Introduction
Informed consent is a fundamental principle that underpins ethical clinical research, serving as a protective measure for participants while ensuring their autonomy and rights are respected. This intricate process involves providing potential study participants with comprehensive information about the research objectives, methodologies, risks, benefits, and alternatives, enabling them to make educated decisions regarding their involvement.
Despite the critical importance of informed consent, recent studies indicate that gaps in understanding persist, particularly concerning complex concepts like the placebo effect. This highlights the urgent need for researchers to refine their consent processes, emphasizing clear communication and ethical practices.
As the landscape of clinical research evolves, so too must the approaches to informed consent, ensuring that all participants are adequately informed and empowered to engage in research activities.
Understanding Informed Consent: Definition and Purpose
Informed agreement serves as a fundamental element of ethical and legal structures in clinical studies, acting as an essential safeguard for individuals involved. This process requires that potential study contributors receive thorough information regarding what is included in informed consent, which encompasses the research's purpose, procedures, risks, benefits, and available alternatives. A recent meta-analysis involving 114 studies revealed that 91.4% of individuals understood the essential elements of knowledgeable agreement.
However, understanding of specific concepts, such as the placebo effect and the ability to articulate associated risks, remained less satisfactory. This gap emphasizes the ongoing necessity for careful approval procedures, as indicated by recent findings which show that following ethical guidelines greatly enhances understanding among those involved. The primary aim of what is included in informed consent is to empower individuals to make informed decisions regarding their participation, thereby upholding their autonomy and rights.
Additionally, fostering trust between researchers and participants is crucial; as one expert noted,
Maybe that’s part of the problem that people are now doing clinical research who haven’t really done clinical research in the past and then the collaborations don’t work.
This emphasizes the necessity for researchers to prioritize clear and effective communication in the approval process. Moreover, data requests can be directed to the Charité Research Ethics Committee, which adds a layer of authority to the informed agreement discussion.
The findings from the aforementioned meta-analysis also reveal that specific shortcomings in understanding the placebo concept and risks necessitate enhanced agreement processes. To evaluate the reliability of findings regarding understanding among subjects, the case study titled 'Quality Assessment of Informed Consent Studies' provides a framework by assessing studies based on metrics such as description of subjects and evaluation methods. Ultimately, knowledgeable agreement on what is included in informed consent not only enhances the integrity of studies by ensuring voluntary participation but also respects the essential rights of individuals within the clinical study landscape.
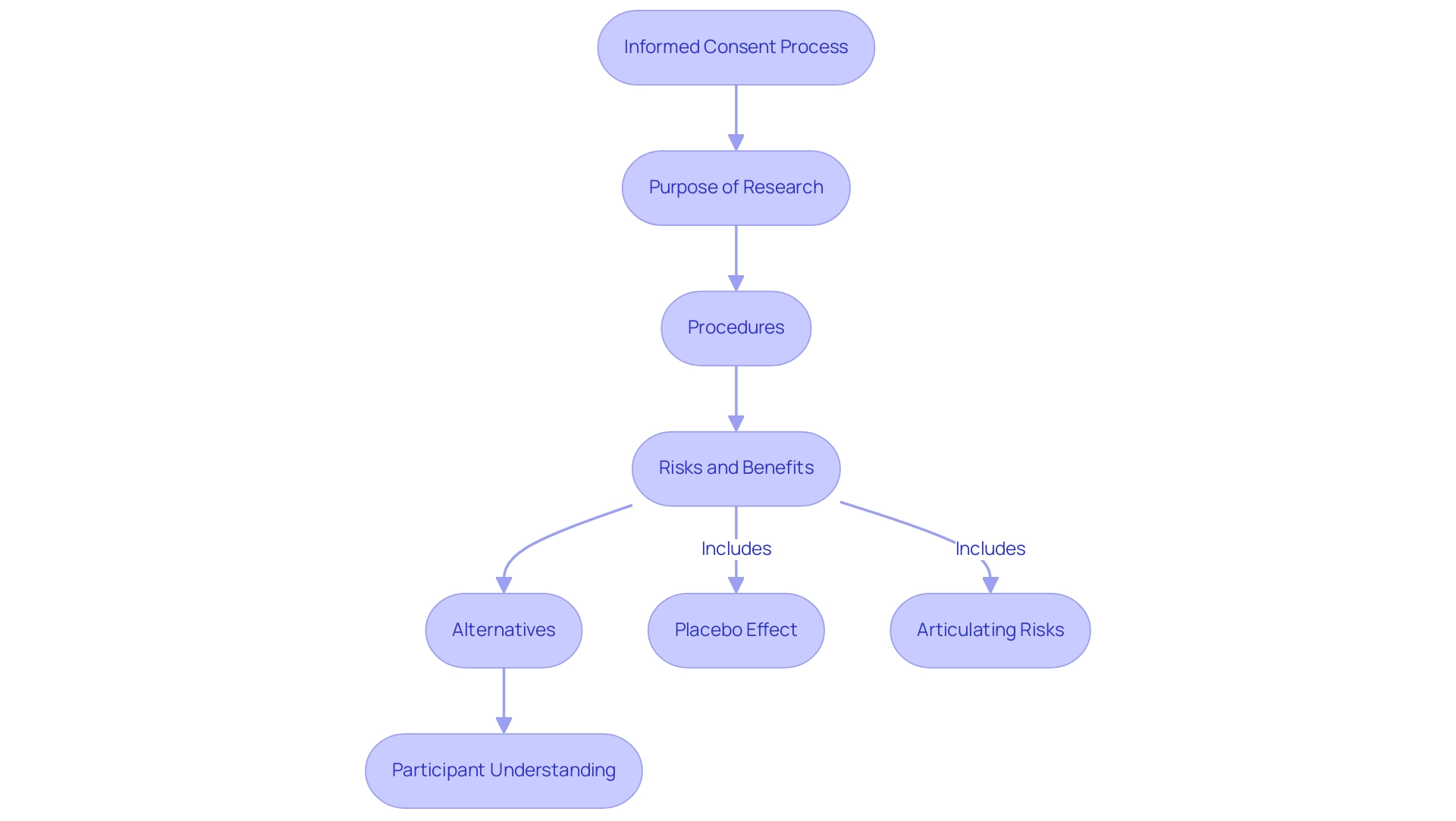
Key Components of the Informed Consent Process
The informed consent procedure is a fundamental element of ethical inquiry, which details what is included in informed consent to ensure understanding and compliance of those involved. Key elements include:
-
Study Purpose: Participants must receive a comprehensive explanation of the study objectives, detailing how the investigation may enhance medical knowledge or improve patient care.
This clarity is essential, as it aligns expectations of those involved with the study objectives. As noted by Nijhawan LP in the review Informed Consent: Issues and Challenges, maintaining clarity in the study purpose is fundamental for ethical research practices.
-
Procedures: Clear, detailed descriptions of the procedures involved in participation are essential. This involves detailing any tests, treatments, or interventions individuals will experience, enabling them to make knowledgeable choices about their involvement.
-
Risks and Benefits: A candid and comprehensive evaluation of possible risks, discomforts, or side effects is essential, along with a clear conversation about any anticipated advantages to individuals or the wider community. Research indicates that 83.5% of participants remembered having fully read the consent form, highlighting the importance of transparent communication.
-
Alternatives: Participants should be informed of alternative treatment options or procedures that may be available outside of the study. This empowers them to weigh their options and make choices that are best for their individual circumstances.
-
Confidentiality: Assurance regarding the protection of personal information is paramount. Participants need to understand the measures in place to maintain confidentiality throughout the study process.
-
Voluntariness: It is essential to emphasize that participation is entirely voluntary. Participants should feel free to withdraw from the study at any time without facing any penalties. This aspect fosters trust and ethical conduct within the investigative environment.
-
Contact Information: Providing clear contact details for the study team is necessary to address any individual questions or concerns. Open lines of communication enhance transparency and comfort for those involved.
Each component plays a vital role in establishing what is included in informed consent to create a transparent and ethical research environment. The case study by Verheggen et al. underscores this, as it assessed understanding among 198 adult patients in 26 trials through follow-up questionnaires, revealing insights into participant comprehension of agreed terms.
Additionally, it is significant that 79% of data sets associated with awareness studies were conducted in middle- or high-income nations, which may affect the relevance of findings across various demographics. Recent advancements in knowledgeable agreement highlight the incorporation of particular details, such as whether the study will involve whole genome sequencing. This requirement mirrors the changing character of knowledgeable agreement methods and the necessity for researchers to keep individuals fully aware.
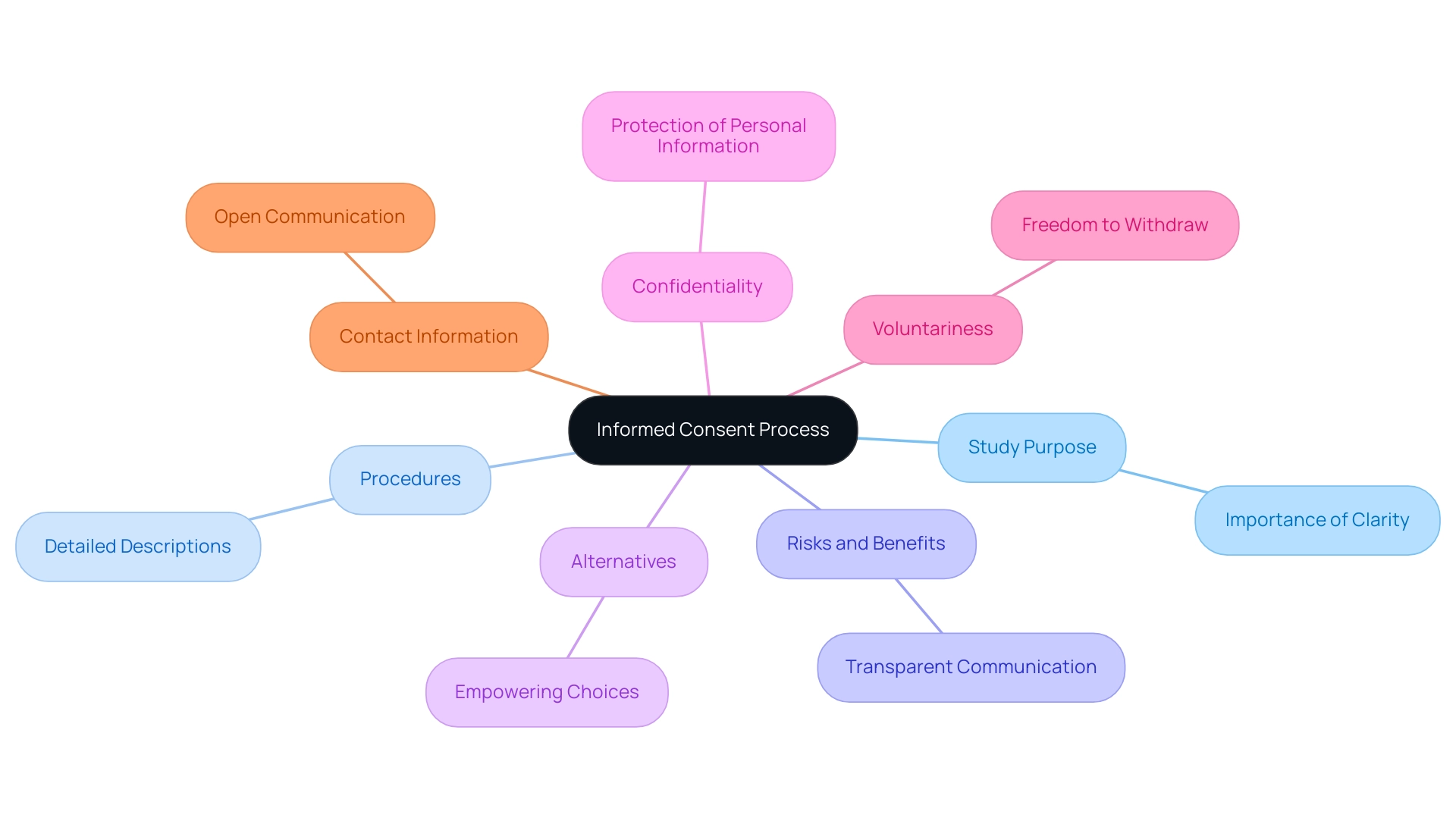
The Role of Ethical Review Boards in Informed Consent
Ethical review boards (ERBs), often known as institutional review boards (IRBs), are essential to the awareness agreement process in healthcare and studies. Their main duty is to carefully assess project proposals to protect the rights and well-being of those involved. This involves a thorough assessment of what is included in informed consent, ensuring that participants are provided with all pertinent information in a clear and understandable format.
ERBs critically analyze the potential risks and benefits associated with studies, ensuring that ethical standards are upheld prior to granting approval. The creation of the Nuremberg Code, after the Nuremberg Trials where Nazi physicians carried out cruel experiments on concentration camp inmates, highlights the importance of these ethical standards in studies. Recent findings indicate that:
- 12.7% of protocols are assigned based on the timing of submission
- 60.0% are assigned based on the current workload of the boards
This highlights the operational factors that influence IRB efficiency.
A comprehensive study of IRBs in the U.S. revealed that their characteristics can be distilled into four key components, with variations observed across institution types. As Tyler M Moore, the lead statistical analyst, noted, 'The role of ERBs is crucial in ensuring that rights of individuals are safeguarded and that the study is conducted ethically.' By acting as an independent oversight entity, ERBs not only enhance public trust in clinical research but also contribute significantly to ensuring the ethical conduct of studies, thus emphasizing the importance of knowledgeable agreement.
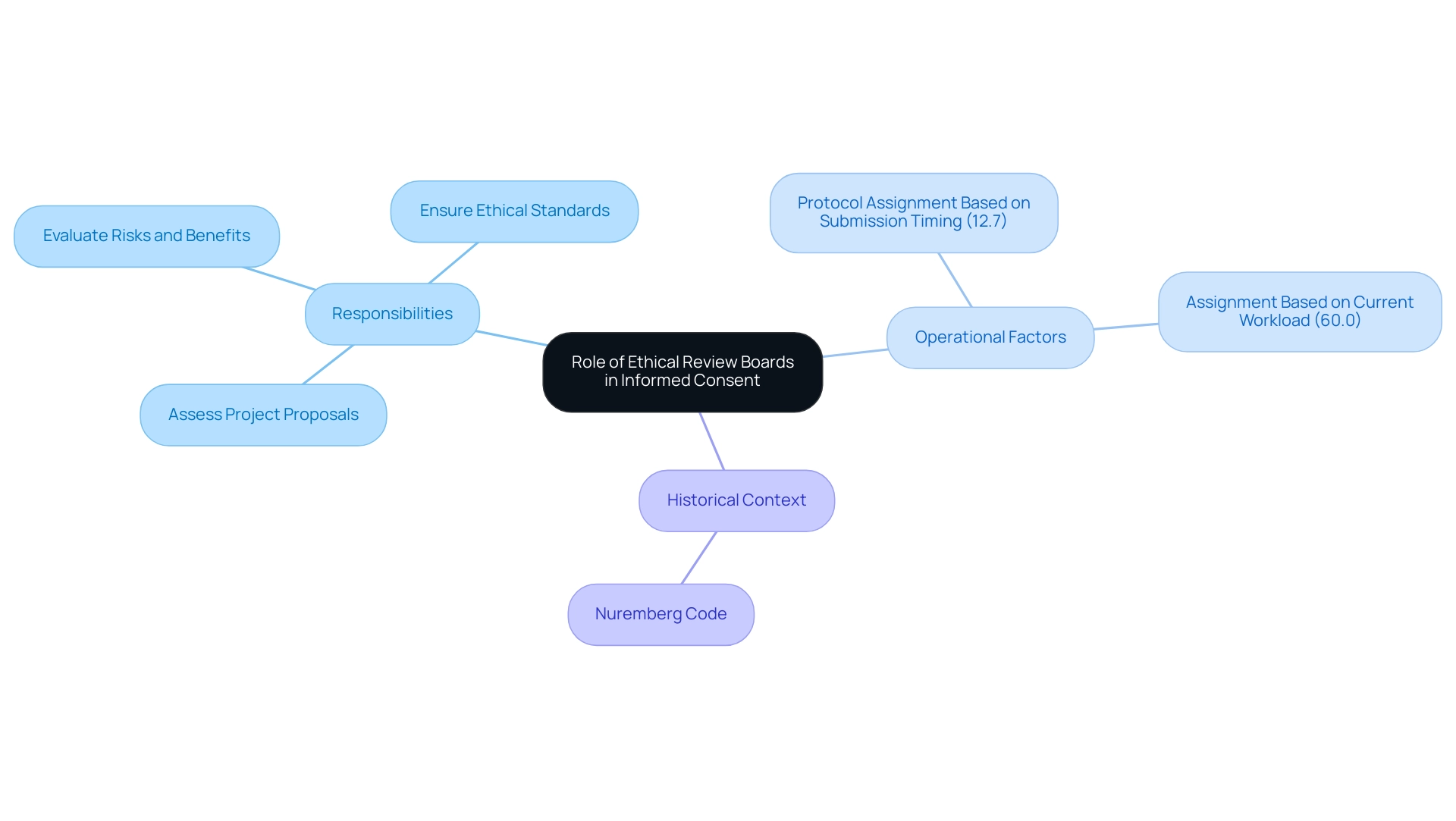
Challenges in the Informed Consent Process
The approval process is filled with obstacles that can obstruct effective communication and limit understanding of those involved. Key issues include:
-
Complex Terminology: The frequent use of technical jargon can obscure the implications of a study, leaving individuals confused.
Simplifying language and offering clear, accessible explanations are essential to enhancing comprehension. L. Hetherington highlighted this point, stating,
Simplifying knowledge agreement is a universal precaution, underscoring the necessity for clarity in communication. -
Cultural Differences: Cultural variations significantly influence how information is interpreted and understood. Researchers must be attuned to these differences and adapt their communication strategies to meet the varied needs of those involved. For example, research has demonstrated that cultural settings can influence understanding of agreements, requiring customized methods to guarantee clarity.
-
Time Constraints: Often, individuals experience time pressures during the consent process, which can lead to incomplete understanding. Allocating sufficient time for thorough discussions and encouraging questions is crucial to fostering informed decision-making.
Research indicates that factors such as providing shorter and simpler Patient Information Leaflets (PIL) can enhance this aspect, with 86% of individuals favoring simplified materials and 54% indicating that more time with individuals would improve the process.
-
Participant Vulnerability: Individuals from at-risk groups may experience excessive pressure to participate, which can undermine the voluntary nature of their agreement.
It is crucial for researchers to guarantee that individuals are completely aware and feel empowered to refuse involvement without consequences. Moreover, the restrictions emphasized in the case study named 'Assessment of Risk of Bias' indicate that the included studies were neither randomized nor blinded regarding outcomes related to knowledge agreement, rendering risk of bias evaluation unfeasible. This raises concerns about the reliability of findings regarding individuals' comprehension of knowledgeable agreement.
Additionally, the research did not assess whether the simplification of approval documents affected individuals' choices to enroll in trials, which provides an important viewpoint on the consequences of simplifying approval. Addressing these challenges is essential to maintain what is included in informed consent within the educated agreement process. By improving communication and understanding, researchers can support the integrity of clinical studies, creating an environment where participants feel respected and knowledgeable.
The Importance of Continuous Training for Research Staff
Ongoing training for investigation personnel is crucial to guarantee they have a comprehensive grasp of what is included in informed consent and its ethical consequences. Regular training sessions act as an essential resource for staff to stay updated on regulatory changes, revised best practices, and effective communication techniques. This dedication to continuous learning not only promotes a culture of ethical awareness and adherence within study groups but also enables staff to confidently respond to individuals' questions and concerns.
Furthermore, training improves the capability of study staff to recognize and address issues related to what is included in informed consent, ultimately leading to better participant experiences and results. The demand for more relevant and personalized learning experiences, as highlighted by Training Industry's CEO, underscores the importance of integrating advancements in VR, AR, and AI technologies into training programs. By investing in ongoing training, research organizations can uphold the highest standards of ethical behavior in clinical trials, ensuring that their teams are well-versed in what is included in informed consent to navigate the intricacies of knowledgeable agreement efficiently.
In fact, recent trends indicate that 41% of learning and development leaders anticipate a larger budget in 2023, which could further enhance training initiatives aimed at improving what is included in informed consent. Furthermore, with less than 5% of businesses adopting leadership development at all levels, it is crucial for research institutions to prioritize training to boost productivity, as evidenced by Yazdanifard and colleagues' findings on the positive impact of employee training and development.
Conclusion
The informed consent process is not only a legal obligation but a fundamental ethical commitment that safeguards the rights and autonomy of research participants. Throughout this discussion, the critical components of informed consent, including the clear communication of study purposes, procedures, risks, benefits, and alternatives, have been emphasized as vital for participant understanding. Despite significant progress, gaps in comprehension persist, particularly concerning complex concepts like the placebo effect. This highlights the pressing need for researchers to refine their consent processes and enhance clarity in communication.
Moreover, the role of ethical review boards is crucial in ensuring that informed consent processes adhere to established ethical standards, thereby reinforcing public trust in clinical research. However, challenges such as complex terminology, cultural differences, time constraints, and participant vulnerability continue to hinder effective communication. Addressing these challenges is imperative to uphold the integrity of the informed consent process, ensuring that participants are not only informed but also empowered to make voluntary decisions regarding their involvement in research.
Continuous training for research staff emerges as a vital strategy to navigate these complexities, equipping them with the knowledge and skills necessary to foster an ethical research environment. By prioritizing education and effective communication, researchers can bridge the gaps in understanding and enhance the informed consent experience for all participants. Ultimately, a commitment to these principles will not only improve the quality of clinical research but also honor the rights and dignity of individuals involved in these studies.




