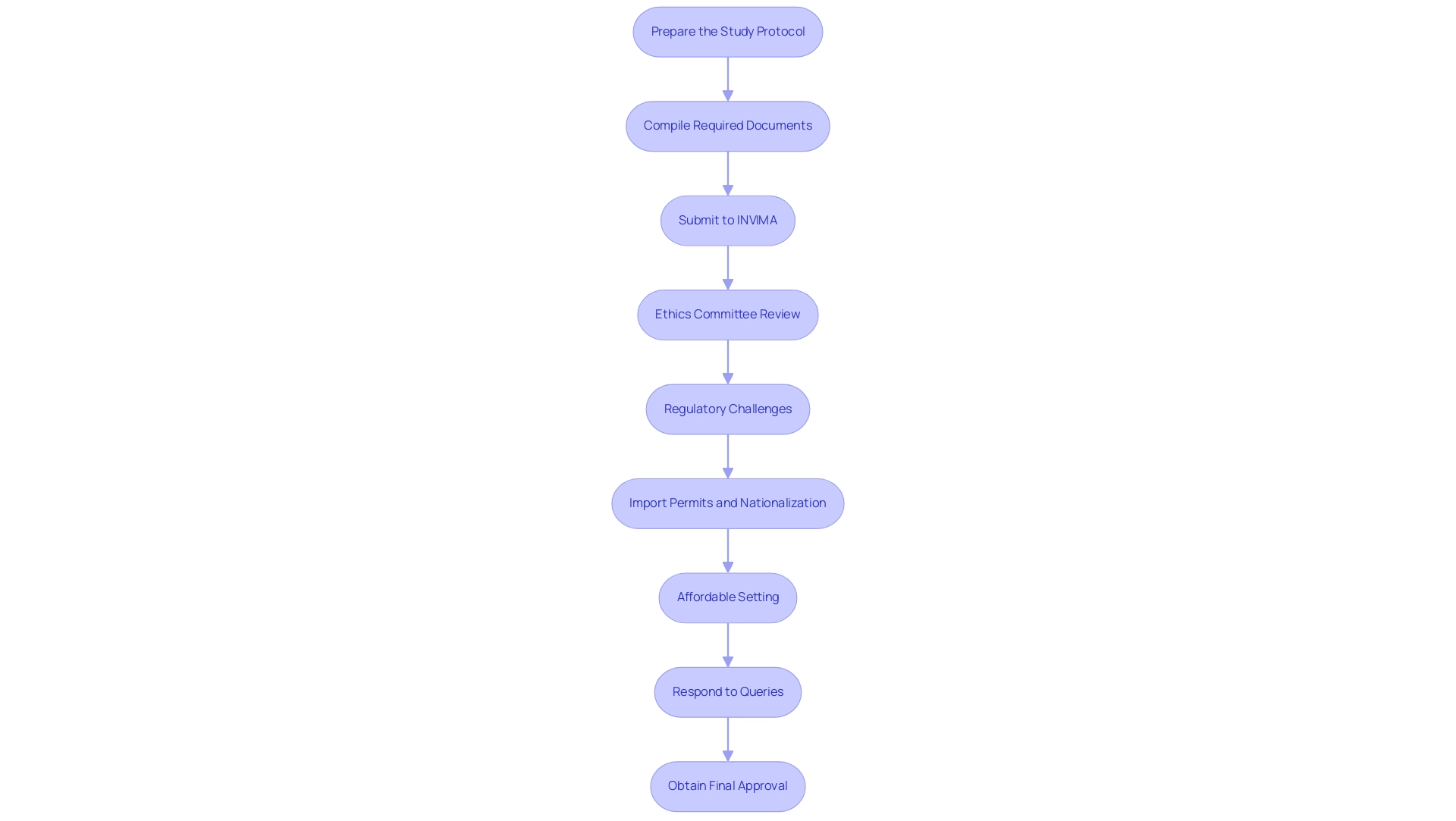
Navigating the approval process for clinical trials in Colombia requires a systematic approach to ensure adherence to standards and facilitate successful execution. Here are the essential steps to follow:
-
Prepare the Study Protocol: Begin by developing a detailed study protocol that clearly outlines the objectives, methodology, and statistical analysis plan.
This document must align with the compliance requirements set forth by INVIMA, as a well-structured protocol lays the groundwork for a successful submission.
-
Compile Required Documents: Gather all necessary documentation, including the informed consent form, investigator's brochure, and any additional materials mandated by INVIMA.
A thorough and structured submission demonstrates a commitment to compliance and simplifies the review process.
-
Submit to INVIMA: File your application electronically through INVIMA's online portal, ensuring all required documents are included.
This step is critical, as approximately 65% of revenue in the medical device sector is influenced by effective collaboration with market intelligence teams; thorough submissions can enhance your competitive edge.
-
Ethics Committee Review: Following submission to INVIMA, securing approval from a local ethics committee is essential.
This review guarantees that the research complies with high ethical standards, highlighting the significance of upholding ethical integrity in medical investigations.
-
Regulatory Challenges: Be aware of the regulatory challenges faced by Medtech companies in Latin America, particularly the lax enforcement of study registration and result reporting.
This emphasizes the necessity for stringent enforcement of reporting requirements to enhance research effectiveness and ensure ethical integrity.
-
Import Permits and Nationalization: Ensure that you also address the import permits and nationalization of investigational devices, which are critical for compliance with Colombian regulations.
These steps are essential for the legal entry and use of medical devices in research trials.
-
Affordable Setting: Colombia's affordable setting enables more comprehensive research without sacrificing quality, making it a beneficial location for trials.
-
Respond to Queries: Be prepared to address inquiries or requests for additional information from INVIMA or the ethics committee promptly.
Timely and comprehensive responses can significantly expedite the approval process and demonstrate your commitment to transparency and collaboration.
-
Obtain Final Approval: After all evaluations are finalized and inquiries adequately resolved, you will receive official authorization to commence the research study.
This finalization is a vital milestone that opens the door for carrying out essential research effectively.
By carefully adhering to these steps and utilizing extensive management services, including feasibility assessments, site selection, and a focus on import permits and reporting obligations, researchers can maneuver through the approval process for investigations in Colombia with enhanced efficiency, thus ensuring regulatory compliance in pivotal studies in Latin America and promoting the successful execution of important medical research.
Engaging with Ethics Committees and Regulatory Authorities
To effectively engage with ethics committees and oversight authorities in the context of clinical trials in Colombia, consider implementing the following strategies:
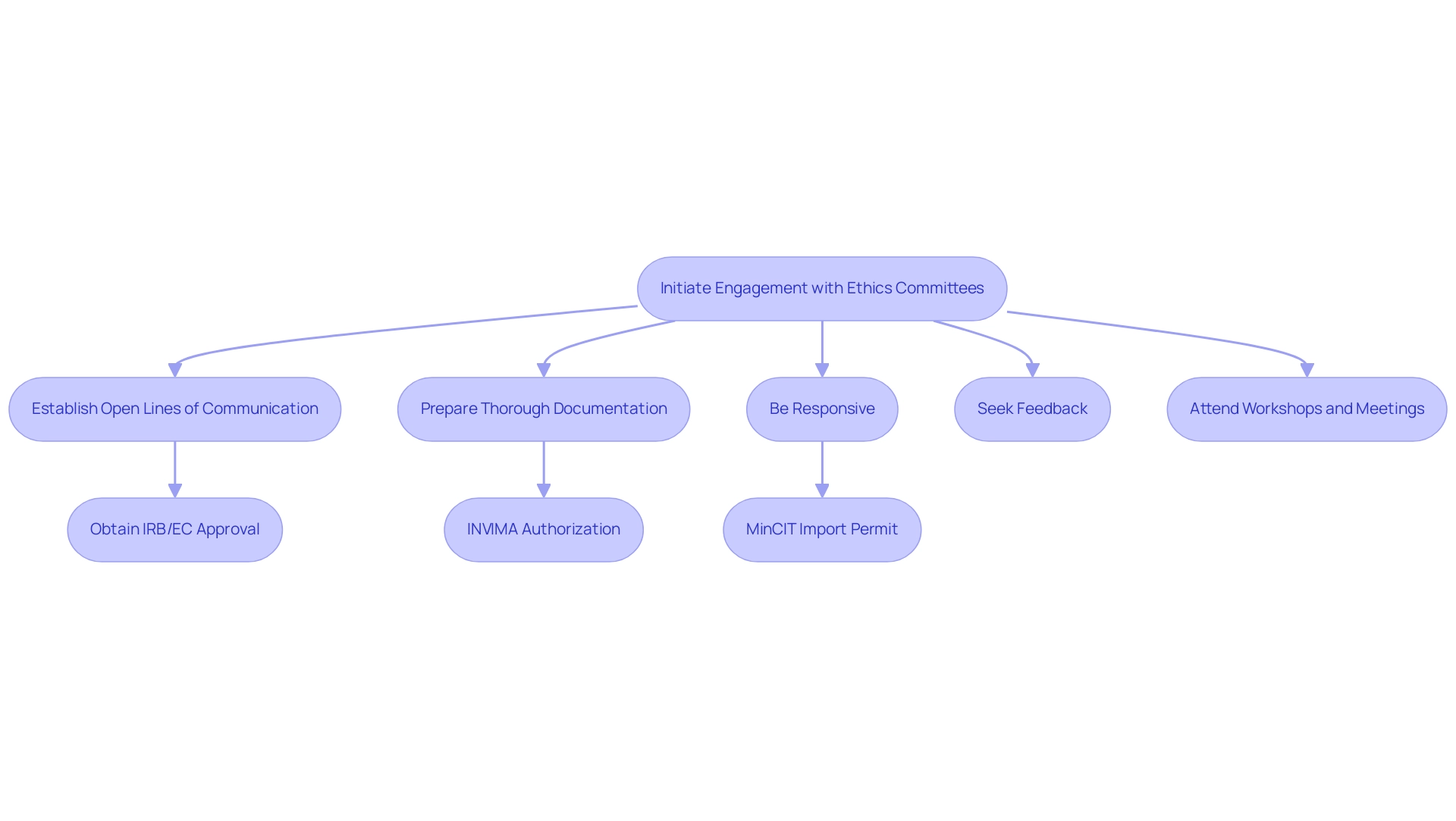
- Establish Open Lines of Communication: Initiate contact early in the study planning process. Establishing rapport can result in more constructive interactions and a clearer understanding of expectations concerning IRB/EC approval, INVIMA compliance, and obtaining an import permit from MinCIT.
- Prepare Thorough Documentation: Ensure all submissions are complete and meticulously organized. Clear and comprehensive documentation reduces the likelihood of queries and possible delays, facilitating smoother reviews by ethics committees and oversight bodies.
- Be Responsive: Address any questions or requests for additional information promptly. Prompt communication demonstrates professionalism and a commitment to compliance, which can positively affect approval times and assist in overcoming the challenges encountered by medical device startups.
- Seek Feedback: After submission, proactively request feedback from ethics committees. Understanding their perspectives can enhance the quality of future submissions, fortifying compliance reviews and promoting a collaborative environment.
- Attend Workshops and Meetings: Actively engage in workshops or meetings arranged by governing bodies. This not only keeps you updated about compliance changes but also offers invaluable networking opportunities with key stakeholders in research management.
In Colombia, the approval process involves several critical steps: obtaining project approval from your site's institutional review board (IRB)/ethics committee (EC), securing authorization from INVIMA (the oversight agency), and acquiring an import permit from the Ministry of Industry and Commerce (MinCIT) for investigational devices. Successful navigation of these steps can significantly influence timelines. Furthermore, our extensive trial management services encompass feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project oversight, and reporting, all of which assist medical device startups in navigating legal obstacles, competition for participant recruitment, and financial limitations.
As emphasized in a 1996 report titled Evaluation of an Ethics Consultation Service: Patient and Family Perspective, incorporating diverse perspectives can enhance the ethical standards of research. By engaging proactively with these committees and oversight bodies, researchers can enhance collaboration while ensuring regulatory compliance in pivotal studies in Latin America, ultimately contributing to the local economy through job creation and healthcare enhancement, and reinforcing the vital role of medtech trials in fostering international cooperation.
Implementing Quality Management Systems in Clinical Trials
Establishing a successful quality management system (QMS) in research studies is crucial for ensuring regulatory compliance in pivotal studies in Latin America, as well as improving the integrity of research results. Our extensive clinical trial management services include essential components such as feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all in accordance with the regulations established by INVIMA, the Colombian National Food and Drug Surveillance Institute. The following steps outline the framework for a successful QMS:
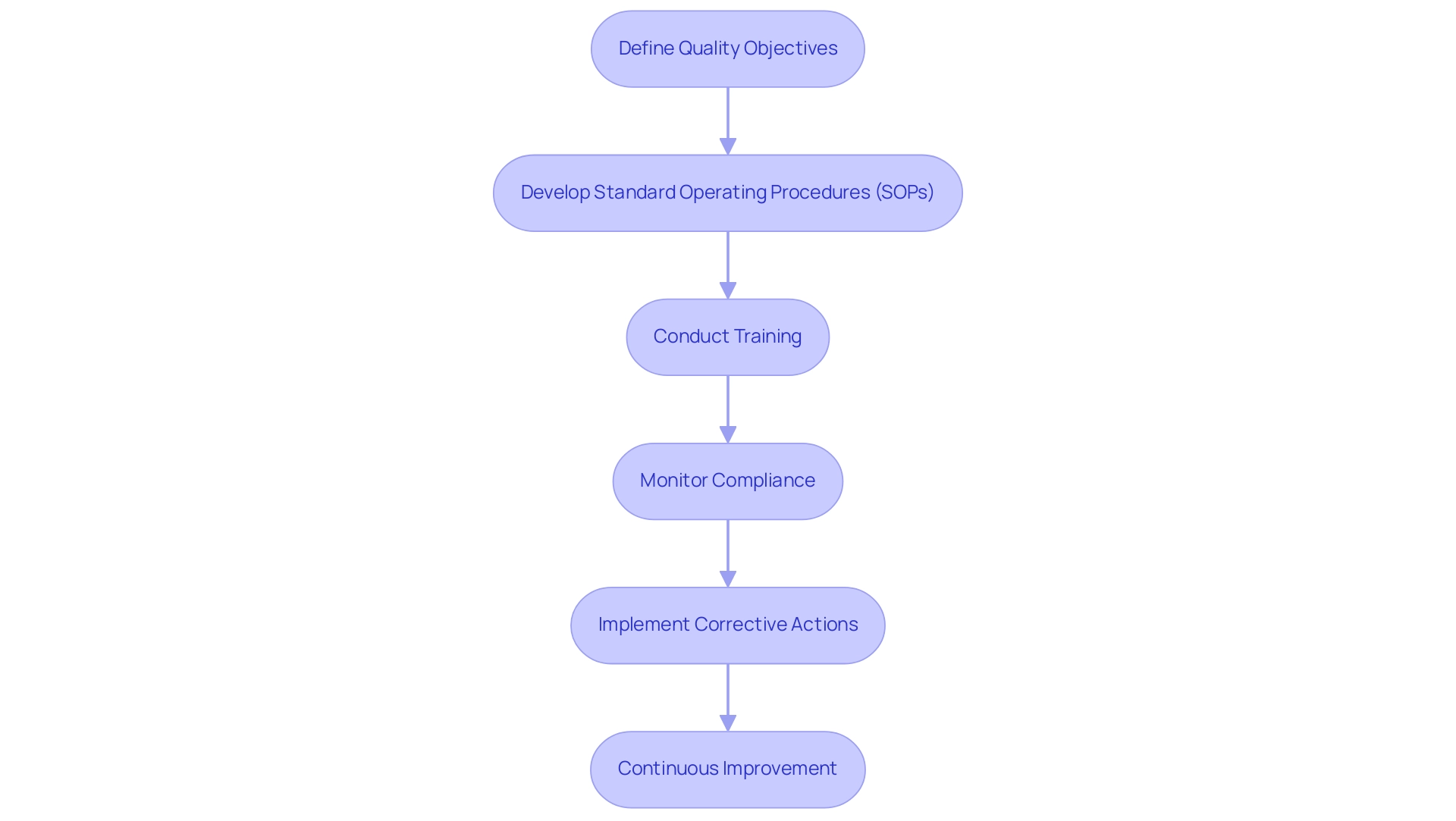
- Define Quality Objectives: Set quality objectives that are not only clear but also aligned with both compliance requirements and the specific aims of the research. This alignment is crucial for maintaining oversight and ensuring all team members understand their targets.
- Develop Standard Operating Procedures (SOPs): Create comprehensive SOPs for all critical processes, including data collection, monitoring, and reporting. These SOPs should be dynamic documents, regularly reviewed and updated to reflect evolving compliance standards and study needs.
- Conduct Training: It is imperative that all team members receive thorough training on the QMS and are well-informed about their roles in upholding quality standards. Training employees on QMS standards, such as ISO 9001, helps ensure compliance and improve overall quality management. This training ensures that everyone is equipped to contribute effectively to the quality objectives.
- Monitor Compliance: Regular compliance monitoring is vital. This can be achieved through systematic audits and inspections that assess adherence to SOPs and regulatory requirements, helping to identify areas for improvement.
- Implement Corrective Actions: In the event of deviations, prompt implementation of corrective actions is crucial. Addressing issues swiftly not only resolves current problems but also prevents future occurrences, thereby enhancing the overall quality of the research.
- Continuous Improvement: Cultivating a culture of continuous improvement is essential. This entails frequently assessing procedures, incorporating input from team members, and adjusting strategies to achieve quality objectives more efficiently.
The influence of quality management systems on research studies cannot be overstated. Research indicates that the cost of poor quality, including rework and recalls, can account for 15 to 35 percent of total business costs in regulated industries. Additionally, Medtech research studies contribute to local economies through job creation, economic growth, and healthcare improvement.
By following these steps and cultivating a trustworthy partnership in quality management, researchers can greatly enhance the reliability and integrity of their studies, thereby ensuring regulatory compliance in pivotal studies in Latin America.
Training and Development for Clinical Research Teams
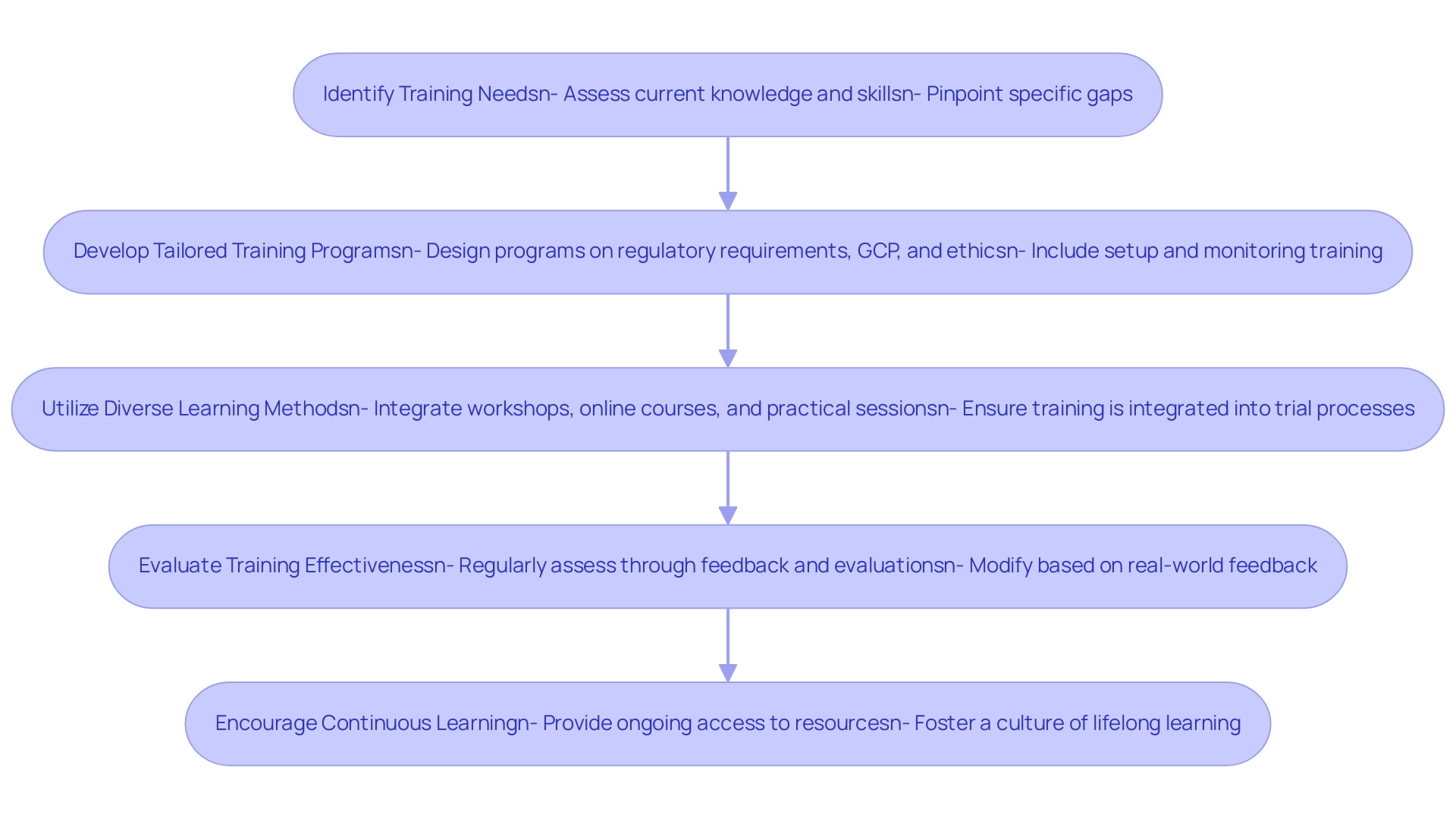
To ensure that clinical research teams are adequately prepared for regulatory compliance, it is essential to implement comprehensive training and development strategies alongside robust clinical study management services:
- Identify Training Needs: Conduct a thorough assessment of the current knowledge and skills among team members to pinpoint specific gaps that require attention. This assessment is crucial, as highlighted in the recent findings by Delaney H et al., which indicate limited evidence on the effectiveness of educational interventions in trial recruitment. The urgency of addressing these gaps is underscored by the statistic that participants in the research assignment had only two hours to examine the study data and present their findings.
- Develop Tailored Training Programs: Design customized training programs that encompass vital topics such as regulatory requirements, Good Clinical Practice (GCP), and ethical considerations. This approach aligns with the ongoing developments in clinical research training, ensuring that programs are relevant for 2024 and beyond. Our service capabilities further enhance this by offering feasibility studies, site selection, and compliance reviews, ensuring that teams are equipped with the necessary insights. Furthermore, integrating training on setup and monitoring processes can provide practical knowledge that complements theoretical learning.
- Utilize Diverse Learning Methods: Integrate a variety of learning modalities, including workshops, online courses, and practical training sessions. This diversity caters to different learning styles, fostering greater engagement and retention. Additionally, our project management services ensure that training is seamlessly integrated into the trial setup and monitoring processes, reinforcing the practical application of knowledge.
- Evaluate Training Effectiveness: Implement a systematic process to regularly assess the effectiveness of training initiatives through participant feedback and performance evaluations. This guarantees that training stays pertinent and effective, permitting modifications based on real-world feedback, similar to our reporting systems for project status and adverse events. Furthermore, incorporating feedback on review documents can enhance the compliance aspect of training.
- Encourage Continuous Learning: Cultivate a culture of lifelong learning by providing ongoing access to resources such as webinars, industry publications, and conferences. As highlighted by Ramkrishna Kumar Singh, MD, MPH, becoming a well-rounded investigator is essential in the changing environment of medical research.
The case examination on research training requirements in China demonstrates the practical relevance of these strategies. It disclosed that even doctors in top hospitals encounter significant challenges in carrying out research, emphasizing the necessity for systematic training programs focused on data management and biostatistics. Moreover, Katherine Ruiz, an expert in ensuring regulatory compliance in pivotal studies in Latin America for medical devices and in vitro diagnostics in Colombia, exemplifies the level of expertise needed to navigate these complexities. By investing in these training and development strategies, alongside comprehensive clinical trial management services, organizations can focus on ensuring regulatory compliance in pivotal studies in Latin America while also upholding the highest standards in their clinical research.
This commitment to education is essential, especially in light of the challenges faced by researchers.
Conclusion
Navigating the regulatory landscape for clinical trials in Latin America is a multifaceted endeavor that requires a thorough understanding of local laws and international guidelines. Researchers must engage with regulatory authorities such as INVIMA in Colombia and ANVISA in Brazil to ensure compliance with the specific mandates governing clinical studies. The importance of securing ethics committee approval and developing informed consent documents cannot be overstated, as these elements are fundamental to maintaining ethical integrity and participant welfare.
Furthermore, the implementation of quality management systems and comprehensive training programs for research teams plays a crucial role in enhancing the reliability and effectiveness of clinical trials. By fostering a culture of continuous improvement and open communication with regulatory bodies, researchers can streamline the approval process and better navigate potential challenges.
Ultimately, the collaborative efforts within the region position Latin America as a promising hub for clinical trials, with the potential to significantly contribute to global healthcare innovation. By understanding and adhering to the intricate regulatory requirements, researchers not only enhance the integrity of their studies but also pave the way for transformative advancements in medical research that can benefit diverse patient populations. The commitment to regulatory compliance and ethical standards is essential for fostering a robust clinical research environment that supports both scientific progress and community health.
Frequently Asked Questions
What is required for ensuring regulatory compliance in pivotal studies in Latin America?
Researchers must thoroughly understand the specific requirements established by each country's regulatory authorities, engage with local agencies, adhere to international guidelines, secure ethics committee approval, develop informed consent documents, maintain documentation and reporting, and notify regulatory bodies of any study changes.
Which regulatory authorities oversee research studies in Colombia and Brazil?
In Colombia, the regulatory authority is INVIMA, while in Brazil, it is ANVISA.
What international guidelines should researchers be familiar with?
Researchers should be familiar with Good Clinical Practice (GCP) and the Declaration of Helsinki, which guide ethical integrity in research studies.
Why is ethics committee approval important before starting a research project?
Ethics committee approval is mandatory as it evaluates the research protocol to ensure it aligns with ethical standards and safeguards participant welfare.
What is the significance of informed consent documents in research?
Informed consent documents must comply with local regulations and communicate the project's objectives, processes, potential risks, and benefits to participants, promoting transparency.
What documentation practices are essential for regulatory compliance?
Researchers must diligently maintain records of all regulatory submissions and communications with regulatory bodies to demonstrate compliance and establish a robust audit trail.
What are the responsibilities of sponsors regarding clinical study suspensions or cancellations in Brazil?
Sponsors must notify ANVISA within a maximum of 15 consecutive days of any decision to suspend or cancel a clinical study.
What services do bioaccess™ and Caribbean Health Group offer to assist with regulatory compliance?
They provide feasibility assessments, site selection, compliance reviews, testing setup, import permits, project management, and reporting.
What case example illustrates the importance of updated guidance in clinical trials?
The guidance issued by ANVISA related to COVID-19 medical evaluations provided researchers with updated technical notes essential for conducting trials in a rapidly evolving landscape.
How can understanding compliance necessities benefit researchers in Latin America?
By understanding compliance necessities and the benefits of the region, researchers can effectively position themselves for successful regulatory compliance in pivotal studies.




