Introduction
Navigating the complexities of informed consent is a critical component of ethical clinical research, as outlined in the regulations set forth by 21 CFR 50.27. This regulation establishes the framework for ensuring that participants are fully informed about the nature, risks, and benefits of their involvement in studies. Key elements such as:
- Clear communication
- Voluntary participation
- Ongoing consent
underscore the necessity for researchers to prioritize participant understanding and autonomy.
However, challenges such as:
- Complex language
- Time constraints
- Varying literacy levels
can hinder effective communication.
This article delves into the essential elements of informed consent, the role of ethics committees, common challenges faced, and best practices that can enhance the informed consent process, ultimately fostering a more ethical and transparent research environment.
Overview of 21 CFR 50.27: Key Regulations for Informed Consent
21 CFR 50.27 details the essential requirements for obtaining informed agreement from individuals in clinical trials, highlighting the ethical duty to ensure that participants are fully aware of the nature, risks, and advantages associated with the research. This regulation requires that approval must be obtained before the initiation of any research procedures. Essential elements of this regulation, specifically detailed in 21 CFR 50.27, involve the requirement for clear language, providing individuals sufficient time to consider their engagement, and guaranteeing that agreement continues to be a dynamic process during the research.
Significantly, recent findings suggest that public access to approval documents could greatly improve trial recruitment efforts, as potential subjects are more inclined to engage when they have access to clear and transparent information about the study. Taichman Sahni Pinborg highlights the necessity for data sharing statements in clinical trials, emphasizing that:
Data sharing statements for clinical trials: a requirement of the International Committee of Medical Journal Editors.
Furthermore, addressing literacy barriers in clinical trial agreements is critical for improving participant comprehension and retention.
The Flesch-Kincaid Grade Level for the Confidentiality clause has been simplified to 5.70, indicating a need for simpler language to enhance understanding. The FDA's authority to revise guidance on awareness procedures aligns with the mandates of the 21st Century Cures Act, which emphasizes patient control over their health data as outlined in 21 CFR 50.27. Proposed amendments could empower patients to request the sharing of their de-identified clinical data with researchers, enhancing transparency and patient autonomy.
Understanding these elements is vital for ensuring compliance with regulations while fostering ethical research practices.
Essential Elements of Informed Consent Under 21 CFR 50.27
The essential elements of knowledgeable consent, as outlined in 21 CFR 50.27, are foundational to ethical clinical research. These elements include:
-
Disclosure of Information: Participants must receive comprehensive details about the study, encompassing its purpose, duration, procedures, potential risks, and expected benefits.
This transparency is crucial for informed decision-making.
-
Researchers are responsible for ensuring that individuals grasp the information provided.
This may involve evaluating their comprehension through targeted questions or discussions, a critical step given that only 24.9% of participants from developed countries demonstrate a clear understanding of the placebo concept, compared to 43.4% in least developed countries.
-
Voluntary Participation: Consent must be obtained freely, without coercion or undue influence.
Participants should be made aware of their right to withdraw from the study at any point without facing any penalties.
-
Documentation of Consent: Proper documentation is essential, typically achieved through a signed consent form that encapsulates the key elements discussed.
This documentation not only safeguards individuals but also acts as a record of adherence to ethical standards.
-
Ongoing Consent: Effective communication is vital throughout the research.
Researchers must inform individuals of any new findings or modifications to the study that could impact their willingness to continue. This dynamic method to agreement ensures that individuals stay informed throughout the research process.
Each of these elements is pivotal in safeguarding ethical standards and protecting the rights of research participants.
However, challenges persist, such as the complexity of information leaflets, which are increasingly laden with GDPR and data protection details. As noted by a research staff member,
Info leaflets are getting more complicated with GDPR/data protection information. It is almost impossible to make it shorter without risking rejection by the ethics committee.
This complexity emphasizes the need for clarity in ongoing agreement communication. Furthermore, a case study on the study selection process illustrates these challenges: the initial search yielded 10,246 records, which were screened to identify eligible studies for inclusion in the meta-analysis, ultimately resulting in 114 studies. This example emphasizes the thorough methodology necessary to guarantee that understanding and documentation are effectively achieved.
Thus, navigating the balance between thoroughness and clarity in approval procedures remains a critical issue in clinical research.
The Role of Ethics Committees in the Informed Consent Process
Ethics committees, often called institutional review boards (IRBs), play an essential role in the knowledge agreement process within clinical research. Their main duty is to carefully examine research proposals, ensuring that the rights and welfare of those involved are prioritized. Before any research can proceed, the IRB assesses the approval documents for clarity, comprehensiveness, and adherence to regulatory standards as mandated by 21 CFR 50.27.
Along with confirming adherence, the committee thoroughly evaluates the risk-to-benefit ratio of the research, ensuring that potential hazards are reduced while enhancing possible advantages for those involved. Researchers are required to submit their informed consent materials for IRB approval, marking this as a vital step in the overall research process. Each Member State must complete its assessment within 45 days from the validation date, providing a relevant timeframe for IRB assessments.
A relevant case study on disclosure requirements in pragmatic randomized controlled trials highlights current regulations that dictate what must be disclosed to potential subjects, including the study's purpose, risks, benefits, and the voluntary nature of involvement. Authors in this field generally agree on the necessity of disclosing these elements; however, there is ongoing debate regarding whether the randomization process itself should be explicitly conveyed to those involved. This debate is further enriched by the analysis conducted by Kim SY and Miller FG, which examines the ethical implications of standard-of-care treatment randomized trials.
As one ethics committee member stated,
if we delete them, we close moral spaces that we actually need to keep open,
emphasizing the ethical complexities faced by IRBs in their oversight roles.
Common Challenges in Obtaining Informed Consent
The informed agreement procedure in clinical research faces several substantial challenges that can affect subject understanding and involvement. These challenges include:
-
Complex Language: The use of technical jargon or overly sophisticated explanations can obstruct comprehension.
Researchers must prioritize clarity, employing straightforward language to facilitate understanding.
-
Time Constraints: Participants often express feeling rushed when signing authorization forms, which may prevent them from fully grasping the information presented.
It is essential to allocate sufficient time for thorough discussions to ensure individuals can make informed decisions.
-
Varying Literacy Levels: Given that individuals have differing literacy levels, it is essential to adjust the approval process accordingly.
Customizing materials to address the literacy needs of all individuals can significantly enhance understanding.
-
Cultural Sensitivity: Awareness of cultural differences is vital, as these can influence views on agreement.
Researchers should be adaptable in their approaches, engaging with individuals in culturally appropriate ways.
-
Continuous Dialogue: Maintaining contact with individuals throughout the research presents a challenge, but it is essential for guaranteeing that awareness remains valid over time.
Continuous dialogue helps reinforce understanding and engagement.
Tackling these challenges in advance is essential for enabling a more efficient understanding process, ultimately improving comprehension and encouraging greater involvement. The intricacies of knowledgeable agreement are further demonstrated by a research examining community-based trials in rural North India, which underscores the cultural elements affecting decision-making in clinical research.
Significantly, a research project disclosed that 18% of individuals acknowledged not having completely read the informational letter (Pope et al.), underlining the urgent need for clearer communication and engagement strategies. Furthermore, the study group included 49.5% Whites and 43.8% Blacks, highlighting the significance of acknowledging diversity in backgrounds when tackling challenges related to understanding.
This report is part of the European Research Project WRAMSOC under the V Framework Program, which underscores the significance of these findings within a broader research context.
Best Practices for Ensuring Effective Informed Consent
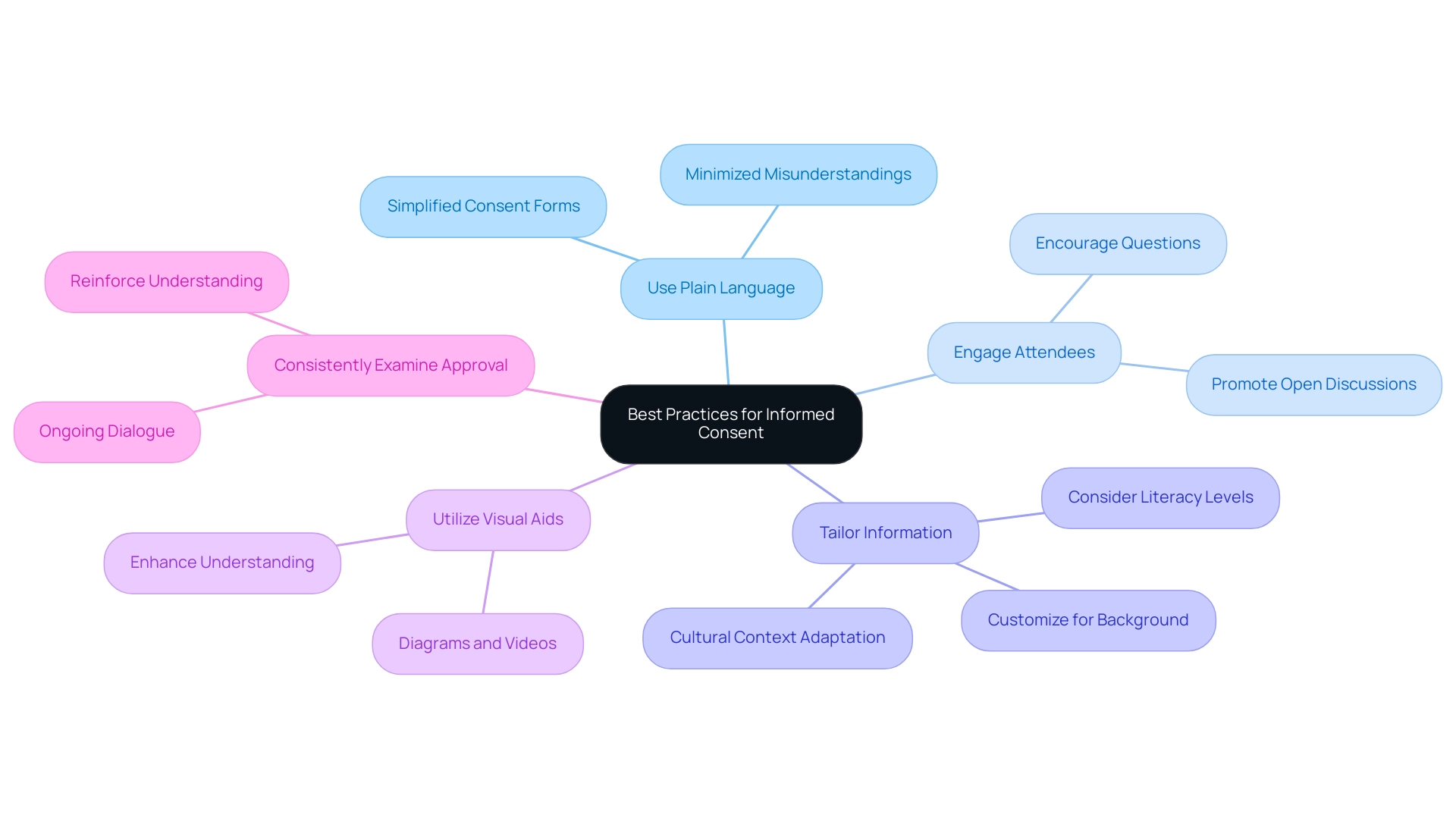
To enhance the informed consent process, researchers should adopt several best practices that uphold the integrity of clinical trials and respect participants' rights:
- Use Plain Language: It is crucial to simplify the consent form and accompanying discussions, ensuring that individuals can easily grasp the content. Utilizing accessible language minimizes misunderstandings and fosters a clearer comprehension of the study’s objectives and implications.
- Engage Attendees: Actively encourage questions and open discussions, allowing individuals to voice their concerns. This interactive approach promotes a sense of comfort and empowerment, enabling individuals to make informed decisions about their participation.
- Tailor Information: Customize the agreement information to align with each participant's background, literacy level, and cultural context. This personalized approach acknowledges diversity and enhances understanding. For instance, considering that the average age of parents or guardians in a neonatology study in the UK was 30.5 years (Snowdon, 1997), researchers should adapt their communication strategies to resonate with this demographic.
- Utilize Visual Aids: Incorporate diagrams, videos, or other visual tools to complement the written agreement form. These aids can significantly enhance understanding and retention of information, making the agreement process more accessible.
- Consistently Examine Approval: Occasionally revisit the approval process throughout the research to ensure that participants stay aware and involved. This ongoing dialogue reinforces their understanding and affirms the voluntary nature of their participation.
By following these best practices, researchers can develop a more effective understanding agreement experience. As emphasized by results from Verheggen's 1996 research involving 198 adult patients, comprehension of clear agreement greatly improves with effective communication. The study emphasized that "most participants in clinical trials understood fundamental components of informed consent such as the nature and benefits of the study, freedom to withdraw at any time and the voluntary nature of participation."
Thus, implementing these strategies not only enhances ethical standards but also enriches the overall integrity of clinical research.
Conclusion
Navigating the informed consent process is essential for ethical clinical research, as emphasized by the regulations outlined in 21 CFR 50.27. This article has explored the key elements of informed consent, including:
- The importance of clear communication
- Voluntary participation
- The need for ongoing consent
By ensuring that participants are well-informed about the nature, risks, and benefits of their involvement in clinical trials, researchers uphold the ethical standards crucial to protecting participant rights.
However, several challenges impede the effectiveness of the informed consent process, such as:
- Complex language
- Time constraints
- Varying literacy levels among participants
Addressing these obstacles is vital for enhancing participant comprehension and fostering engagement. The role of ethics committees in reviewing and approving informed consent materials also plays a significant part in ensuring that ethical standards are met and participant welfare is prioritized.
Implementing best practices, such as:
- Simplifying language
- Engaging participants in discussions
- Utilizing visual aids
can significantly improve the informed consent experience. By tailoring information to meet the diverse needs of participants and maintaining open lines of communication throughout the study, researchers can promote a more ethical and transparent research environment. Ultimately, prioritizing informed consent not only safeguards participant autonomy but also enhances the integrity of clinical research, fostering trust and cooperation between researchers and participants alike.
Frequently Asked Questions
What is the purpose of 21 CFR 50.27 in clinical trials?
21 CFR 50.27 outlines the essential requirements for obtaining informed consent from individuals in clinical trials, ensuring that participants are fully aware of the nature, risks, and benefits associated with the research.
What are the key elements of informed consent as per 21 CFR 50.27?
The key elements include: 1. Disclosure of Information: Comprehensive details about the study must be provided. 2. Understanding: Researchers must ensure participants grasp the information. 3. Voluntary Participation: Consent must be given freely, with the right to withdraw at any time. 4. Documentation of Consent: Proper documentation through a signed consent form is required. 5. Ongoing Consent: Continuous communication about new findings or changes in the study is essential.
How does 21 CFR 50.27 address participant comprehension?
Researchers are responsible for ensuring that participants understand the information provided, which may involve assessing comprehension through targeted questions or discussions.
What recent findings suggest about public access to clinical trial approval documents?
Recent findings indicate that public access to approval documents could enhance trial recruitment, as potential subjects are more likely to participate when they have access to clear and transparent information about the study.
What role do data sharing statements play in clinical trials?
Data sharing statements are required by the International Committee of Medical Journal Editors and emphasize the importance of transparency in clinical trials.
What challenges exist regarding literacy in clinical trial agreements?
Addressing literacy barriers is critical for improving participant comprehension, as complex language in agreements may hinder understanding.
How has the Flesch-Kincaid Grade Level for the Confidentiality clause been adjusted?
The Flesch-Kincaid Grade Level for the Confidentiality clause has been simplified to 5.70, indicating a need for simpler language to enhance understanding.
What does the 21st Century Cures Act emphasize regarding patient control?
The 21st Century Cures Act emphasizes patient control over their health data, aligning with the mandates of 21 CFR 50.27 and suggesting that patients could request sharing of their de-identified clinical data with researchers.
Why is ongoing consent important in clinical trials?
Ongoing consent ensures that participants remain informed about any new findings or changes in the study that may affect their willingness to continue participating.
What is a significant challenge in the approval procedures for clinical trials?
A significant challenge is balancing thoroughness and clarity in approval procedures, as information leaflets can become overly complex due to the inclusion of GDPR and data protection details.




