Introduction
Informed consent stands as a foundational pillar in the realm of clinical research, embodying the ethical commitment to participant autonomy and informed decision-making. This essential process transcends mere regulatory compliance; it fosters a transparent dialogue between researchers and participants, ensuring that individuals are fully aware of the study's purpose, potential risks, and their rights.
As the landscape of clinical trials evolves, the importance of clear and comprehensible consent documents cannot be overstated, particularly in light of recent findings that highlight significant gaps in participant understanding.
This article delves into the intricacies of informed consent, exploring its key elements, the step-by-step process for obtaining it, special considerations for vulnerable populations, and best practices for documentation. By examining these facets, the discussion aims to reinforce the critical role of informed consent in upholding ethical standards and enhancing the integrity of clinical research.
Understanding Informed Consent: Definition and Importance
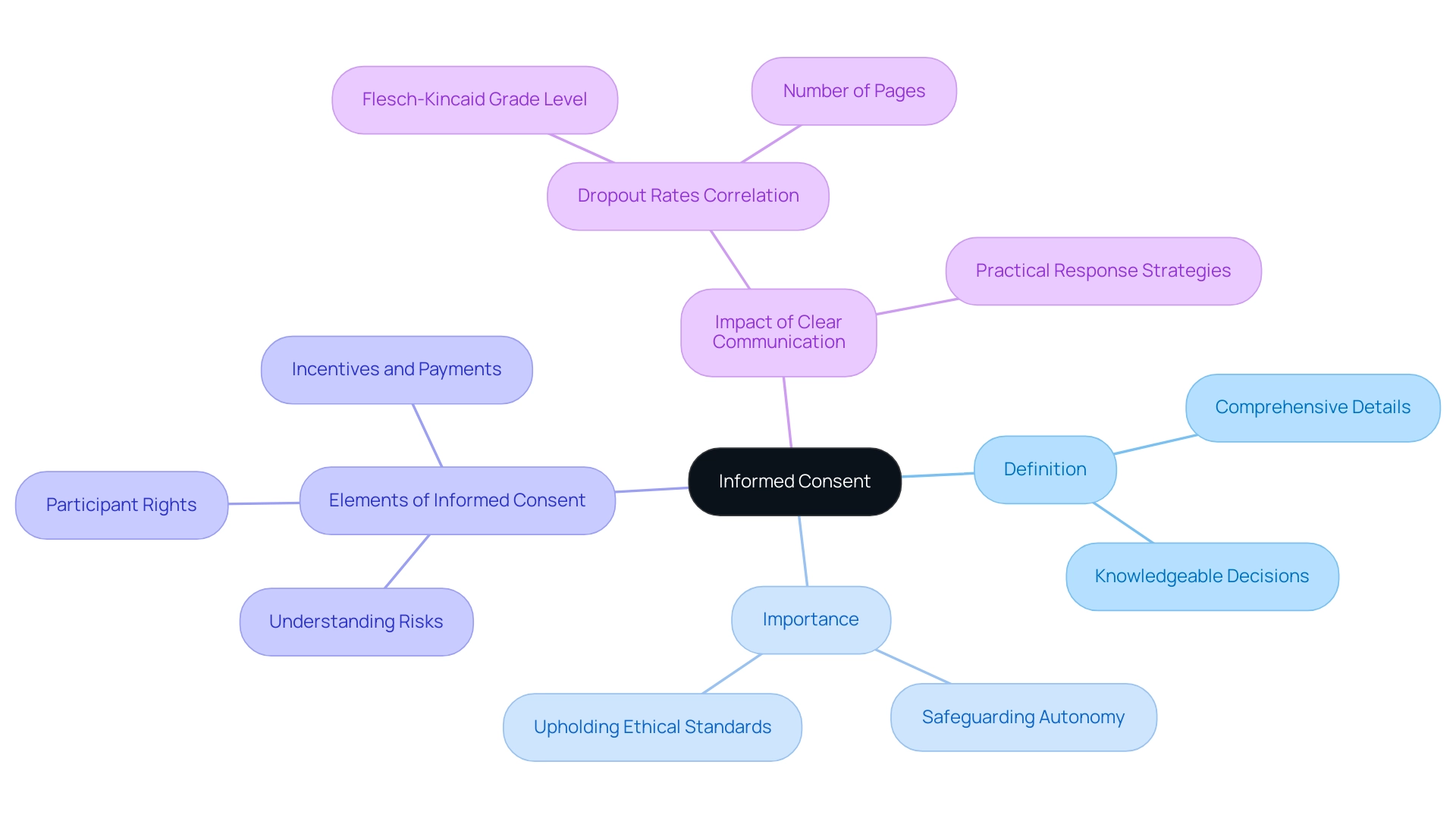
Informed agreement is an essential procedure in clinical research, and your informed consent must describe the comprehensive details about the research that researchers provide to potential subjects. This allows individuals to make knowledgeable decisions regarding their involvement. Instead of being a simple formality, your informed consent must describe a continuous conversation that ensures individuals completely understand the study's purpose, inherent risks, possible advantages, their rights as contributors, and the incentives and payments for their time, inconvenience, and discomfort.
The importance of knowledgeable approval is highlighted by the fact that your informed consent must describe its role in safeguarding participant autonomy and upholding ethical standards in research practices. As emphasized by recent discoveries released in June 2024, your informed consent must describe not only a regulatory necessity but also its essential role in the ethical structure of clinical trials. Researchers are ethically bound to ensure that your informed consent must describe individuals' rights to make informed choices about their participation, which is essential to the credibility of clinical research and the trust placed in the scientific community.
A notable study titled 'Association Between Form Metrics and Dropout Rates' revealed that each additional increase in the Flesch-Kincaid Grade Level of forms correlated with a 16% rise in dropout rates, while the number of pages did not significantly predict dropout rates. This highlights the significance of clear communication, as your informed consent must describe the necessary aspects in the approval process. As Reviewer #2 noted, 'The response strategies provided by the authors are practical, feasible and drawn from interview data collected from experts with direct experience in relevant fields.'
Acknowledging these factors guarantees that your informed consent must describe educated agreement as a fundamental element of ethical research practices.
Key Elements of an Informed Consent Document
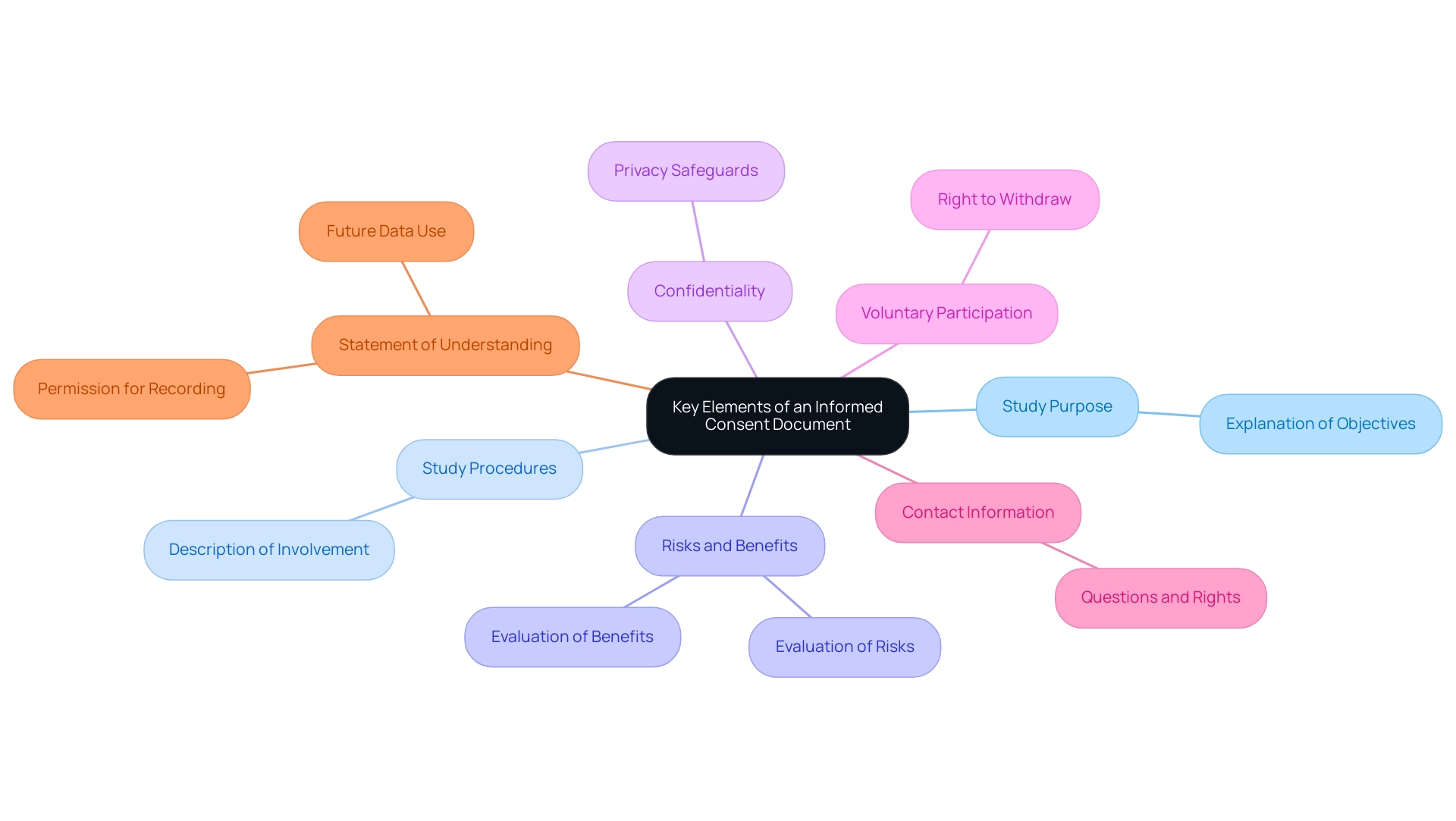
An effective informed consent document must encompass several key elements to ensure that individuals are well-informed and their rights are protected:
- Study Purpose: A comprehensive explanation outlining the objectives of the study, ensuring individuals grasp why the research is being conducted.
- Study Procedures: A thorough description of what involvement entails, detailing any tests, treatments, or interventions that individuals will undergo.
- Risks and Benefits: A transparent evaluation of the potential risks and benefits associated with participation, allowing individuals to make an informed decision.
- Confidentiality: Clear information about how individuals' privacy will be safeguarded, including specifics on data use and sharing practices.
- Voluntary Participation: A strong statement affirming that involvement is entirely voluntary, with reassurance that individuals can withdraw at any time without facing any penalties.
- Contact Information: Essential details for whom individuals can reach out to with questions regarding the study or their rights, fostering open communication.
- Statement of Understanding: An explicit statement confirming that individuals understand the information provided, including their permission for audio/video recording and future data use.
Incorporating these elements not only promotes transparency but also means that your informed consent must describe the trust cultivated between researchers and individuals. Notably, recent findings indicate that only 53% of individuals reported understanding critical aspects, such as the concept of a placebo, underscoring the need for clarity in consent documents. Moreover, ethics committees highlight the significance of openness, especially considering recent conversations about insufficient disclosure, where individuals may not initially be aware of the project's complete intent to prevent skewing responses, yet are assured a debriefing at the end of their engagement.
This ethical consideration is essential for maintaining trust and integrity in research.
Additionally, a case study titled 'Reliability Testing of the Abstraction Tool' demonstrates the practical application of these elements. Two reviewers independently evaluated 250 approval documents to assess the quality of awareness, achieving high agreement (>90%) across all items, which underscores the effectiveness of structured approval documents in enhancing participant comprehension.
The Informed Consent Process: Step-by-Step Guide
To effectively obtain informed consent in healthcare and research, follow this structured approach:
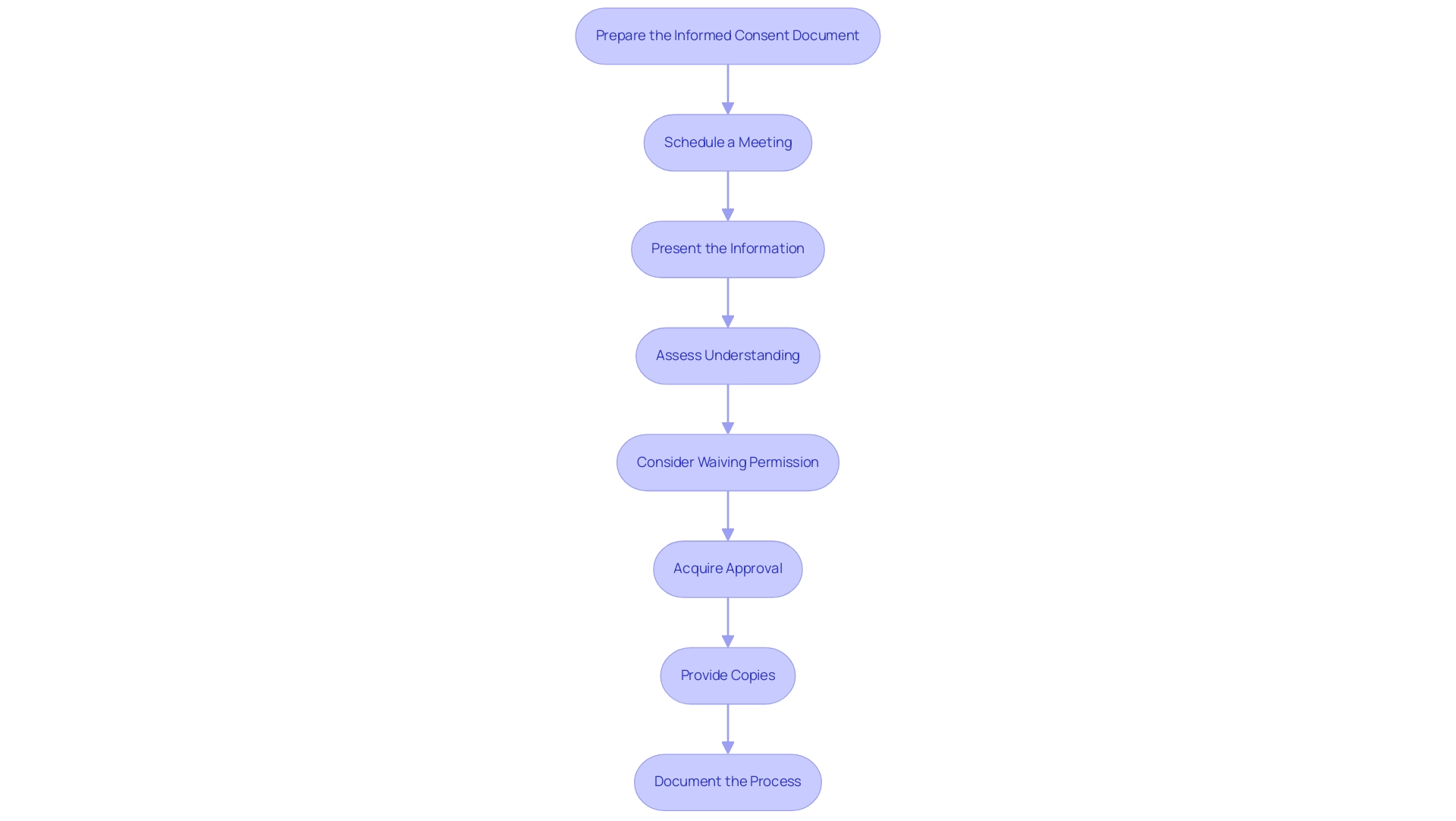
- Prepare the Informed Consent Document: Your informed consent must describe all essential elements as outlined by federal regulations (45 CFR 46.116), which dictate the necessary information for compliance and transparency in the consent process.
- Schedule a Meeting: Organize a private and confidential gathering with prospective individuals to discuss the research in detail, fostering an environment conducive to open dialogue.
- Present the Information: Clearly articulate the study's purpose, procedures, potential risks, and benefits. It is essential to motivate individuals to ask questions and ensure that these inquiries are met with comprehensive responses.
- Assess Understanding: To confirm comprehension, ask participants to paraphrase the information provided. This step is crucial in validating that they understand the details necessary for knowledgeable decision-making, which your informed consent must describe.
- Consider Waiving Permission: Acknowledge that under certain conditions, as pointed out by Amelia Licari, knowledgeable approval can be waived, permitting “research without permission” under strict regulations. This is a significant factor in specific research situations where acquiring approval may not be feasible.
- Acquire Approval: Once individuals are at ease and agree to take part, provide them with the informed agreement document for their signature, ensuring they fully understand what they are agreeing to.
- Provide Copies: After signing, give individuals a copy of the signed document for their records, reinforcing their rights and the transparency of the process.
- Document the Process: It is essential to meticulously record the date, method of agreement, and any relevant discussions or issues raised by individuals involved. This record-keeping is vital for compliance and ethical accountability in research.
Integrating insights from the case study titled "5 Essential Ethical Guidelines for Accurate Research in 2024," it is vital to follow ethical principles that direct the approval process, particularly in developing nations where compliance challenges and apprehension about study procedures may occur. By following these steps, researchers can ensure that your informed consent must describe the awareness agreement process, making it not only comprehensive but also considerate of participants' rights, ultimately improving the integrity and ethical standards of clinical research.
Special Considerations in Informed Consent
When navigating the complexities of informed agreement in special populations, including children or individuals with diminished decision-making capacity, researchers must adhere to several essential guidelines:
- Parental/Guardian Consent: It is imperative to secure consent from a parent or legal guardian while also obtaining assent from the child when appropriate. This dual approach respects the child's emerging autonomy and acknowledges the legal limitations of minors, as highlighted in the case titled 'Informed Consent for Children in Research,' which emphasizes the need for parental permission and the concept of 'assent' to ensure children understand their rights.
- Simplified Language: Researchers should utilize age-appropriate language and tailored materials to facilitate understanding, ensuring that the information provided is accessible to the child’s cognitive level.
- Support for Decision-Making: For individuals with diminished capacity, it is crucial to offer additional support and resources that empower them to comprehend their choices fully. This can involve involving caregivers or advocates who can assist in the decision-making process.
- Ongoing Consent: Consent is not a one-time event but a continuous dialogue. Researchers should encourage inquiries and reassess the individual's comprehension throughout the research, promoting an environment where individuals feel empowered to express concerns or withdraw. Your informed consent must describe the essential considerations for upholding ethical integrity and ensuring that all individuals are treated with the highest respect during the agreement process. Recent statistics show that 15.1% of individuals in research reported a decrease in understanding about approval protocols, highlighting the necessity for clarity and continuous involvement. This statistic emphasizes the crucial significance of effective communication in the decision-making process. Moreover, specialists like Amelia Licari stress that although emergency clinical studies might serve as exceptions to the usual approval requirements, the core principles of respect and understanding must always dominate, especially when interacting with vulnerable groups.

Documenting Informed Consent: Best Practices and Requirements
To ensure thorough documentation of informed agreement, it is essential to adhere to the following best practices:
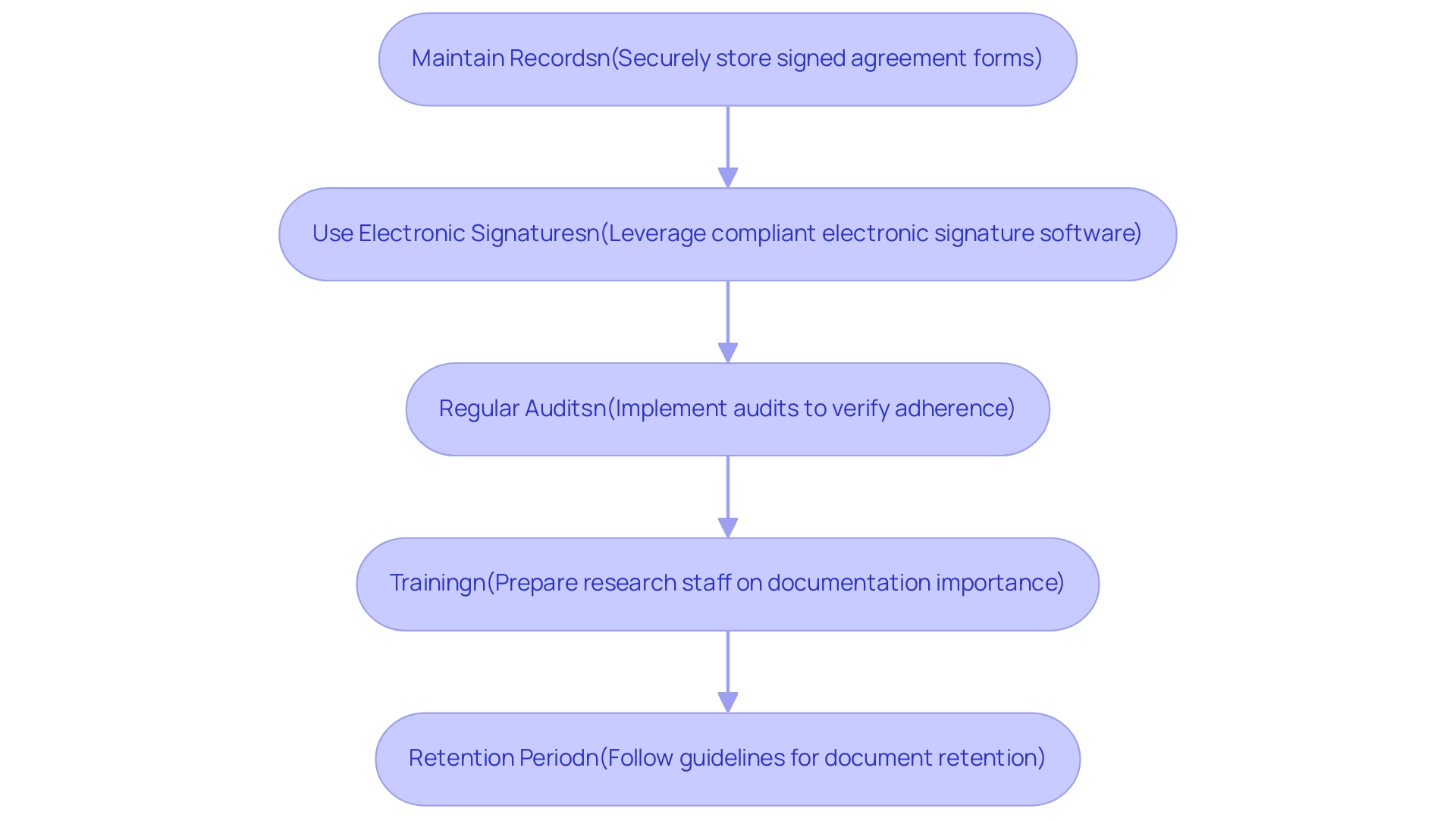
- Maintain Records: Securely store signed agreement forms in a location that is accessible only to authorized personnel, preserving confidentiality and integrity.
- Use Electronic Signatures: When applicable, leverage electronic signature software that meets legal standards for electronic records, ensuring a streamlined process while maintaining compliance.
- Regular Audits: Implement regular audits of documentation to verify adherence to regulatory requirements, identifying any discrepancies and rectifying them promptly.
- Training: Invest in comprehensive preparation for research staff on the critical nature of knowledgeable agreement documentation, emphasizing that your informed consent must describe its importance and the specific requirements pertinent to your study. Dr. Ezekiel J. Emanuel stresses that effective training is vital for upholding high standards in awareness practices.
- Retention Period: Follow institutional guidelines concerning the retention period of approval documents, ensuring adherence to legal and ethical standards by keeping records for the specified duration.
By incorporating these best practices, researchers can improve the integrity of the approval process and ensure compliance with changing regulatory standards. Recent research suggests that simplified language and shorter documents—averaging 8.4 words in length and achieving a readability score of 61.9—significantly enhance comprehension. A case study on informed consent language for COVID-19 vaccine trials highlighted that lengthy and complex documents often fail to adequately inform participants, reinforcing the need for ongoing evaluation and improvement in consent practices.
Conclusion
Informed consent is not merely a procedural requirement but a fundamental ethical obligation that ensures participants are fully informed and empowered in clinical research. This article has explored the intricate layers of informed consent, emphasizing its critical role in protecting participant autonomy and maintaining the integrity of research practices. Key elements such as study purpose, risks, voluntary participation, and confidentiality must be clearly articulated to foster trust and understanding between researchers and participants.
The step-by-step process for obtaining informed consent underscores the importance of clear communication and participant engagement. It is essential to not only present information but also to verify understanding through interactions that encourage questions and dialogue. Special considerations for vulnerable populations, including children and individuals with diminished decision-making capacity, further highlight the need for tailored approaches that respect the rights and dignity of all participants.
Best practices in documenting informed consent are crucial for compliance and ethical accountability. By maintaining meticulous records, utilizing electronic signatures, and conducting regular audits, researchers can ensure that the informed consent process is transparent and adheres to evolving regulatory standards. Ultimately, the commitment to informed consent reinforces the ethical framework of clinical trials, fostering trust and credibility within the scientific community. As the landscape of clinical research continues to evolve, prioritizing informed consent remains essential for protecting participant rights and enhancing the quality of research outcomes.
Frequently Asked Questions
What is informed consent in clinical research?
Informed consent is an essential procedure that describes comprehensive details about the research, allowing individuals to make knowledgeable decisions regarding their involvement. It is a continuous conversation ensuring participants understand the study's purpose, risks, benefits, rights, and any incentives or payments.
Why is informed consent important in clinical research?
Informed consent safeguards participant autonomy and upholds ethical standards in research practices. It is both a regulatory necessity and a crucial element of the ethical framework of clinical trials, ensuring individuals can make informed choices about their participation.
What are the key elements that must be included in an informed consent document?
The key elements include: 1. Study Purpose: Explanation of the study's objectives. 2. Study Procedures: Description of what participation entails. 3. Risks and Benefits: Evaluation of potential risks and benefits. 4. Confidentiality: Information on privacy protection and data use. 5. Voluntary Participation: Assurance that involvement is voluntary and can be withdrawn at any time without penalty. 6. Contact Information: Details for inquiries about the study or rights. 7. Statement of Understanding: Confirmation that individuals understand the information provided.
How does communication impact informed consent?
Clear communication is crucial, as studies have shown that an increase in the complexity of consent forms correlates with higher dropout rates. Ensuring clarity in consent documents fosters trust and enhances participant comprehension.
What recent findings highlight the need for clarity in informed consent?
Recent findings indicate that only 53% of individuals reported understanding critical aspects of informed consent, such as the concept of a placebo. This underscores the importance of clear and transparent consent documents.
How do ethics committees view informed consent?
Ethics committees emphasize the significance of openness and adequate disclosure in informed consent, ensuring that individuals are fully aware of the project's intent and are assured a debriefing at the end of their participation.
What evidence supports the effectiveness of structured informed consent documents?
A case study demonstrated that two reviewers independently evaluated 250 approval documents with a high agreement (>90%) across all items, indicating that structured approval documents effectively enhance participant comprehension.




