Overview
The article focuses on understanding the regulatory requirements for First-In-Human (FIH) studies in Latin America, particularly highlighting the advantages and processes involved in conducting these trials in Colombia. It emphasizes the importance of adhering to local regulations, such as obtaining ethical approvals and following Good Clinical Practice (GCP) guidelines, which not only facilitate expedited approvals but also enhance the credibility and success of medical research in the region.
Introduction
Navigating the regulatory landscape for First-In-Human (FIH) studies in Latin America presents both challenges and opportunities for researchers.
Colombia, in particular, has emerged as a key player in this arena, bolstered by its efficient regulatory processes and competitive advantages.
With INVIMA at the helm, the country offers a streamlined approval timeline and significant cost savings compared to more established markets.
However, understanding the intricacies of local regulations, ethical considerations, and logistical requirements is crucial for successful trial execution.
As the demand for innovative clinical research grows, Colombia's strategic position and commitment to regulatory compliance become increasingly relevant, making it an attractive destination for global clinical trials.
Navigating the Regulatory Landscape for FIH Studies in Latin America
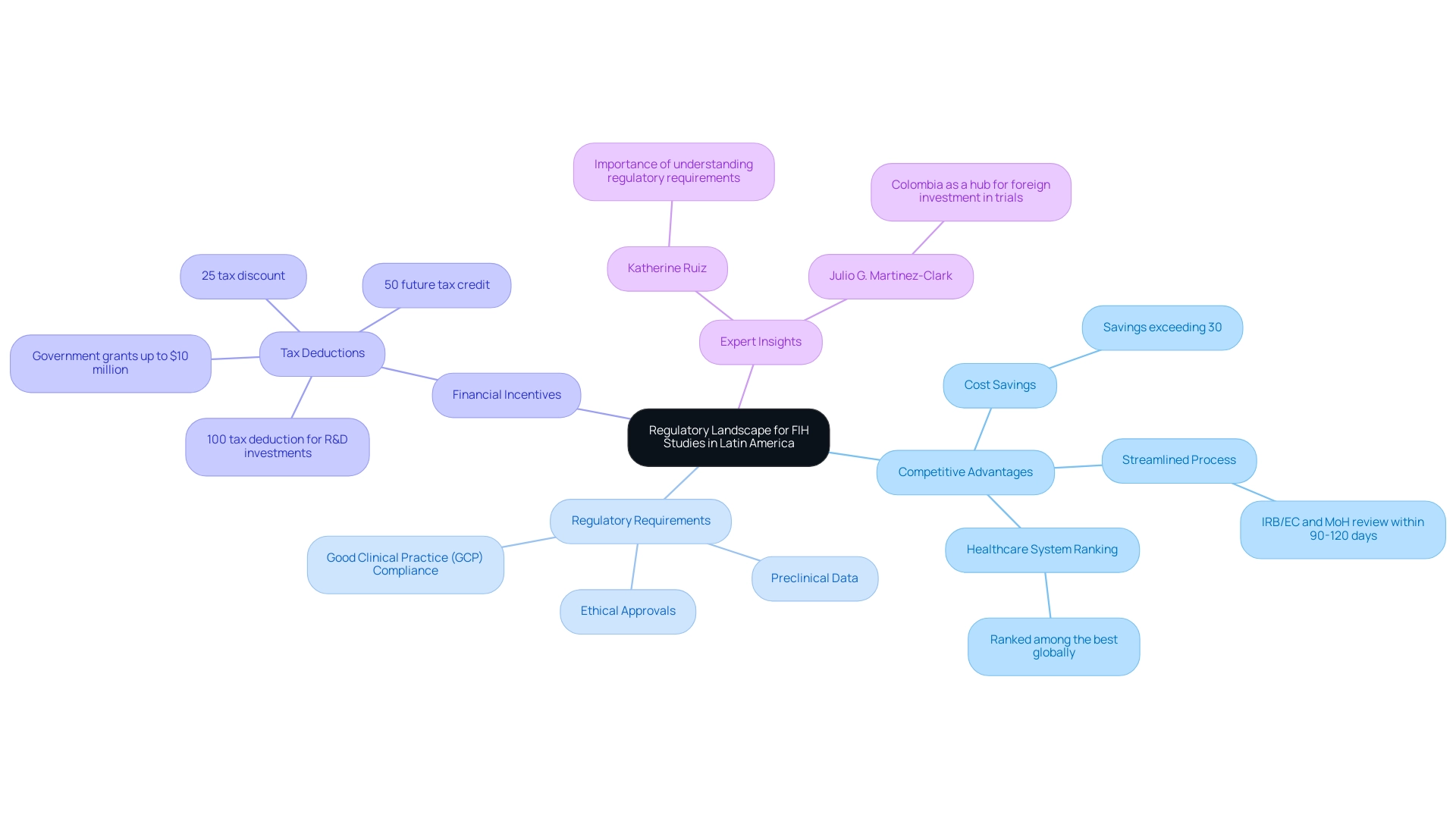
The regulatory requirements for FIH studies in Latin America present an intricate landscape shaped by several national entities, with the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) in that country playing a crucial role. The country offers several competitive advantages for conducting FIH trials, including:
- Cost savings exceeding 30% compared to trials in North America or Western Europe.
- A streamlined regulatory process that enables IRB/EC and MoH (INVIMA) review within 90-120 days.
- A healthcare system ranked among the best globally.
It is essential for researchers to thoroughly understand the regulatory requirements for FIH studies in Latin America, which include comprehensive preclinical data, ethical approvals, and strict adherence to Good Clinical Practice (GCP) guidelines.
Adhering to the regulatory requirements for FIH studies in Latin America is not just a procedural formality; it is vital for ensuring that such studies conform to both local and international standards, facilitating expedited approvals and fostering an environment conducive to innovative medical research. Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics, emphasizes the importance of this understanding for successful navigation of the regulatory landscape. Furthermore, as Julio G. Martinez-Clark, CEO of bioaccess, expressed, "We will showcase this nation as an illustration of a country dedicated to drawing more foreign investment in research trials."
This statement reflects a growing acknowledgment of Colombia's strategic significance in the global research landscape, supported by a population of over 50 million with 95% coverage under universal healthcare, enhancing patient recruitment. It is also vital to acknowledge that only 10% of annual funding into health research targets the health needs of the poorest 90% of the world's population, highlighting the importance of regulatory compliance in addressing health disparities. Furthermore, sponsors must detail how the study was monitored and how investigators were trained to comply with GCP guidelines, which is crucial for understanding the regulatory requirements for FIH studies in Latin America.
Additionally, with 52 percent of global trials occurring outside the U.S., Latin America is becoming an increasingly significant hub for trials, which emphasizes the need to adhere to the regulatory requirements for FIH studies in Latin America. Notably, this country also offers attractive R&D tax incentives, including:
- A 100% tax deduction for investments in science, technology, and innovation projects.
- A 25% tax discount.
- A 50% future tax credit.
- Approximately $10 million in government grants.
Moreover, hospitals in the nation must undergo a rigorous ICH/GCP certification process before conducting research with pharmaceutical drugs, ensuring high standards of quality and compliance.
Key Steps in the Approval Process for FIH Clinical Trials in Colombia
The approval process for First-In-Human (FIH) studies in the nation adheres to the regulatory requirements for FIH studies in Latin America, involving a structured procedure consisting of several essential steps. Initially, the preparation of the Clinical Trial Application (CTA) is essential, which includes compiling vital documents such as the study protocol and the investigator’s brochure that outline the study's objectives and methodology. Considering the distinct challenges and opportunities for clinical research in Latin America, especially in relation to the regulatory requirements for FIH studies in Latin America, Paraguay emerges as a promising destination despite its restricted study activities.
Once the CTA is prepared, it is submitted to INVIMA, the national regulatory authority recognized as a Level 4 health authority by PAHO/WHO, which conducts a thorough review to ensure compliance with the regulatory requirements for FIH studies in Latin America. Obtaining ethical approval from a recognized ethics committee is also mandatory to safeguard participant rights and welfare. Throughout this process, it is crucial to promptly address any queries raised by INVIMA or the ethics committee to avoid delays.
The overall activation timelines of the study can vary significantly; for instance, the APHINITY study, which involved 42 countries, had a median RA approval time of 53 days and an EC/IRB approval time of 56 days. Typically, the entire approval timeline for FIH studies in Colombia is influenced by the regulatory requirements for FIH studies in Latin America and ranges from 2 to 6 months, heavily dependent on the submission's complexity and quality. As highlighted by interventional cardiologist Juan Andres Delgado Restrepo, the quality of the approval process is paramount, surpassing the mere emphasis on speed.
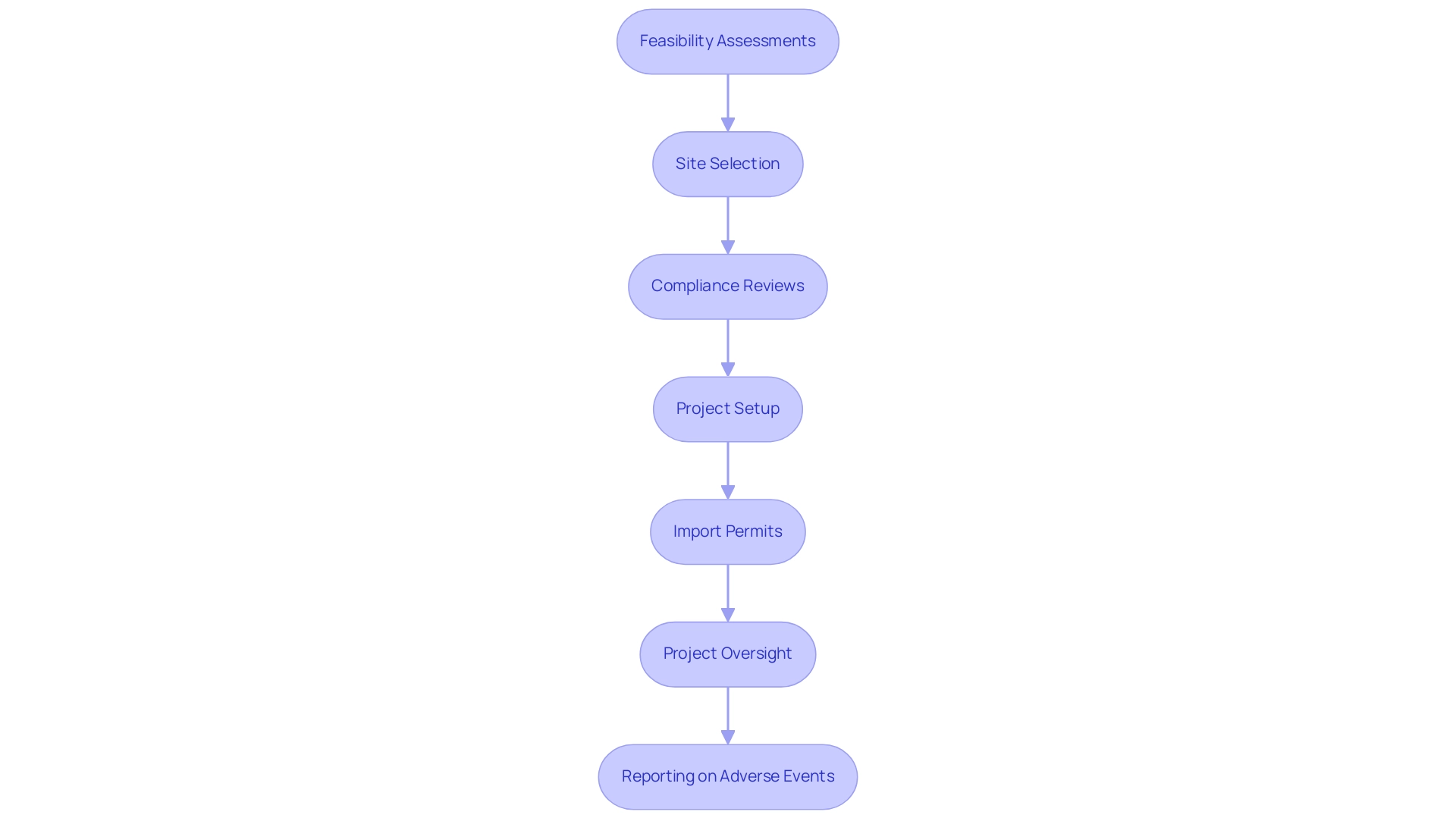
A well-prepared application, including proper citing options and collections, can facilitate a smoother approval experience and contribute significantly to the overall success of the endeavor. Moreover, partnerships such as that between bioaccess™ and Caribbean Health Group are crucial in establishing Barranquilla as a prominent location for clinical research in Latin America, considering the regulatory requirements for FIH studies in Latin America and backed by the Colombian Minister of Health. Moreover, our service capabilities include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Test setup
- Import permits
- Project management
- Thorough reporting on project status and adverse events
Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics, plays a crucial role in ensuring adherence to regulatory standards and enhancing the overall efficiency of the approval process.
Selecting the Right Sites and Investigators for Successful FIH Trials
Choosing suitable sites for first-in-human (FIH) studies necessitates careful assessment of each location's capabilities, including access to diverse patient populations and the sufficiency of infrastructure, along with previous experience in similar research. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, underscored the significance of bioaccess® during its initial human study in Colombia, stressing their proficiency in managing the intricacies of medical evaluations. bioaccess® provides a variety of specialized services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This ensures that each study complies with the regulatory requirements for FIH studies in Latin America while being managed with the utmost care.
Ethical considerations in site selection remain paramount to avoid past mistakes, further underscoring the need for collaboration with seasoned investigators. Individuals with a proven history in medical research and a thorough understanding of local regulations greatly improve the study's credibility and operational efficiency. Furthermore, taking into account cultural and socio-economic factors in the region can result in enhanced patient protection and overall study quality.
The nation, with its geographical benefits and competitive expenses, has become more appealing for medical studies, especially amid persistent patient recruitment difficulties encountered in the U.S. Initiatives such as the Knowledge Societies Policy Handbook demonstrate how Latin American nations, including Colombia, are transforming into knowledge societies, positively influencing the clinical study landscape and site selection criteria. This evolution aligns with the comprehensive services provided by bioaccess®, which are designed to meet the specific requirements of each research type.
Therefore, conducting thorough due diligence on potential sites and investigators is crucial to ensure alignment with research objectives and the regulatory requirements for FIH studies in Latin America, paving the way for successful trials.
Logistical Considerations for Conducting FIH Studies in Colombia
Conducting first-in-human (FIH) studies in the country necessitates meticulous logistical planning, particularly in relation to the regulatory requirements for FIH studies in Latin America, including the importation of investigational devices and adherence to local regulations. With Paraguay having the lowest urbanization rate in South America at 61.6%, compared to an average of 84.6% for the region, understanding the regional landscape is crucial for effective logistical planning in the country. Sponsors must ensure that all imported materials comply with INVIMA standards, the National Food and Drug Surveillance Institute, and are accompanied by appropriate documentation, including import permits.
As Ian Sanne observes, 'Navigating the complexities of regulatory compliance is essential for the success of research studies in emerging markets.' Engaging with local vendors and service providers can significantly enhance the efficiency of these processes, helping to mitigate potential delays. The worldwide allocation of medical studies is transitioning towards developing countries, which provide ethical, high-quality, and affordable research opportunities, making Colombia an appealing choice for U.S. MedTech startups.
Establishing a robust supply chain is essential, as is the development of contingency plans to effectively manage unforeseen logistical challenges. This strategic method not only optimizes operations but also protects the integrity and advancement of research studies in the region. Our comprehensive clinical research management services include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Project setup—including ethics committee and health ministry approvals
- Import permits
- Project oversight
- Reporting on serious and non-serious adverse events
This ensures that we address all pertinent regulatory requirements effectively.
Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes the importance of understanding the regulatory requirements for FIH studies in Latin America to facilitate successful study execution.
Effective Patient Recruitment and Ethical Considerations in FIH Trials
Effective patient recruitment for First-in-Human (FIH) studies necessitates a well-defined strategy that emphasizes collaboration with local healthcare providers and partnerships with organizations like bioaccess™ and Caribbean Health Group. These collaborations not only enhance the recruitment process but also position Barranquilla as a leading destination for clinical trials in Latin America, supported by the Colombian Minister of Health. Engaging with community organizations and patient advocacy groups broadens outreach while ensuring adherence to local laws and ethical standards, particularly the imperative of obtaining informed consent from participants.
A transparent communication plan that proactively addresses potential patient concerns fosters trust and significantly encourages participation. Ethical considerations must stay crucial throughout the recruitment process, protecting patient rights and ensuring the safe execution of the research. Notably, in total, 3861 articles were screened for inclusion in the research, underscoring the thoroughness required in developing effective recruitment strategies.
Recent research emphasizes the effectiveness of customized study recruitment solutions, such as those discussed in the case study titled 'Implementing Tailored Study Recruitment Solutions,' which demonstrates how deploying bespoke strategies enables sponsors to track and manage patient recruitment efficiently. These solutions generate key metrics and insights that help recruitment teams monitor patient journeys and take timely actions, leading to improved recruitment efficiency. Furthermore, the integration of metaheuristics in optimizing complex recruitment plans has shown promise, providing various options that meet budgetary constraints and recruitment targets.
However, Medtech companies in Latin America face unique challenges, including language barriers and fragmentation of resources, which can hinder effective communication and collaboration on regulatory requirements for FIH studies in Latin America. As Simon Francis Thomsen observes, the incorporation of strong ethical frameworks into recruitment strategies is essential for preserving public trust and adherence in research activities. Adopting these practices paves the way for more successful recruitment efforts while adhering to ethical standards, particularly in the context of clinical trials conducted in Colombia, exemplified by successful outcomes from PAVmed's first-in-human implantations of the PortIO™ Intraosseous Infusion System.
Conclusion
Colombia's regulatory landscape for First-In-Human (FIH) studies stands out as a beacon of opportunity for researchers seeking to conduct clinical trials in Latin America. With INVIMA leading the charge, the country offers a combination of efficient approval processes, significant cost savings, and a robust healthcare system that enhances patient recruitment. Understanding the intricacies of local regulations, ethical considerations, and logistical requirements is essential for researchers aiming to navigate this complex environment successfully.
The advantages of conducting FIH trials in Colombia are further underscored by the structured approval process and the strategic selection of sites and investigators. By adhering to Good Clinical Practice (GCP) guidelines and engaging with experienced local partners, sponsors can ensure that their trials not only meet regulatory standards but also uphold ethical practices that protect patient rights. This commitment to quality and compliance is crucial in fostering an environment where innovative clinical research can thrive.
As the global clinical research landscape evolves, Colombia's strategic position as a destination for FIH studies becomes increasingly significant. The country's competitive advantages, including attractive tax incentives and a commitment to regulatory compliance, make it an appealing option for international sponsors. By leveraging these strengths, Colombia can continue to position itself as a leader in the region, driving advancements in medical research while addressing health disparities and improving patient outcomes. The future of clinical trials in Colombia is bright, and those who embrace the opportunities presented by its regulatory framework are poised for success.
Frequently Asked Questions
What are the key regulatory requirements for First-In-Human (FIH) studies in Latin America?
Key regulatory requirements include comprehensive preclinical data, ethical approvals, and strict adherence to Good Clinical Practice (GCP) guidelines.
What advantages does Colombia offer for conducting FIH trials?
Colombia offers several competitive advantages, including cost savings exceeding 30% compared to trials in North America or Western Europe, a streamlined regulatory process with reviews completed within 90-120 days, and a healthcare system ranked among the best globally.
What is the role of INVIMA in FIH studies in Colombia?
INVIMA, the national regulatory authority, is crucial for conducting thorough reviews of Clinical Trial Applications (CTAs) to ensure compliance with regulatory requirements for FIH studies.
How long does the approval process for FIH studies typically take in Colombia?
The approval timeline for FIH studies in Colombia typically ranges from 2 to 6 months, depending on the complexity and quality of the submission.
What financial incentives are available for conducting research and development in Colombia?
Financial incentives include a 100% tax deduction for investments in science, technology, and innovation projects, a 25% tax discount, a 50% future tax credit, and approximately $10 million in government grants.
What steps are involved in the approval process for FIH studies?
The approval process involves preparing a Clinical Trial Application (CTA), obtaining ethical approval from a recognized ethics committee, and addressing any queries from INVIMA or the ethics committee promptly.
Why is regulatory compliance important for FIH studies in Latin America?
Regulatory compliance is essential to ensure that studies conform to both local and international standards, facilitating expedited approvals and fostering an environment conducive to innovative medical research.
What is the significance of GCP certification for hospitals in Colombia?
Hospitals must undergo a rigorous ICH/GCP certification process to ensure high standards of quality and compliance before conducting research with pharmaceutical drugs.
How does the population and healthcare coverage in Colombia impact clinical trials?
Colombia has a population of over 50 million with 95% coverage under universal healthcare, enhancing patient recruitment for clinical trials.
What expert insights are available regarding the regulatory landscape for FIH studies in Latin America?
Experts like Katherine Ruiz emphasize the importance of understanding regulatory requirements to navigate the landscape successfully, while industry leaders recognize Colombia's strategic significance in attracting foreign investment for research trials.




