Introduction
In the rapidly evolving landscape of medical devices, compliance with regulatory standards is paramount, particularly in Latin America where diverse regulations present both challenges and opportunities. Companies aiming to penetrate this market must navigate a complex web of local requirements governed by authorities such as:
- INVIMA in Colombia
- ANVISA in Brazil
- COFEPRIS in Mexico
Understanding the nuances of these regulations is crucial for ensuring the safety and efficacy of medical products while facilitating a successful market entry. Through strategic partnerships with local experts and a robust compliance framework, organizations can enhance their operational efficiency and accelerate their growth in this competitive region.
This article delves into the essential steps for achieving regulatory compliance, the importance of local partnerships, and the ongoing monitoring required to maintain adherence to evolving standards, ultimately guiding companies towards success in the Latin American medical device market.
Establishing Compliance: Key Steps for Medical Device Companies in Latin America
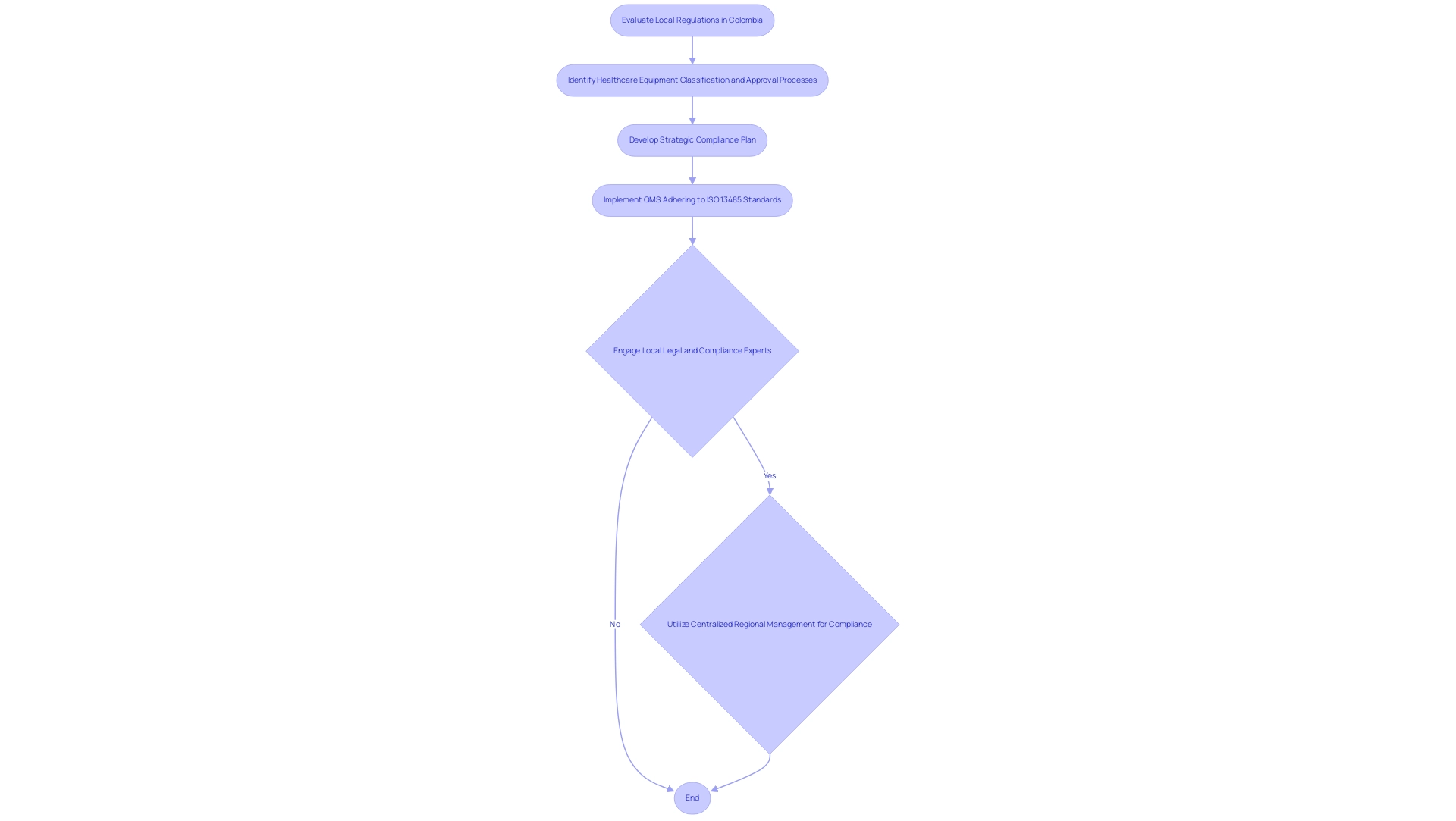
To ensure Medical Device Trials Compliance Latin America, healthcare product companies must start their process in Colombia with a thorough evaluation of the various local regulations and standards that oversee the market, particularly those established by INVIMA (Colombia National Food and Drug Surveillance Institute). This evaluation must include the specific requirements concerning healthcare equipment classification, registration, and approval processes relevant to each country in the region. INVIMA, acknowledged as a Level 4 health authority by PAHO/WHO, plays a crucial role in ensuring the safety and efficacy of health products, including therapeutic instruments.
The agency is responsible for categorizing healthcare instruments based on their risk levels, which affects the registration and approval timeline. Notably, US manufacturers control more than 50% of the Mexican import market, highlighting the competitive landscape in which companies must navigate these regulations. A strategic compliance plan should be developed for Medical Device Trials Compliance Latin America, which includes:
- Specialized training for staff on legal obligations
- Establishment of a quality management system (QMS) that adheres to ISO 13485 standards
As highlighted by Monica Mabel Guaita, CEO of MMGC SRL, "Effective navigation of Medical Device Trials Compliance Latin America is essential for success in the healthcare equipment market." Considering the complexities of navigating these regulations, involving local legal and compliance experts in Medical Device Trials Compliance Latin America, such as Katherine Ruiz, a specialist in affairs for medical devices and in vitro diagnostics, is essential. Their insights can significantly enhance a company's understanding of Medical Device Trials Compliance Latin America requirements, thus ensuring a more efficient market entry.
Furthermore, the case study on centralized regional management of compliance affairs illustrates how manufacturers can group countries and manage compliance issues more efficiently, reducing costs and delays in registrations. This approach enables manufacturers to start in one country and expand to others, optimizing documentation use and facilitating market entry across the region.
Navigating the Regulatory Landscape for Medical Device Trials in Latin America
Navigating the regulatory landscape in Latin America, which has a population of around 650 million individuals, necessitates a comprehensive understanding of the specific regulatory authorities overseeing approvals for health-related equipment in each nation.
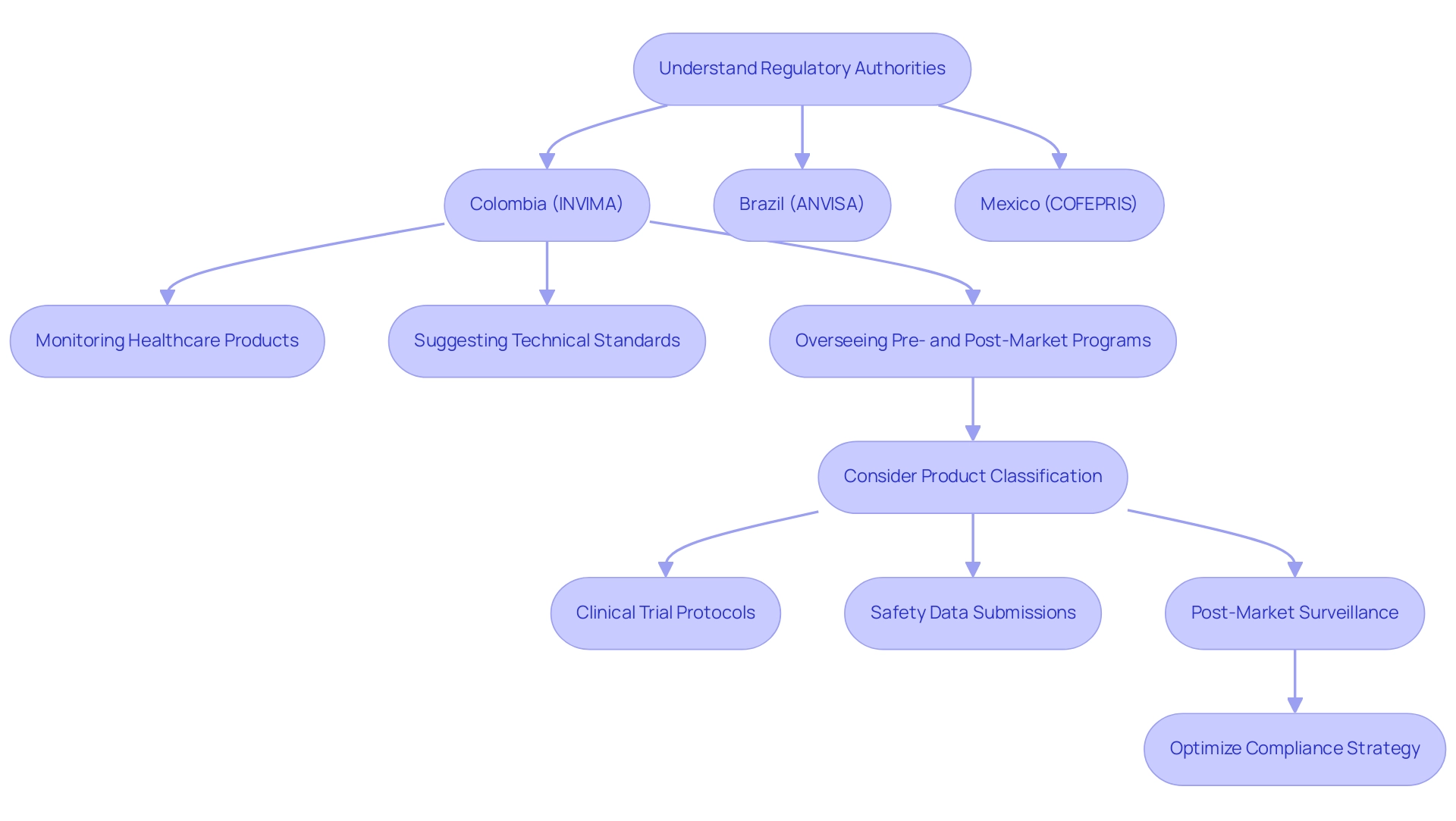
In Colombia, the INVIMA (Colombia National Food and Drug Surveillance Institute), created under the Ministry of Health and Social Protection, plays a crucial role in inspecting and overseeing the promotion and production of health products, including healthcare tools. The Directorate for Healthcare Instruments and other Technologies within INVIMA is tasked with:
- Monitoring and controlling healthcare products
- Suggesting technical standards
- Overseeing pre- and post-market programs to ensure adherence to health regulations
As a Level 4 regional reference health authority acknowledged by PAHO/WHO, INVIMA ensures the safety, efficacy, and quality of medical devices through rigorous oversight and adherence to health standards.
In Brazil, ANVISA (Agencia Nacional de Vigilância Sanitária) supervises the compliance process, while in Mexico, the responsibility lies with COFEPRIS (Comisión Federal para la Proteccion contra Riesgos Sanitarios). Familiarity with these organizations is essential for ensuring compliance with local regulations.
Furthermore, the categorization of the medical instrument plays a crucial role in establishing the oversight pathway, as different classes may be subject to distinct levels of scrutiny and approval requirements. Companies must be prepared for potential discrepancies in approval timelines, which can be influenced by factors such as:
- Clinical trial protocols
- Safety data submissions
- Post-market surveillance obligations
Creating a clear timeline for submissions and approvals is essential for managing expectations and streamlining the process.
Significantly, products that have previously obtained clearance from the US FDA or EU can accelerate the compliance process in Mexico, highlighting the interconnectedness of global standards. A case study titled 'Mexico’s Approval Process' illustrates that Mexico's framework for health products differs significantly from that of Mercosur countries, with a more straightforward approval process for products already authorized by the US FDA or EU.
By strategically addressing these key considerations and understanding the distinctions in compliance frameworks, companies can navigate the complexities of medical device trials compliance in Latin America more effectively, leveraging insights from experts like Katherine Ruiz to enhance their strategies.
The Role of Local Partnerships in Ensuring Compliance
Forming alliances with local consultants, legal advisors, and clinical research organizations is vital for improving Medical Device Trials Compliance Latin America. As demonstrated by Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, and Dr. John B. Simpson of Avinger, collaboration with LATAM CRO experts not only facilitates Medical Device Trials Compliance Latin America by providing access to diverse patient populations for clinical trials but also offers valuable insights into local market dynamics, which are critical for tailoring strategies to regional needs. Local advisors typically provide services such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Project management
All of which are essential for ensuring Medical Device Trials Compliance Latin America while navigating the legal landscape effectively.
For example, Katherine Ruiz, a compliance affairs expert with extensive experience at INVIMA, emphasizes the significance of understanding the specific legal landscape for Medical Device Trials Compliance Latin America in order to navigate the often complex approval processes with greater efficiency. Cultivating strong connections with key stakeholders is essential for establishing trust and ensuring smoother interactions with oversight authorities regarding Medical Device Trials Compliance Latin America. Furthermore, the recent classification of INVIMA as a Level 4 health authority by PAHO/WHO highlights its proficiency in regulating healthcare products, which is vital for achieving Medical Device Trials Compliance in Latin America, making local collaborations even more essential.
As emphasized by Phyllis Meng, CEO of Pure Global, integrating local insights is invaluable, enabling companies to leverage data from over 30 markets effectively. Furthermore, Pure Global's successful acquisition of two Class II Medical Device Certificates in Brazil illustrates how local collaborations can result in favorable outcomes in the context of Medical Device Trials Compliance Latin America. The backing from platforms like Greenlight Guru, a quality management system for medical equipment firms, further improves the regulatory environment, promoting sustainable growth in the area.

Understanding Regulatory Requirements for Medical Device Trials
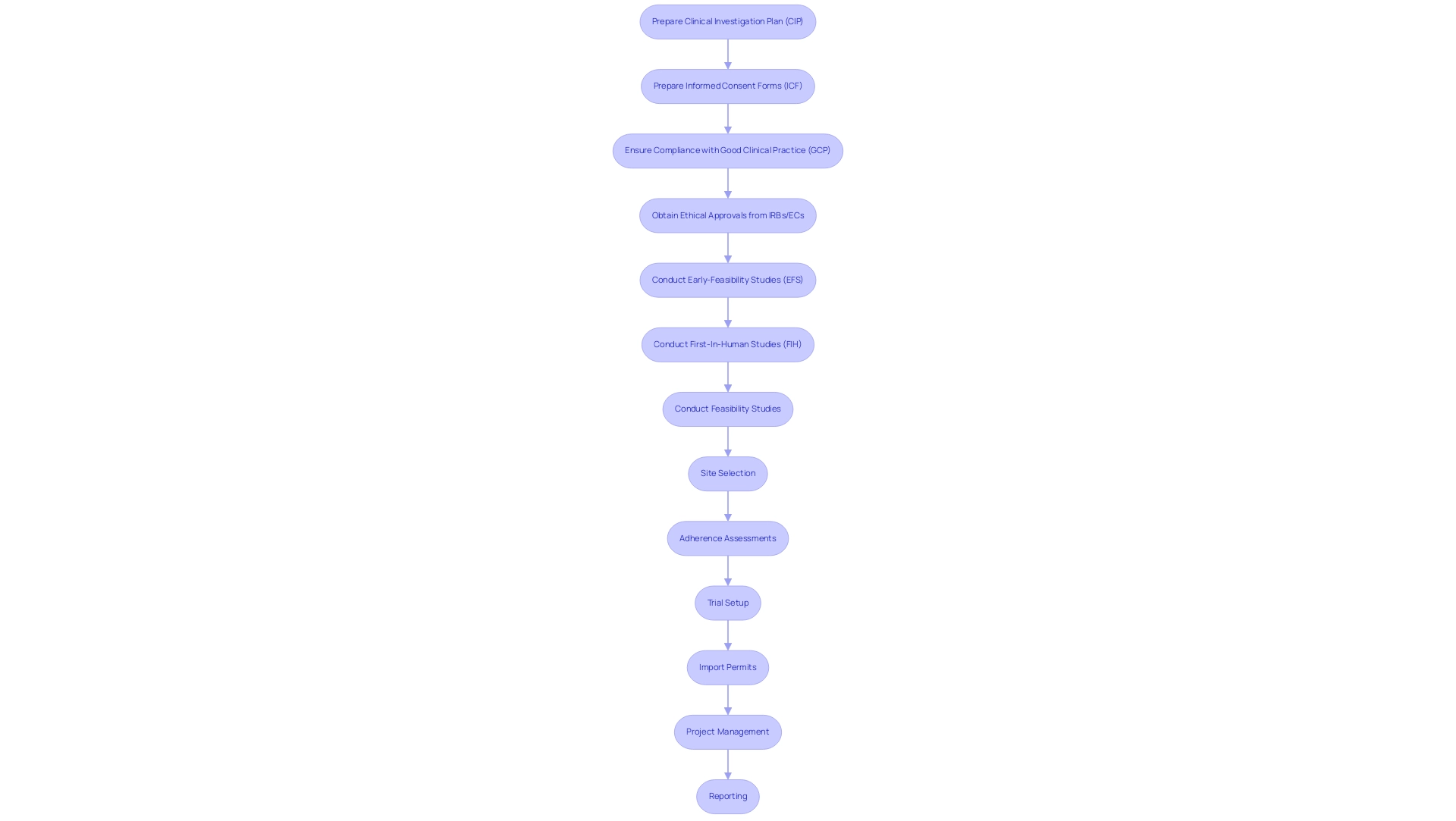
Medical device trials compliance in Latin America requires strict adherence to specific legal requirements, notably the preparation of vital documentation such as the Clinical Investigation Plan (CIP) and Informed Consent Forms (ICF). To achieve Medical Device Trials Compliance Latin America, companies conducting these trials must ensure that all protocols are fully compliant with Good Clinical Practice (GCP) guidelines, which are essential for maintaining the integrity and quality of clinical data. Furthermore, obtaining ethical approvals from Institutional Review Boards (IRBs) or Ethics Committees (ECs) is a fundamental step in achieving Medical Device Trials Compliance in Latin America.
In Colombia, the country stands out as the only one in Latin America with a GCP institutional-level certification, which guarantees that the clinical data exported meets high-quality standards for Medical Device Trials Compliance Latin America. According to Julio G. Martinez-Clark, co-founder and CEO of bioaccess®, 'Thanks to the efforts made by the different actors in the health sector, Colombia is a powerhouse in clinical research in Latin America.' This is underscored by the fact that five Colombian research centers are classified among the 15 best in Latin America, which highlights the quality of clinical trials and the importance of Medical Device Trials Compliance Latin America conducted in the country.
bioaccess® offers extensive clinical trial management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Feasibility studies
- Site selection
- Adherence assessments
- Trial setup
- Import permits
- Project management
- Reporting
To ensure Medical Device Trials Compliance Latin America, it is imperative to meticulously record all trial-related activities and document any adverse events to maintain transparency and adherence to regulations throughout the trial lifecycle. Such diligence not only enhances the credibility of the research but also aligns with the best practices recognized in the region, ensuring the successful execution of Medical Device Trials Compliance Latin America.
Furthermore, it is advantageous to evaluate the benefits of performing clinical trials in nations such as Chile, which provides a varied patient population, cost-effectiveness, rapid regulatory approval, and high-quality data gathering for healthcare companies.
Ensuring Ongoing Compliance and Monitoring Post-Approval
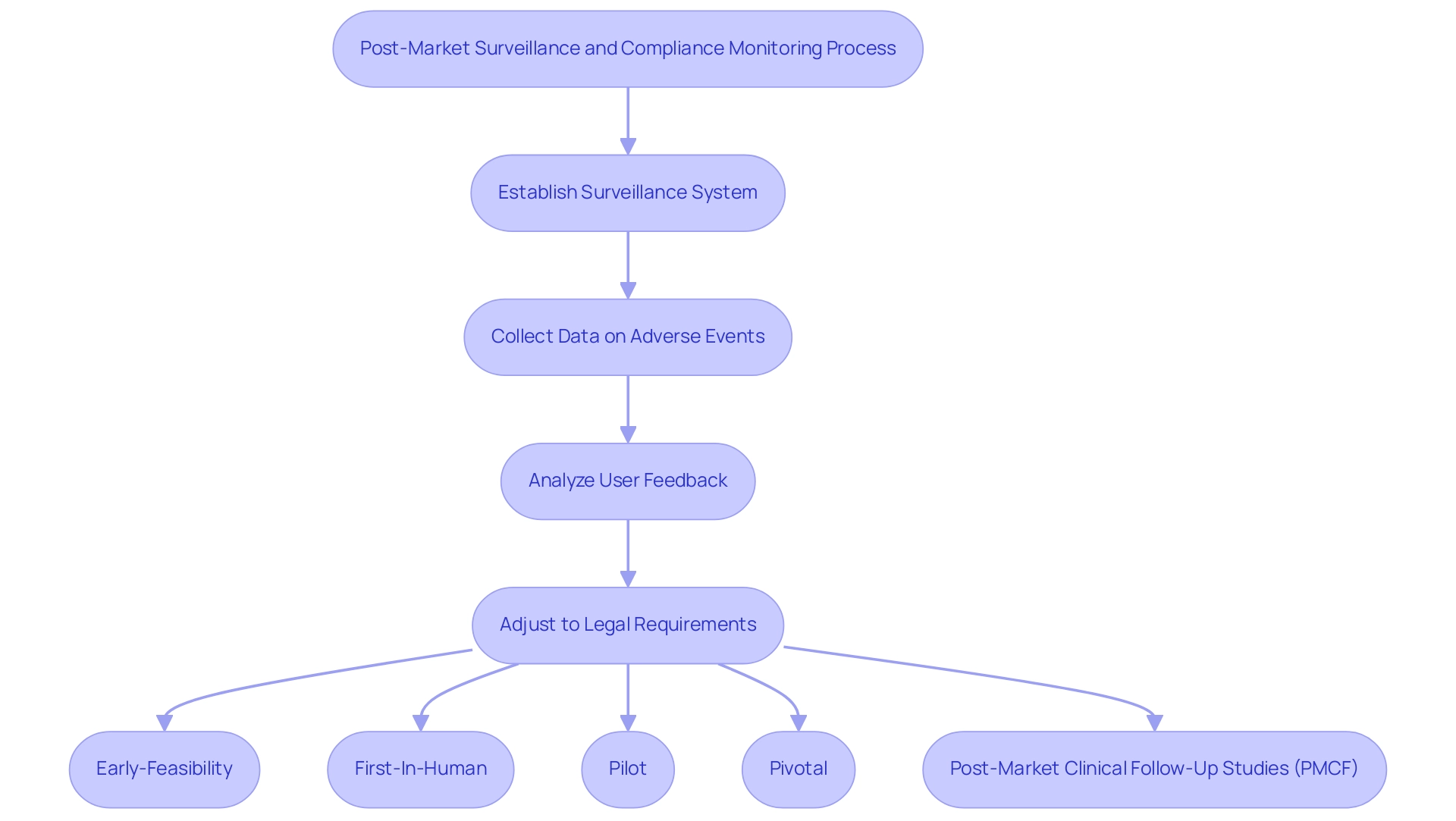
Following the approval of a healthcare instrument, it is essential for companies to establish a strong post-market surveillance system to actively monitor the instrument's performance and safety in real usage contexts. This process demands a systematic approach to collecting and analyzing data on adverse events, as well as user feedback, to swiftly identify any potential issues. As L.M. Koch et al. highlight, efficient post-market monitoring is crucial for guaranteeing the safety and effectiveness of healthcare products in practical settings.
In Latin America, bioaccess® leverages over 20 years of Medtech expertise and a flexible, specialized approach to guide companies through various clinical studies while ensuring Medical Device Trials Compliance Latin America, including:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Clinical Follow-Up Studies (PMCF)
According to recent findings, the adverse event reporting rates for healthcare products are projected to evolve in 2024, highlighting the need for vigilance in tracking safety after approval. Businesses need to stay flexible, adjusting to alterations in legal requirements and incorporating best practices into their adherence strategies, especially considering INVIMA's strict supervision as a Level 4 health authority acknowledged by PAHO/WHO.
The case study on emerging global markets illustrates that as global health expenditure rises, domestic medical device companies have opportunities to expand their market presence beyond the U.S., highlighting the importance of Medical Device Trials Compliance Latin America in ensuring effective post-market surveillance.
Regular internal audits and comprehensive training for staff on adherence protocols are essential components that foster a culture of following regulatory standards. Additionally, insights from Gretton et al. regarding machine learning research related to maximum mean discrepancy can inform ongoing compliance monitoring strategies.
As the global medical device market anticipates a growth rate of 5.9% annually until 2030, with sales expected to approach $800 billion, the importance of effective post-market surveillance cannot be overstated. This proactive approach not only safeguards public health but also positions companies favorably within the increasingly competitive landscape of emerging global markets, aligning with the services offered by bioaccess®.
Conclusion
Navigating the regulatory landscape for medical devices in Latin America is a multifaceted endeavor that requires a strategic approach to compliance. Companies must start with a comprehensive assessment of the regulations specific to each country, particularly the roles of:
- INVIMA in Colombia
- ANVISA in Brazil
- COFEPRIS in Mexico
By understanding the classification and approval processes, organizations can better position themselves to meet the requirements set forth by these regulatory authorities, which is essential for ensuring the safety and efficacy of their products.
Moreover, forming local partnerships with regulatory experts and clinical research organizations is invaluable. These collaborations not only facilitate compliance but also provide critical insights into local market dynamics. Leveraging the expertise of professionals familiar with the regulatory landscape allows companies to navigate the complexities of the approval process more effectively, ultimately enhancing their operational efficiency and market entry strategies.
Finally, ongoing compliance and post-market surveillance are crucial for maintaining the integrity of medical devices once they are on the market. A robust post-market system that actively monitors device performance is necessary to identify potential issues quickly and adapt to evolving regulatory requirements. This proactive stance ensures that organizations remain compliant while safeguarding public health.
In summary, success in the Latin American medical device market hinges on a thorough understanding of local regulations, the establishment of strategic partnerships, and a commitment to ongoing compliance. By embracing these principles, companies can not only navigate the challenges but also seize the opportunities presented by this dynamic and growing market.
Frequently Asked Questions
What is the first step for healthcare product companies to ensure Medical Device Trials Compliance in Latin America?
Companies must start their process in Colombia with a thorough evaluation of local regulations and standards, particularly those established by INVIMA (Colombia National Food and Drug Surveillance Institute).
What role does INVIMA play in medical device compliance?
INVIMA categorizes healthcare instruments based on their risk levels, oversees the registration and approval processes, and ensures the safety and efficacy of health products.
What should a strategic compliance plan include for Medical Device Trials in Latin America?
A strategic compliance plan should include specialized training for staff on legal obligations and the establishment of a quality management system (QMS) that adheres to ISO 13485 standards.
Why is it important to involve local legal and compliance experts in the compliance process?
Involving local experts can significantly enhance a company's understanding of Medical Device Trials Compliance requirements, ensuring a more efficient market entry.
How can manufacturers manage compliance issues more efficiently across different countries?
Manufacturers can group countries for centralized regional management of compliance affairs, which helps reduce costs and delays in registrations.
Which regulatory authorities oversee medical device compliance in Brazil and Mexico?
In Brazil, the regulatory authority is ANVISA (Agencia Nacional de Vigilância Sanitária), while in Mexico, it is COFEPRIS (Comisión Federal para la Protección contra Riesgos Sanitarios).
What documentation is essential for achieving Medical Device Trials Compliance in Latin America?
Essential documentation includes the Clinical Investigation Plan (CIP) and Informed Consent Forms (ICF), along with compliance with Good Clinical Practice (GCP) guidelines.
What unique certification does Colombia hold regarding GCP?
Colombia is the only country in Latin America with a GCP institutional-level certification, ensuring that clinical data exported meets high-quality standards.
What is the importance of post-market surveillance after the approval of a healthcare instrument?
Post-market surveillance is crucial for actively monitoring the instrument's performance and safety, collecting data on adverse events, and ensuring ongoing compliance with regulations.
How can companies stay compliant with evolving legal requirements in Latin America?
Companies should conduct regular internal audits, provide comprehensive training for staff on adherence protocols, and remain flexible to adjust to changes in legal requirements.




