Introduction
The journey of a new drug from conception to market is a complex and rigorous process, with the Investigational New Drug (IND) application serving as a critical gateway. This pivotal document represents a drug that, while not yet approved for general use, is undergoing essential clinical trials to evaluate its safety and efficacy. Researchers must navigate a detailed and demanding application process, which requires a comprehensive submission of preclinical data and adherence to regulatory standards set forth by the FDA.
As the pharmaceutical landscape evolves, understanding the nuances of different types of IND applications—including commercial, research, and emergency pathways—becomes vital for researchers aiming to advance medical science. This article delves into the intricacies of the IND process, outlining essential steps, timelines, and the importance of strategic planning to ensure successful submissions and ultimately enhance patient care.
What is an Investigational New Drug (IND)?
An Investigational New Drug (IND) represents a pivotal point in the drug development lifecycle, specifically referring to a drug that has not yet received FDA approval for general use but is undergoing clinical trials aimed at assessing its safety and efficacy. Before starting human testing, researchers are required to submit a comprehensive request that meets the IND research term to the FDA, which demands meticulous attention to detail and adherence to national requirements. This application must encompass extensive data derived from rigorous preclinical studies, providing foundational insights into the drug's biological activity and potential risks.
By securing IND status, researchers gain the authorization necessary to commence clinical trials, a critical juncture that highlights the importance of the IND research term in ensuring the drug can be studied safely within human populations. This method not only protects participant welfare but also establishes the foundation for subsequent stages of drug development, ultimately aiding the progress of medical science and patient care. It is important to note that cross-agency performance, including compliance inspection activities, plays a significant role in the IND research term procedure.
As Amsberry aptly states, 'Preparing a regulatory-compliant IND submission is extremely labor intensive and time-consuming.' Successful preparation and submission of IND research terms require substantial strategic planning and precise timing. Moreover, comprehensive clinical trial management services—such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
are essential in facilitating this procedure.
These services are crucial for ensuring that the IND application meets all regulatory requirements, particularly in the context of Colombia, where experts like Katherine Ruiz specialize in Regulatory Affairs for medical devices and in vitro diagnostics. Specific procedures involved in trial setup and approval include obtaining ethics committee clearance and health ministry approval, which are vital for compliance. Resources such as the CDER Learn Courses are also available to help industry professionals enhance their understanding of the IND process, focusing on the Chemistry, Manufacturing, and Controls (CMC) perspective.
Types of IND Applications: Understanding the Options
The landscape of Investigational New Drug (IND) applications is defined by three primary types:
- Commercial IND: This request is submitted by pharmaceutical companies aiming to develop a drug for commercial sale. It encompasses extensive documentation and must comply with rigorous regulatory standards, reflecting the commercial intent behind the development.
- Research IND: The research term, typically submitted by investigators or academic institutions, is focused on conducting clinical research for academic or non-commercial purposes. This form of software is crucial for advancing scientific knowledge and often involves innovative methodologies that contribute to the broader medical field.
- Emergency IND: In urgent situations where no alternative treatments are available, an emergency IND permits the use of an investigational drug. This pathway is essential for addressing critical health crises and underscores the flexibility of the regulatory framework to meet urgent needs.
Understanding these distinctions is vital for researchers, as it allows them to determine the most suitable approach for their specific study objectives. Given the labor-intensive nature of preparing a compliant IND application, as emphasized by industry expert Amsberry, "Preparing a regulatory-compliant IND application is extremely labor intensive and time-consuming. Successful preparation and submission of INDs require substantial strategic planning and precise timing."
Engaging with regulatory consultants like Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, can significantly streamline the preparation and submission process. Her insights into comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are crucial for ensuring alignment with the latest FDA regulations and guidance. This strategic planning becomes even more pertinent as trends in the research IND requests evolve, particularly with emerging data on research IND submissions for 2024.
Remarkably, in 2018, 103 IND requests for innovative biopharmaceuticals received approval in China, emphasizing the importance of efficient IND procedures in the global arena. Additionally, resources such as the CDER Learn Courses offer industry professionals the opportunity to enhance their understanding of the IND process, connecting practical training to the theoretical aspects discussed.
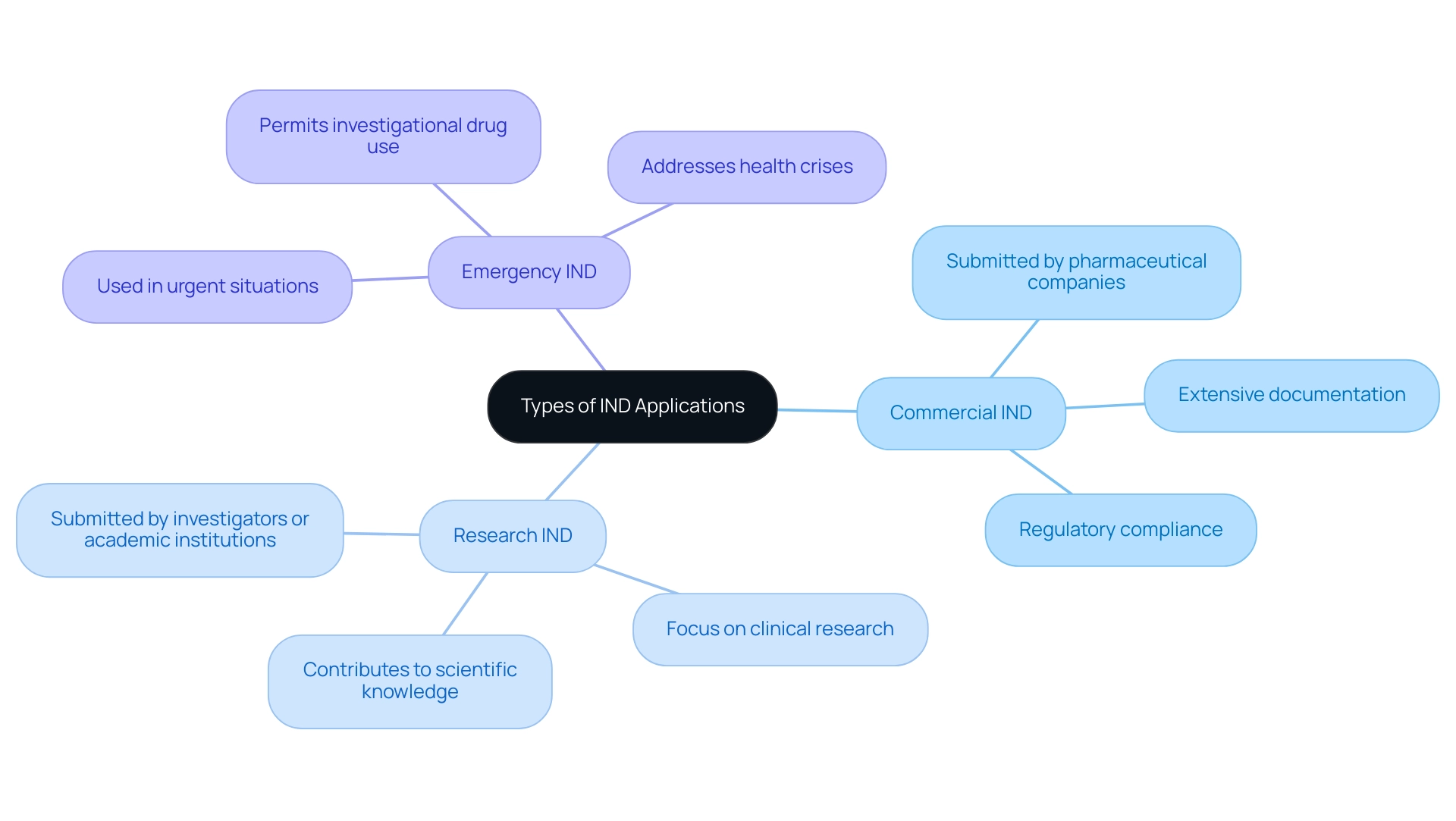
Categories of IND: Navigating the Regulatory Framework
IND applications are categorized into three distinct types, each with unique regulatory requirements and implications for clinical research:
- Type A: These applications pertain to new chemical entities that have not been previously studied in any clinical context. As such, they often require extensive preliminary data and a robust regulatory framework to ensure safety and efficacy.
- Type B: This category includes requests for drugs that have undergone prior testing but necessitate new studies aiming at different indications or populations. This type is particularly relevant for repurposing existing drugs, which can accelerate the research timeline if managed effectively.
- Type C: These programs involve drugs that are similar to already existing therapies, focusing on enhancements in efficacy or safety profiles. Understanding the nuances of Type C applications is critical for navigating competitive therapeutic landscapes.
Recognizing the distinctions among these categories is essential, as each type carries specific regulatory requirements that can significantly impact the IND research term process. Our comprehensive clinical trial management services, which encompass feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting, are designed to assist researchers through these complexities. For example, our service of reviewing and offering feedback on research documents ensures adherence to national requirements, which is crucial for the approval of IND submissions.
Additionally, our reporting services keep stakeholders informed about project status, inventory, and any adverse events, thereby enhancing transparency and facilitating timely decision-making.
Notably, only about 9% of IND submissions are placed on clinical hold, indicating that thorough preparation can mitigate potential delays. As highlighted in the case study titled "Regulatory Documentation Verification," preparing a regulatory-compliant IND application is labor-intensive and requires strategic planning, with all necessary documentation—such as Informed Consent Forms, IRB authorization, and Investigator Brochures—essential to include. Regulatory experts, including Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasize that successful preparation and submission of the IND research term require substantial strategic planning and precise timing.
Collaborating with external regulatory specialists can streamline the procedure and ensure compliance with the latest FDA regulations and guidance. Furthermore, for those looking to deepen their understanding of the IND, CDER offers courses on Chemistry, Manufacturing, and Controls (CMC) related to the IND.
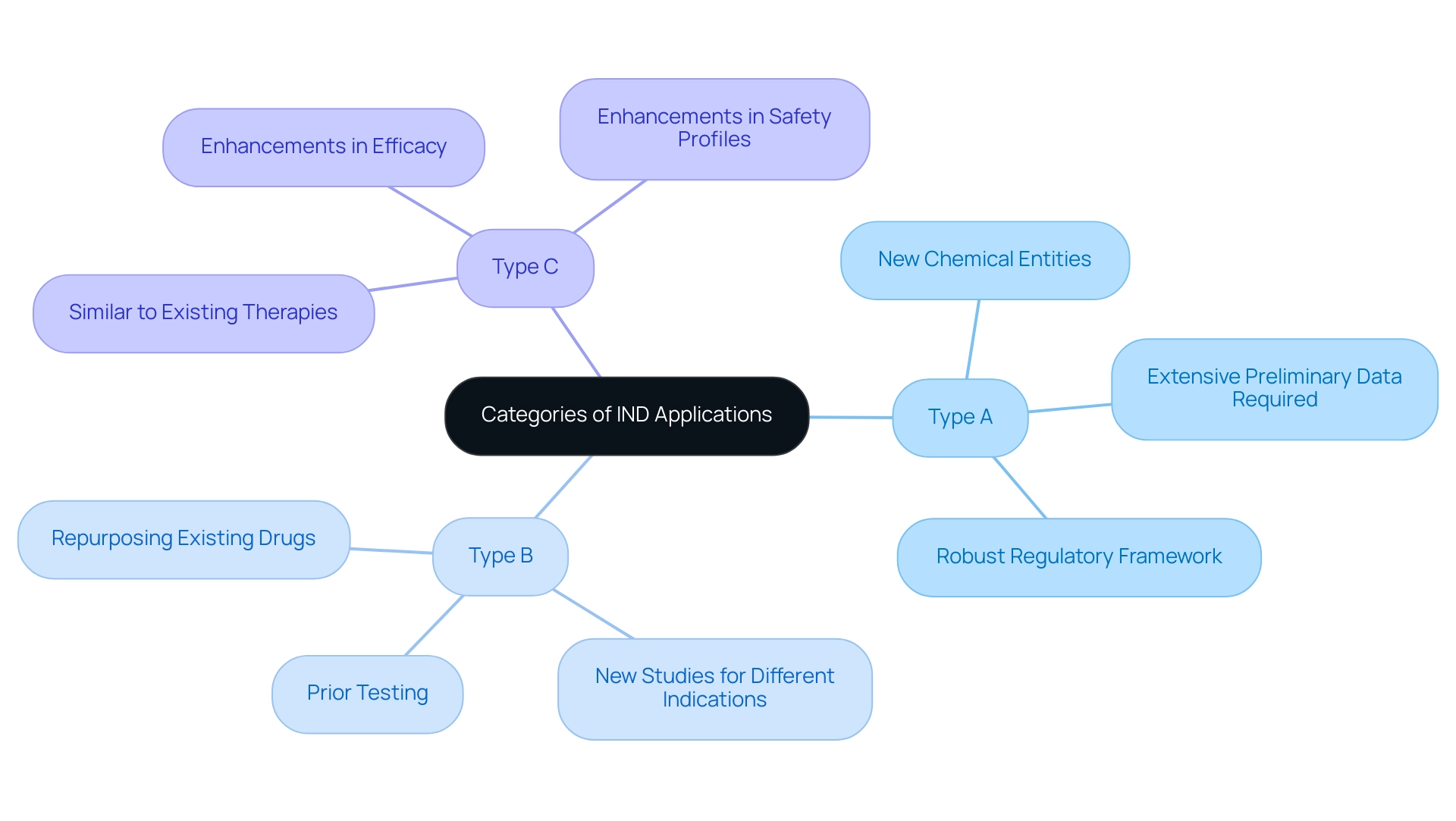
How to Complete an IND Application: A Step-by-Step Guide
Completing an Investigational New Drug (IND) submission is a multi-faceted process that encompasses several critical steps:
- Compile Preclinical Data: Gather comprehensive information from laboratory and animal research to establish the drug’s safety and efficacy. This compilation should provide a robust foundation for your project, as it plays a crucial role in demonstrating the need for human trials.
- Draft the IND Submission: Develop a detailed IND submission that includes essential sections such as administrative information, study protocols, investigator information, and informed consent documents. Each component must be meticulously crafted to meet FDA standards and facilitate a smooth evaluation.
- Submit to the FDA: Ensure that your request is complete and submit it electronically, if feasible. This not only accelerates the submission procedure but also aligns with current best practices for regulatory compliance. There are several types of IND research term submissions to consider: Research IND, Emergency Investigational New Drug (EIND), and Treatment IND, each serving different purposes in the drug development process.
- Respond to FDA Queries: Be prepared to address any inquiries or concerns raised by the FDA during their review. This responsiveness is essential, as major issues may lead the agency to revise cautionary statements or necessitate additional submissions for any changes.
In the context of effective clinical trial management, services such as feasibility assessments, site selection, compliance reviews, trial setup, import permits, and project management are essential for the success of the IND research term applications. For instance, trial setup involves coordinating with various stakeholders to ensure that the research is properly designed and compliant with regulatory requirements. Import permits are crucial for the legal entry of investigational devices, while project management ensures that all aspects of the study are executed efficiently and within timelines.
The expertise of professionals such as Katherine Ruiz in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia adds substantial value to this endeavor. By following these steps diligently, you can enhance the likelihood of a successful submission, which is crucial considering that the average time taken to complete IND processes can range from several months to over a year. The systematic investigation process in clinical trials, as outlined in the case study, further emphasizes the importance of thorough preparation in the journey of drug development.
Wuxi AppTec aptly states, > every drug can be made and every disease can be treated <, highlighting the critical nature of these steps. In 2024, continuing to adapt and refine these steps will be essential for navigating the complexities of IND submissions effectively.
Timeline for IND Applications: What to Expect
The timeline for the IND research term submissions is critical for project planning and resource allocation. It generally encompasses several key phases:
- Pre-Submission Preparation: This phase typically spans from 6 months to 1 year, during which researchers compile the necessary safety and efficacy data and draft their proposal. Thorough preparation during this stage is vital for a smooth submission process.
- FDA Review: Once submitted, the FDA has a statutory period of 30 days to review the IND request. During this time, the agency may reach out for additional information or clarification on specific aspects of the submission. Recent statistics indicate that while the initial review period is set at 30 days, actual approval timelines can vary significantly based on the complexity of the submission, with some submissions taking several months to over a year for final approval. This step is crucial as it determines whether the application will move forward to the next stage.
- Clinical Trial Initiation: Upon approval, researchers can commence their clinical trials. The timeline for this phase is influenced by the research design and can vary widely depending on the nature of the trial. Grasping these timelines is crucial for efficient project planning, especially concerning thorough clinical trial management services, including feasibility assessments, site selection, and the trial establishment and initiation stages, which are vital for regulatory compliance as part of the IND research term. Additionally, the importance of compliance reviews and project management cannot be overstated in ensuring that all regulatory requirements are met. This includes the review and feedback on study documents to comply with country requirements, as well as meticulous reporting of study status and inventory, along with serious and non-serious adverse events. Furthermore, the FDA's commitment to advancing innovative therapies is illustrated by the recent granting of Regenerative Medicine Advanced Therapy (RMAT) designation to two cell therapies and one gene therapy, showcasing the agency's support for breakthrough treatments. As noted by Erica Kratz,
The Food and Drug Administration is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs.
This underscores the importance of navigating the IND process with diligence and awareness of the FDA's review protocols, particularly in light of the ongoing need for a better understanding of safety and efficacy evidence required for pharmaceutical product development.

Conclusion
The journey through the Investigational New Drug (IND) application process is a multifaceted and demanding endeavor, crucial for advancing medical research and patient care. From the initial compilation of preclinical data to the meticulous drafting of the application, each step requires careful planning and adherence to regulatory standards. Understanding the distinctions among the various types of IND applications—commercial, research, and emergency—enables researchers to tailor their approach effectively, ensuring that their objectives align with regulatory expectations.
Moreover, recognizing the different categories of IND applications—Type A, B, and C—further emphasizes the need for strategic preparation. Each type presents unique challenges and requirements that can impact the progress of clinical research. Engaging with experienced regulatory consultants and utilizing comprehensive clinical trial management services can significantly streamline the process, mitigating potential delays and enhancing compliance with FDA regulations.
The timeline for IND applications underscores the importance of thorough preparation and responsiveness throughout the review process. With a typical pre-submission phase lasting from six months to a year, and the FDA's review taking an additional 30 days or longer, meticulous planning is essential for timely project execution. As the pharmaceutical landscape continues to evolve, understanding and navigating the complexities of the IND process remains paramount for researchers dedicated to bringing innovative therapies to market.
In conclusion, the IND application process is not merely a regulatory hurdle; it is a vital gateway that supports the development of new therapies, ultimately enhancing patient care and advancing medical science. By prioritizing strategic planning, compliance, and effective project management, researchers can contribute to a future where groundbreaking treatments become a reality.
Frequently Asked Questions
What is an Investigational New Drug (IND)?
An Investigational New Drug (IND) is a drug that has not yet received FDA approval for general use but is undergoing clinical trials to assess its safety and efficacy.
What is required before starting human testing for an IND?
Researchers must submit a comprehensive request to the FDA that meets the IND research term, including extensive data from rigorous preclinical studies that provide insights into the drug's biological activity and potential risks.
Why is securing IND status important?
Securing IND status allows researchers to commence clinical trials, ensuring that the drug can be safely studied in human populations, protecting participant welfare, and establishing the foundation for further drug development.
What are the main types of IND applications?
The three primary types of IND applications are: 1. Commercial IND: Submitted by pharmaceutical companies for drugs intended for commercial sale. 2. Research IND: Submitted by investigators or academic institutions for non-commercial clinical research. 3. Emergency IND: Permits the use of an investigational drug in urgent situations where no alternative treatments are available.
What challenges are associated with preparing an IND application?
Preparing a regulatory-compliant IND application is labor-intensive and time-consuming, requiring substantial strategic planning and precise timing.
What clinical trial management services are essential for IND preparation?
Essential services include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What role do regulatory consultants play in the IND process?
Regulatory consultants, such as Katherine Ruiz, can streamline the preparation and submission of IND applications by providing expertise in compliance with the latest FDA regulations and guidance.
What resources are available for understanding the IND process?
Resources like the CDER Learn Courses offer industry professionals opportunities to enhance their understanding of the IND process, particularly focusing on the Chemistry, Manufacturing, and Controls (CMC) perspective.

