Overview
To ensure data integrity in clinical research, it is essential to implement accurate data management practices, maintain consistency and completeness, and leverage technology for real-time monitoring and validation. The article outlines various strategies, including developing management plans, conducting regular audits, utilizing electronic data capture systems, and adhering to regulatory compliance, all of which reinforce the importance of meticulous data handling to produce reliable research outcomes.
Introduction
In the realm of clinical research, ensuring data integrity is not merely a regulatory requirement but a cornerstone of scientific credibility. For Medtech companies in Latin America, the stakes are particularly high due to the unique challenges posed by regulatory complexities and resource limitations. The accuracy, consistency, and reliability of data are critical in navigating these hurdles and producing trustworthy results.
This article delves into the foundational principles of data integrity, explores the necessary procedures for maintaining it, and highlights the role of technology in enhancing data quality. Furthermore, it addresses the regulatory landscape that governs clinical research and the common challenges faced by researchers, offering practical solutions to uphold the integrity of their studies.
As the Medtech sector continues to evolve, understanding and implementing these principles will be vital for fostering innovation and ensuring the success of clinical trials in this dynamic region.
Understanding the Foundations of Data Integrity in Clinical Research
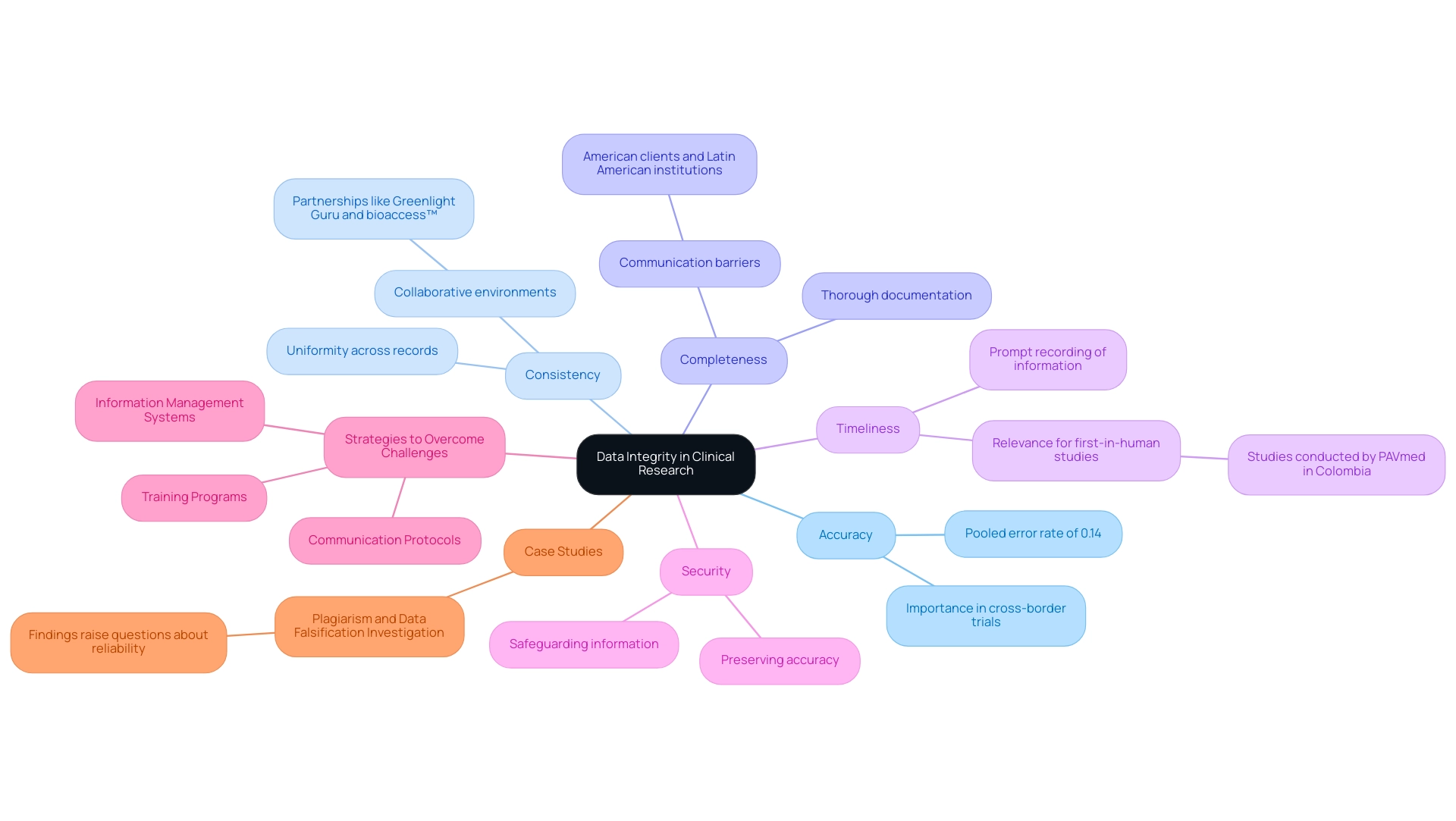
Understanding how to ensure data integrity in clinical research is crucial for Medtech firms operating in Latin America, where obstacles like regulatory challenges and scattered resources frequently hinder advancement. Understanding how to ensure data integrity in clinical research involves recognizing the accuracy, consistency, and dependability of information gathered throughout a study, which is essential for overcoming these challenges. The foundational principles supporting information integrity include:
- Accuracy: Data must be recorded precisely to reflect true results. A pooled error rate for double-entry found to be 0.14% (95% CI: 0.08, 0.20) underscores how meticulous entry processes can significantly enhance accuracy, especially when navigating the complexities of cross-border clinical trials.
- Consistency: Uniformity across all records and sources is essential to prevent discrepancies that could compromise findings, particularly in collaborative environments like those fostered by partnerships such as Greenlight Guru and bioaccess™.
- Completeness: Thorough documentation of all essential information points is crucial; insufficient information can lead to misinterpretations of findings, further exacerbating communication barriers between American clients and Latin American institutions.
- Timeliness: Prompt recording of information ensures its relevance and accuracy, enabling researchers to make informed decisions based on the most up-to-date details, which is vital for the first-in-human studies like those conducted by PAVmed in Colombia.
- Security: Safeguarding information from unauthorized access or modifications is essential for preserving its accuracy, ensuring that findings are credible and reliable.
To tackle these challenges, Medtech companies can adopt several strategies:
- Implementing robust information management systems that facilitate real-time entry and monitoring
- Fostering training programs that enhance local staff's understanding of information accuracy principles
- Establishing clear communication protocols that bridge gaps between American clients and Latin American institutions
These principles are supported by various NIH awards, highlighting their credibility and significance. Understanding these principles is essential for researchers to learn how to ensure data integrity in clinical research, allowing them to design and execute studies that produce valid results stakeholders can trust.
As Maryam Y Garza, PhD, MPH, MMCi from the Department of Biomedical Informatics indicates:
We hope that our analysis presents a robust and persuasive case for the assessment and dissemination of information precision in medical studies.
Recent investigations into data reliability issues, such as the notable case of plagiarism and data falsification at a prominent cancer lab, illustrate the serious implications of neglecting these principles. This situation brings up worries regarding the dependability of findings from reputable organizations and highlights the importance of understanding how to ensure data integrity in clinical research to maintain the authenticity of medical investigations, especially in the evolving environment of Latin America's Medtech industry.
Connecting the divide between innovation and implementation is essential for the future success of medical studies in this area.
Step-by-Step Procedures for Ensuring Data Integrity
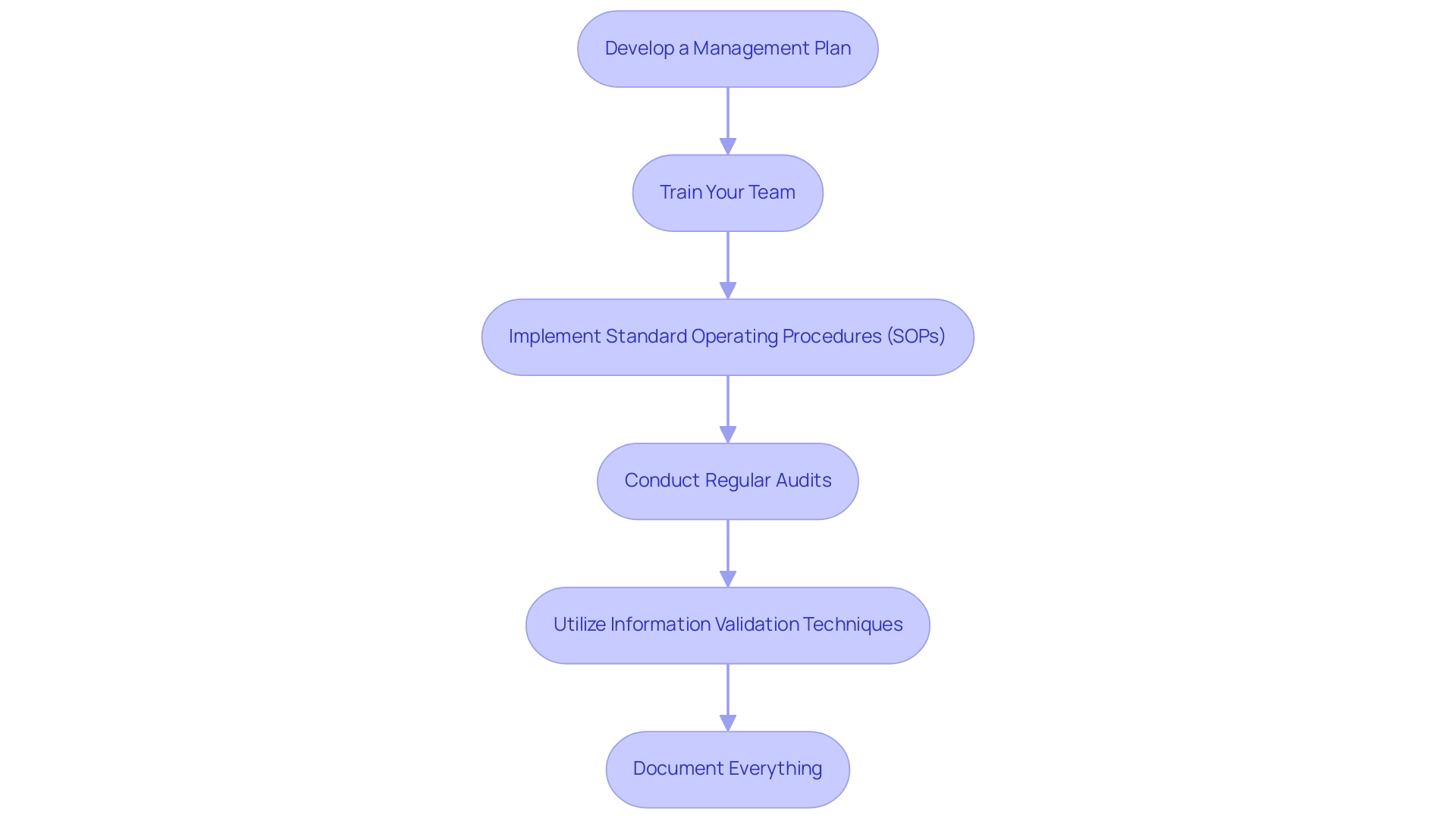
To uphold the integrity of information in clinical research, it is crucial to implement the following procedures:
- Develop a Management Plan: Clearly outline how information will be collected, stored, and analyzed, aligning with the NIH policy that mandates a Management and Sharing Plan upon grant application submission as of January 25, 2023. Following this policy not only guarantees compliance but also strengthens the robustness of information management practices.
- Train Your Team: It is essential to ensure that all team members grasp the principles and procedures related to information integrity. Effective training programs are vital. As Binny Krishnankutty from Dr. Reddy's Laboratories emphasizes, CDM has evolved in response to the ever-increasing demand from pharmaceutical companies to fast-track the drug development process and from regulatory authorities to implement quality systems for high-quality information generation. This evolution highlights the necessity for thorough training on SOPs to navigate the complexities of contemporary information management.
- Implement Standard Operating Procedures (SOPs): Create detailed SOPs for information collection, entry, and management to promote consistency and reliability throughout the research process. These SOPs should be regularly updated to reflect technological advancements and regulatory changes that influence information management.
- Conduct Regular Audits: Regularly reviewing information for accuracy and completeness is imperative. This proactive method enables researchers to recognize and correct inconsistencies swiftly, tackling one of the major challenges the industry encounters in preserving information reliability amid swift technological shifts.
- Utilize Information Validation Techniques: Employ techniques such as double input entry, automated validation scripts, and peer reviews to enhance accuracy and reliability.
- Document Everything: Maintain comprehensive records of all data-related activities. Comprehensive documentation guarantees openness and traceability, which are essential for efficient information management.
By following these optimal practices, researchers can greatly enhance the quality of their information, which is essential for understanding how to ensure data integrity in clinical research, ultimately leading to more dependable and significant outcomes in medical studies. The situation of Datavant demonstrates this, as their platform links medical information with real-world insights, improving study quality and visibility through a network of more than 500 partners.
Leveraging Technology to Enhance Data Integrity
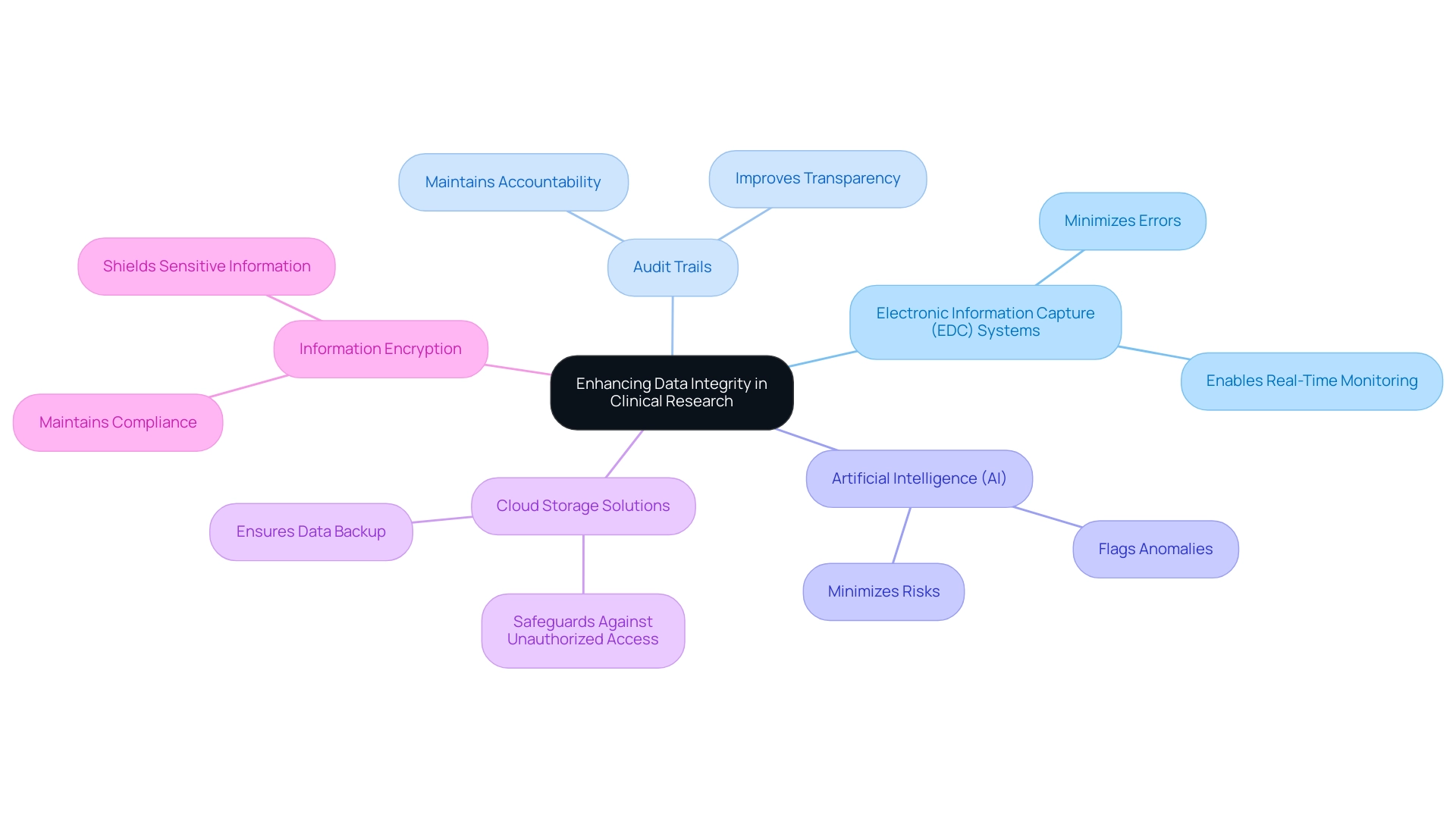
Technology serves as a cornerstone in fortifying data integrity within clinical research through several key mechanisms:
- Electronic Information Capture (EDC) Systems: These systems play a vital role in streamlining the collection process by minimizing errors and enabling real-time monitoring. The use of EDC systems is crucial for understanding how to ensure data integrity in clinical research, ensuring that information is both accurate and readily available for analysis.
- Audit Trails: The implementation of audit trails, which automatically log all modifications to information, is essential for maintaining accountability and traceability. This feature not only improves transparency but also enables comprehensive evaluations during audits, which is essential for understanding how to ensure data integrity in clinical research.
- Artificial Intelligence (AI): AI technologies are increasingly being utilized to sift through extensive datasets in search of anomalies. By flagging potential discrepancies before they escalate, AI contributes significantly to maintaining the overall quality of information and minimizing risks associated with erroneous content.
- Cloud Storage Solutions: Secure cloud services offer a dual advantage of ensuring information is both backed up and easily accessible, while simultaneously safeguarding it from unauthorized access. This level of security is critical in safeguarding sensitive patient information and maintaining compliance with regulatory standards.
- Information Encryption: The practice of encrypting sensitive information offers an additional layer of security, effectively shielding it from potential breaches. This is particularly important in clinical research, where the confidentiality of patient records is paramount.
By incorporating these advanced technologies, researchers can significantly improve the reliability of their information, which is crucial for understanding how to ensure data integrity in clinical research, addressing the issues emphasized by Liu et al., who pointed out that 'having incomplete information on patients’ records has posed the greatest threat to patient care.' Furthermore, as highlighted in the report on the interconnections among quality dimensions, consistency in entry is crucial; when information is recorded inconsistently, it undermines the accuracy of medical records and the validity of secondary uses. Moreover, the analysis recognized five categories of digital health information quality results, highlighting the significance of technology in maintaining information reliability.
These technological innovations not only uphold the reliability of research trials but also illustrate how to ensure data integrity in clinical research by guaranteeing that the information gathered meets the highest standards of quality.
Navigating Regulatory Compliance for Data Integrity
Navigating the regulatory environment is crucial for understanding how to ensure data integrity in clinical research, particularly in light of the FDA's commitment to safeguarding human participant safety and rights during all stages of investigations. This focus significantly impacts compliance measures, which must be prioritized:
-
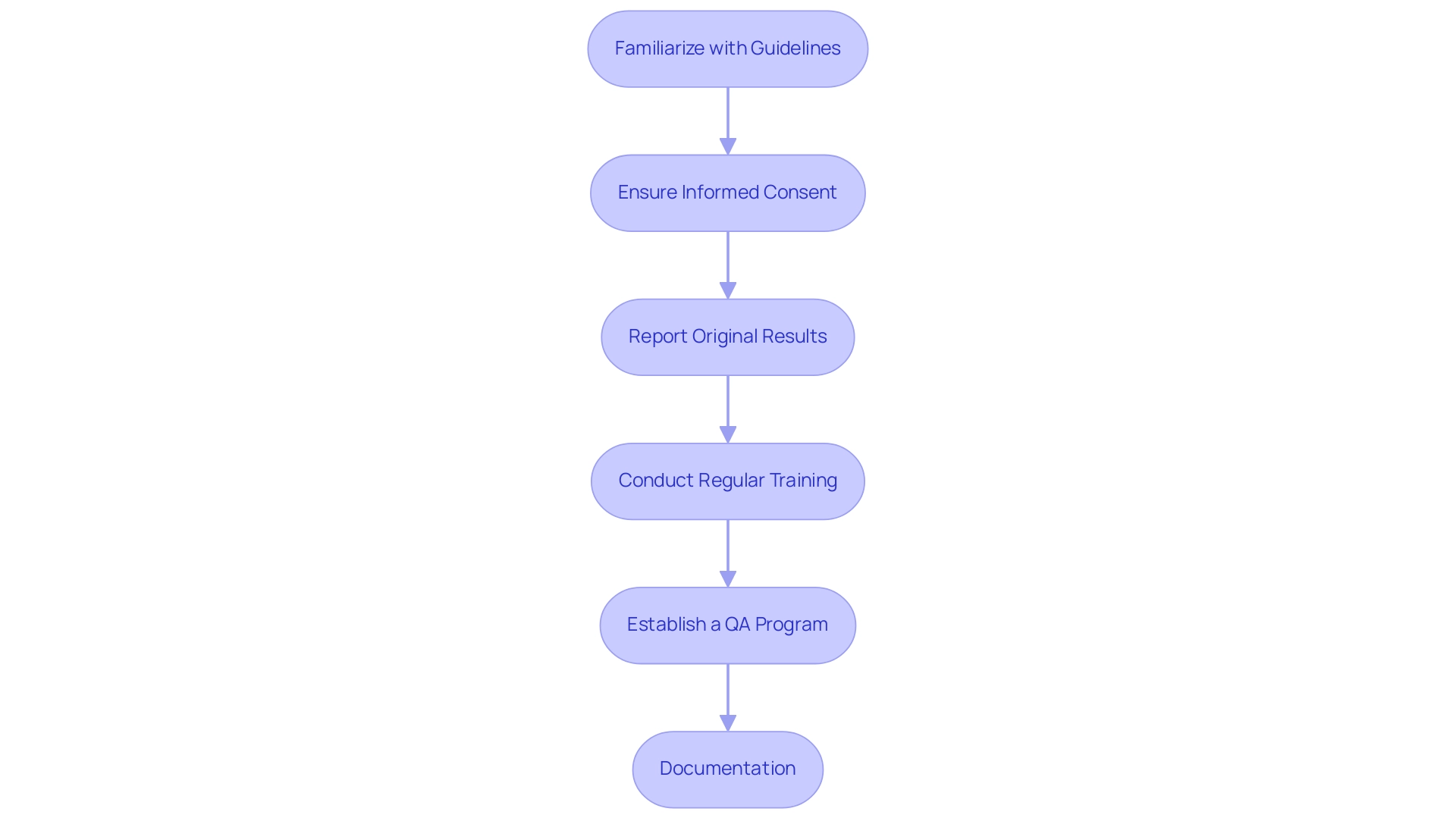
Familiarize Yourself with Relevant Guidelines: It is imperative to understand the FDA regulations, ICH-GCP guidelines, and local regulations that impact your research.
This knowledge is foundational for compliance and effective management, aligning with our comprehensive trial management services that include feasibility studies, site selection, trial setup, and import permits.
-
Ensure Informed Consent: The FDA requires that informed consent documents clearly state that a description of the trial will be available on ClinicalTrials.gov. This transparency promotes ethical standards and guarantees that participants are fully informed about the study and the use of their information, which is essential for maintaining trust and integrity.
-
Report Original Results: The FDA recommends that researchers report original results and provide a description of the non-reference standard without revising the original table. This practice is crucial for preserving information reliability and transparency in clinical research, which is essential for understanding how to ensure data integrity in clinical research through rigorous project management and oversight. Additionally, regular reporting on study status and any serious or non-serious adverse events is crucial for compliance and participant safety.
-
Conduct Regular Training: Ongoing training for staff on compliance and information reliability principles is essential. This not only reinforces understanding but also ensures that all team members are equipped to adhere to the necessary regulations, an approach supported by the expertise of our team, including professionals like Ana Criado and Katherine Ruiz.
-
Establish a Quality Assurance Program: Implementing a robust quality assurance (QA) program is crucial in monitoring compliance and swiftly addressing any non-conformities.
A robust QA framework strengthens the commitment to regulatory compliance and information reliability, ensuring that all elements of the trial setup and approval processes are effectively managed.
-
Documentation: Meticulous record-keeping of compliance activities and decisions is vital to demonstrate adherence to regulations. This practice is reinforced by the FDA's Certificate of Confidentiality, which protects the privacy of study participants by safeguarding identifiable, sensitive information.
Mandatory Certificates of Confidentiality (CoCs) are necessary for federally funded studies, while optional CoCs may be provided for non-federally funded projects, emphasizing the significance of safeguarding participant information while ensuring adherence.
By strictly following these compliance protocols and utilizing our extensive trial management services, including project oversight and reporting, researchers can learn how to ensure data integrity in clinical research while maintaining information accuracy and cultivating trust in their outcomes, ultimately aiding the progress of medical studies.
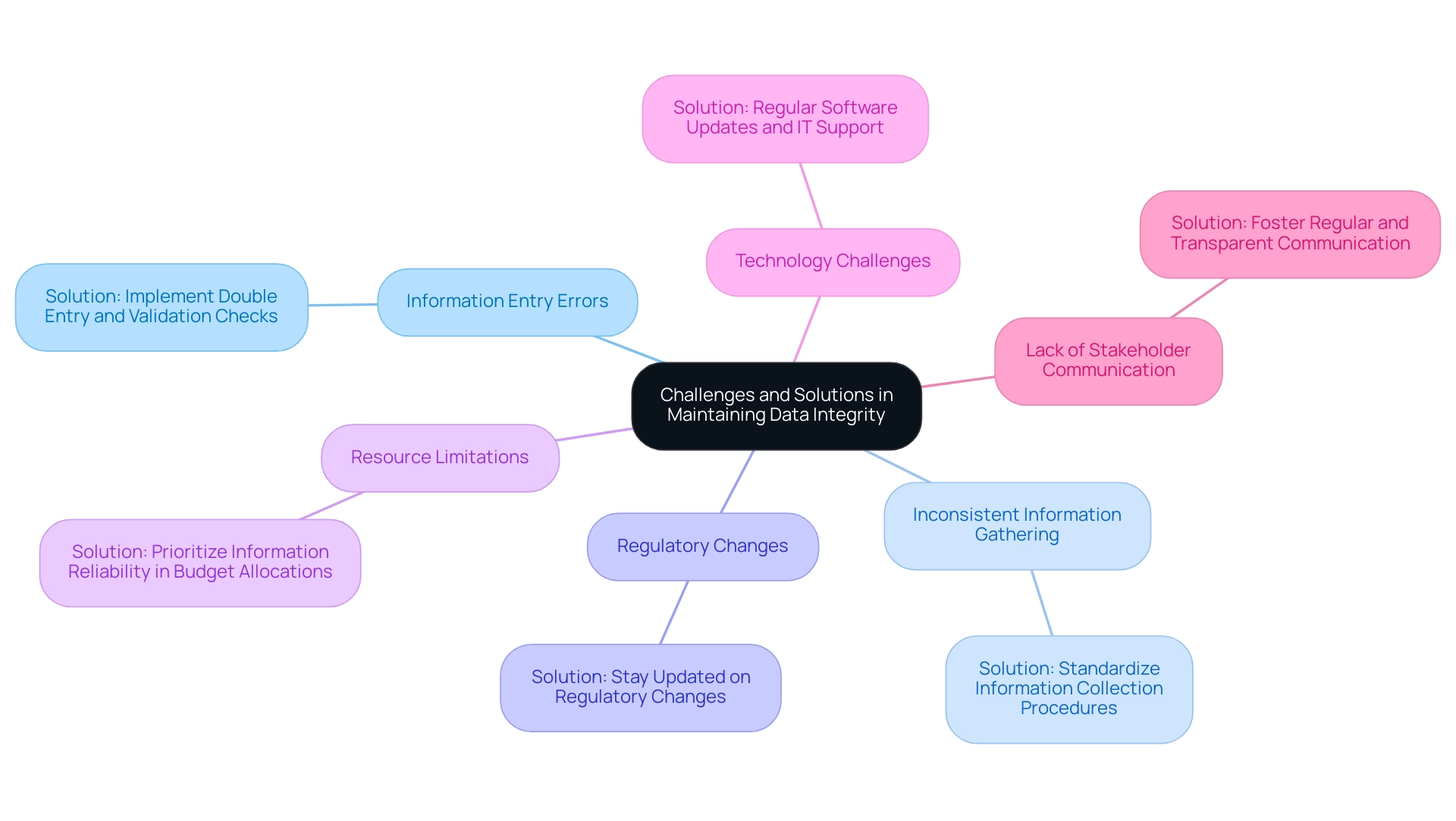
Challenges and Solutions in Maintaining Data Integrity
Understanding how to ensure data integrity in clinical research presents several significant challenges that can affect the overall quality of findings.
-
Information Entry Errors: Human mistakes during entry are a significant issue, with recent findings suggesting that the error rates for the date of the last follow-up visit are more than two-fold higher than those for either first or last treatment visit dates. These errors can potentially affect the interpretation of research results.
- Solution: Implementing double entry alongside rigorous validation checks can mitigate these risks, supported by our comprehensive management services.
-
Inconsistent Information Gathering: Variability in information collection methods across different sites can lead to discrepancies that undermine integrity.
- Solution: Standardizing information collection procedures ensures consistency and reliability across all participating sites, which is crucial for compliance with regulatory bodies like INVIMA, Colombia's Level 4 health authority overseeing medical devices.
Our services include detailed protocols for site selection and training to ensure adherence to these standards.
- Regulatory Changes: The clinical research landscape is continually evolving, making it challenging to keep up with new regulations.
- Solution: Keeping updated on regulatory changes, including those from INVIMA, and modifying processes as needed is crucial for compliance and preserving information accuracy.
Our team provides ongoing reviews and feedback on study documents to ensure compliance with current regulations.
- Resource Limitations: Insufficient resources can significantly hinder efforts in information management.
- Solution: Prioritizing information reliability in budget allocations and seeking additional funding opportunities can enhance resource availability for management tasks.
Our project management services concentrate on enhancing resource distribution to support information integrity initiatives, ultimately promoting economic growth and job creation in local communities.
-
Technology Challenges: Technical issues, such as software malfunctions or system outages, can disrupt information collection and management processes.
- Solution: Regular updates to software and providing robust IT support can address these technical challenges promptly, ensuring uninterrupted study management and monitoring, which are integral to our service capabilities.
-
Lack of Stakeholder Communication: Effective communication among stakeholders is crucial for the success of trials, as poor communication can lead to misunderstandings and delays.
- Solution: Fostering regular and transparent communication among stakeholders can enhance collaboration and address issues promptly. Our comprehensive project management approach includes regular status reporting and stakeholder engagement strategies to improve overall study outcomes, specifically focusing on how to ensure data integrity in clinical research.
By recognizing these challenges and implementing effective solutions tied to our service capabilities, researchers can enhance the maintenance of data integrity throughout their studies, thereby improving the credibility and reliability of clinical research outcomes, while also contributing positively to the local economy through the successful execution of Medtech clinical studies.
Conclusion
Ensuring data integrity in clinical research is essential, particularly for Medtech companies in Latin America facing unique regulatory and resource challenges. The article has outlined the foundational principles of data integrity—accuracy, consistency, completeness, timeliness, and security—each vital for producing credible research outcomes. By implementing robust data management plans, training staff, and utilizing advanced technologies such as electronic data capture systems and artificial intelligence, researchers can significantly enhance the reliability of their data.
Navigating the regulatory landscape is equally important, as adherence to guidelines and maintaining transparent communication with stakeholders can fortify the integrity of clinical trials. Regular audits and comprehensive documentation further contribute to compliance and trust in research findings. Addressing common challenges, such as data entry errors and inconsistent data collection, with practical solutions ensures that the integrity of data is upheld throughout the research process.
Ultimately, the commitment to data integrity not only fosters innovation and enhances the quality of clinical research but also builds trust among stakeholders and participants. As the Medtech sector continues to evolve, prioritizing these principles will be critical for the success and credibility of clinical trials in Latin America, paving the way for advancements in healthcare and improved patient outcomes.
Frequently Asked Questions
Why is data integrity important in clinical research for Medtech firms in Latin America?
Data integrity is crucial for Medtech firms in Latin America to ensure the accuracy, consistency, and reliability of information gathered during studies, which helps overcome regulatory challenges and resource limitations.
What are the foundational principles of data integrity in clinical research?
The foundational principles include: 1. Accuracy: Data must be recorded precisely to reflect true results. 2. Consistency: Uniformity across all records is essential to prevent discrepancies. 3. Completeness: Thorough documentation of all essential information is crucial. 4. Timeliness: Prompt recording of information ensures relevance and accuracy. 5. Security: Safeguarding information from unauthorized access is essential.
What strategies can Medtech companies adopt to ensure data integrity?
Strategies include: - Implementing robust information management systems for real-time entry and monitoring. - Fostering training programs to enhance local staff's understanding of information accuracy. - Establishing clear communication protocols between American clients and Latin American institutions.
What procedures should be implemented to uphold the integrity of information in clinical research?
Key procedures include: 1. Developing a Management Plan for data collection, storage, and analysis. 2. Training the team on principles and procedures related to information integrity. 3. Implementing Standard Operating Procedures (SOPs) for data management. 4. Conducting regular audits for accuracy and completeness. 5. Utilizing information validation techniques like double entry and peer reviews. 6. Documenting all data-related activities comprehensively.
How does the NIH policy affect data management practices in clinical research?
The NIH policy mandates a Management and Sharing Plan upon grant application submission, which ensures compliance and strengthens information management practices.
What role does training play in maintaining data integrity in clinical research?
Effective training programs are vital for ensuring all team members understand the principles and procedures related to information integrity, which helps navigate the complexities of information management.
What are some examples of validation techniques used to enhance data integrity?
Examples of validation techniques include double input entry, automated validation scripts, and peer reviews, which help enhance the accuracy and reliability of data.
Why is comprehensive documentation important in clinical research?
Comprehensive documentation guarantees openness and traceability, which are essential for efficient information management and maintaining the integrity of the research process.

