Introduction
In the intricate world of medical device development, the process of design transfer stands as a critical juncture between innovation and production. This essential phase involves transforming conceptual design inputs into tangible manufacturing workflows, ensuring that each device meets stringent regulatory standards and fulfills its intended purpose.
With the oversight of regulatory bodies like INVIMA, compliance becomes paramount, as it directly impacts patient safety and device efficacy. Key components of this process, such as:
- Meticulous documentation
- Quality control measures
- Cross-functional collaboration
play a significant role in facilitating a seamless transition from design to production. As the landscape of medical technology evolves, understanding the nuances of design transfer is vital for manufacturers aiming to enhance patient outcomes while navigating complex regulatory frameworks.
Understanding Design Transfer in Medical Device Development
The design transfer medical device process is pivotal in the medical industry as it involves converting design inputs into effective production workflows. This meticulous transformation ensures that products are manufactured according to the intended specifications, adhering to all regulatory requirements set forth by authorities such as INVIMA (Colombia National Food and Drug Surveillance Institute), which is responsible for overseeing the marketing and manufacturing of health items. INVIMA's role includes monitoring medical devices through its Directorate for Medical Devices and other Technologies, making compliance with their standards essential for patient safety and efficacy.
Key components of the creation process involve:
- The thorough documentation of specifications
- Formulation of detailed manufacturing procedures
- Implementation of stringent quality control measures
These components are essential for a smooth transition from creation to production. Significantly, a comprehensive list of 365 ICD-9 and ICD-10 codes associated with embolization procedures has been created for cross-referencing patient profiles, improving the accuracy of development.
This master list plays a crucial role in ensuring that equipment is aligned with specific patient needs and conditions. With a statistically significant p-value of < .05 indicating positive influences on selection, it is clear that successful transfer can markedly improve patient outcomes. This statistic suggests that the careful selection of parameters directly correlates with enhanced efficacy and safety in medical devices.
Janet Whipple, a partner at Validant, emphasizes that engineers must take into account the risk factors that are introduced by human use or human error, illustrating the need to consider human interactions during the development phase. Additionally, recent studies have underscored that a well-defined vision and a robust value proposition are crucial for the scrutiny and adoption of innovative medical technologies. Moreover, particular compliance mandates from INVIMA, like alignment with technical standards for production and quality assurance, must be incorporated into the transition phase.
The case study titled 'Use of Vascular Quality Initiative Registry Data for Regulatory Decisions' illustrates how utilizing registry data can aid regulatory decisions, emphasizing the significance of real-world evidence in the development phase. Ultimately, mastering the design transfer medical device transition procedure not only meets regulatory compliance, including the rigorous standards set by INVIMA, but also significantly enhances the overall safety and effectiveness of medical instruments.

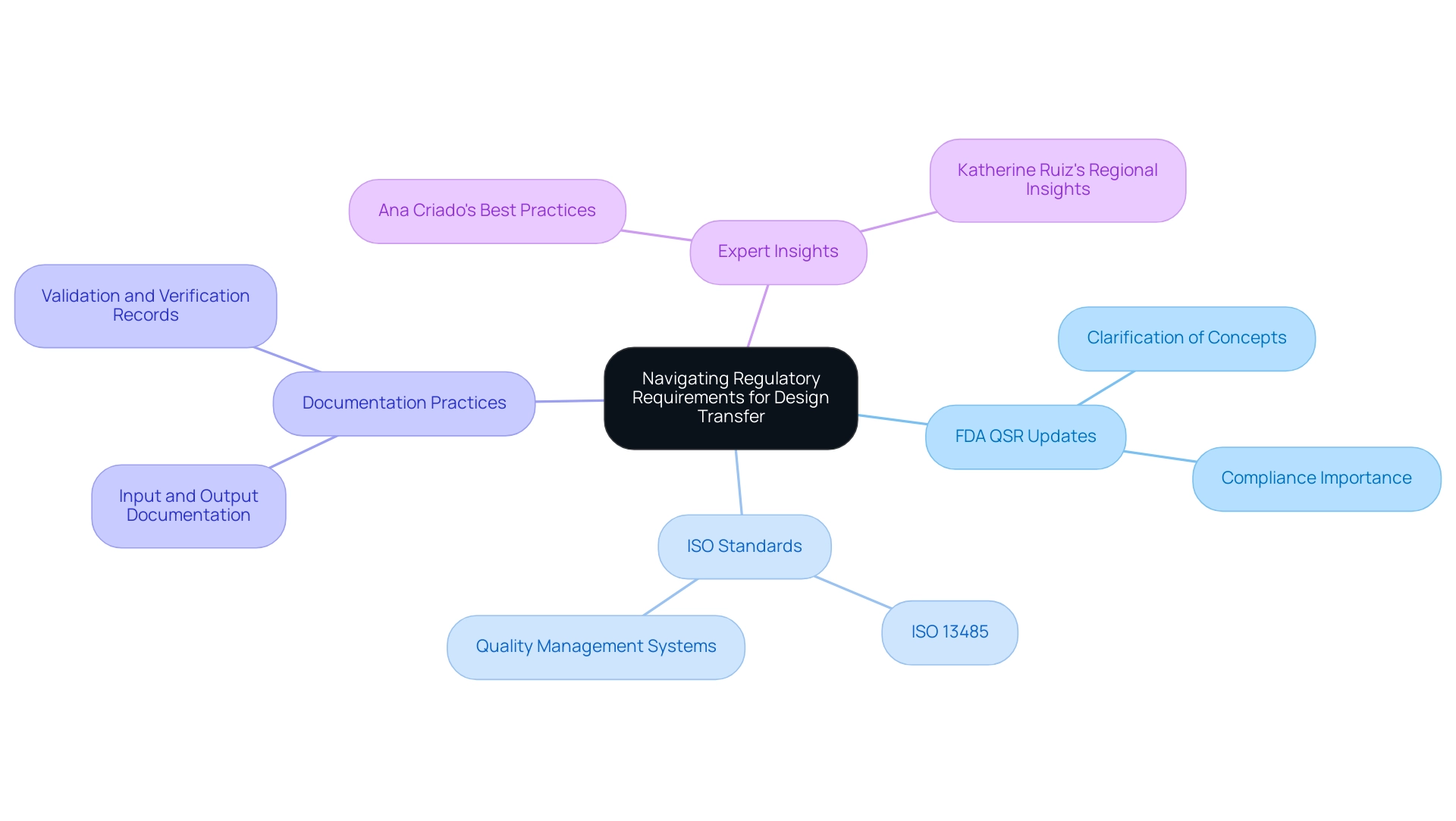
Navigating Regulatory Requirements for Design Transfer
Navigating regulatory requirements is crucial in the development phase for medical device manufacturers. Compliance with the FDA Quality System Regulation (QSR) is paramount, particularly with the upcoming updates effective February 2, 2026. The FDA has recently clarified key concepts by moving certain definitions from § 820.15 to § 820.3(b), enhancing the readability and interpretation of the Quality Management System Regulation (QMSR).
As stated by the FDA, 'FDA agrees with this comment and has revised the rule to remove § 820.15 and move the clarification of certain concepts and terms to § 820.3(b).' This change reflects the FDA's commitment to improving clarity for manufacturers. A relevant case study titled 'Clarification of Concepts in QMSR' underscores this point, showing how the FDA's revisions were driven by a need for enhanced readability and practical application.
Adherence to ISO standards, specifically ISO 13485, further ensures that quality management systems are effectively implemented. Manufacturers must meticulously document input, output, and changes throughout the development process to facilitate regulatory review. With the FDA's updates, it is crucial to adapt documentation practices to align with the clarified definitions and requirements, ensuring comprehensive and accurate documentation for design transfer medical device processes that can withstand scrutiny during inspections.
Additionally, maintaining thorough records of validation and verification activities is crucial for demonstrating compliance with safety and effectiveness standards. Comprehending and efficiently applying these regulatory requirements is crucial to preventing delays in market approval and ensuring the safety of medical products. Experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, highlight the importance of staying informed on these evolving regulations.
Ana's extensive experience in Regulatory Affairs informs best practices in documentation and compliance, particularly in light of the FDA's recent updates, while Katherine's insights into the Colombian regulatory landscape further enhance the understanding of compliance in regional contexts.
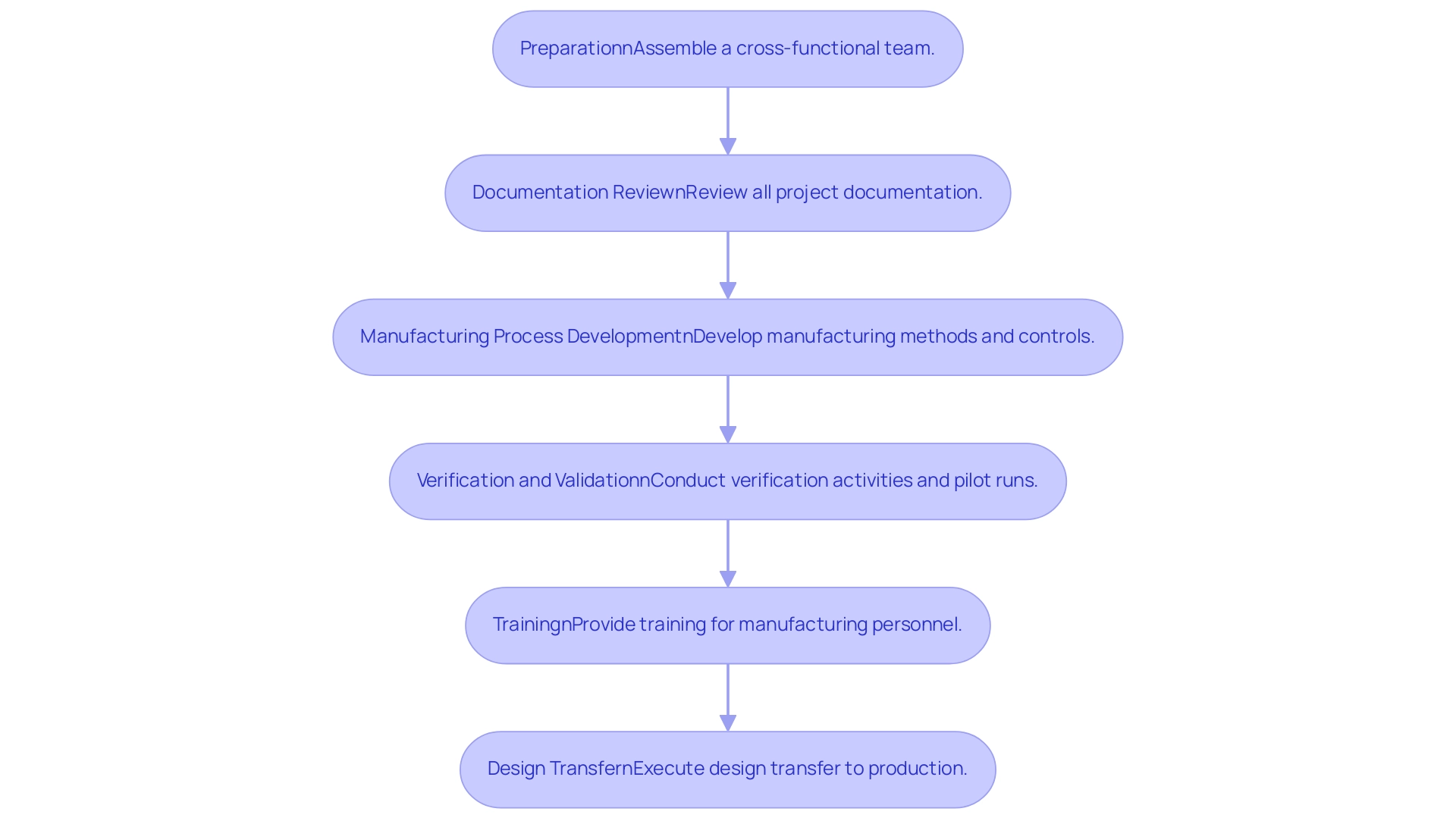
Step-by-Step Process for Effective Design Transfer
- Preparation: Assemble a cross-functional team including engineering professionals, quality assurance personnel, and Regulatory Affairs experts. This joint method is essential during the design transfer medical device phase, ensuring that diverse viewpoints and skills guide decision-making.
- Documentation Review: Conduct a thorough review of all documentation related to the project, including specifications, verification and validation plans, and risk management files. It is vital to make certain that all documents are up to date and adhere to regulatory standards, as they constitute the foundation of the control framework. A quality history file is crucial for recording the development-to-production sequence, ensuring traceability of modifications.
- Manufacturing Process Development: Develop the manufacturing method in alignment with the design specifications. This phase involves creating detailed work instructions, establishing robust controls, and identifying critical quality attributes that will ensure product consistency and compliance. Notably, service bureaus can provide parts within just two or three days, significantly improving efficiency compared to the weeks or months it took previously.
- Verification and Validation: Conduct verification activities to ensure that the manufacturing system is capable of producing devices that meet established requirements. This may involve conducting pilot runs and testing prototypes, as these steps are vital for identifying potential issues before full-scale production. The iterative nature of design control is illustrated by the Waterfall Design Process, which begins with Design Input development and culminates in a finalized design transmitted for mass manufacturing.
- Training: Provide comprehensive instruction for manufacturing personnel on the new methods, ensuring they understand the quality standards necessary for production. Janet Whipple, a partner at Validant, emphasizes that "engineers must take into account the risk factors that are introduced by human use or human error," underlining the need for thorough training to mitigate these risks.
- Design transfer medical device: After successful verification, formally execute the design transfer of the manufacturing process to production. It is critical to monitor the initial production runs closely to detect any issues that might arise, facilitating timely adjustments that uphold product integrity.
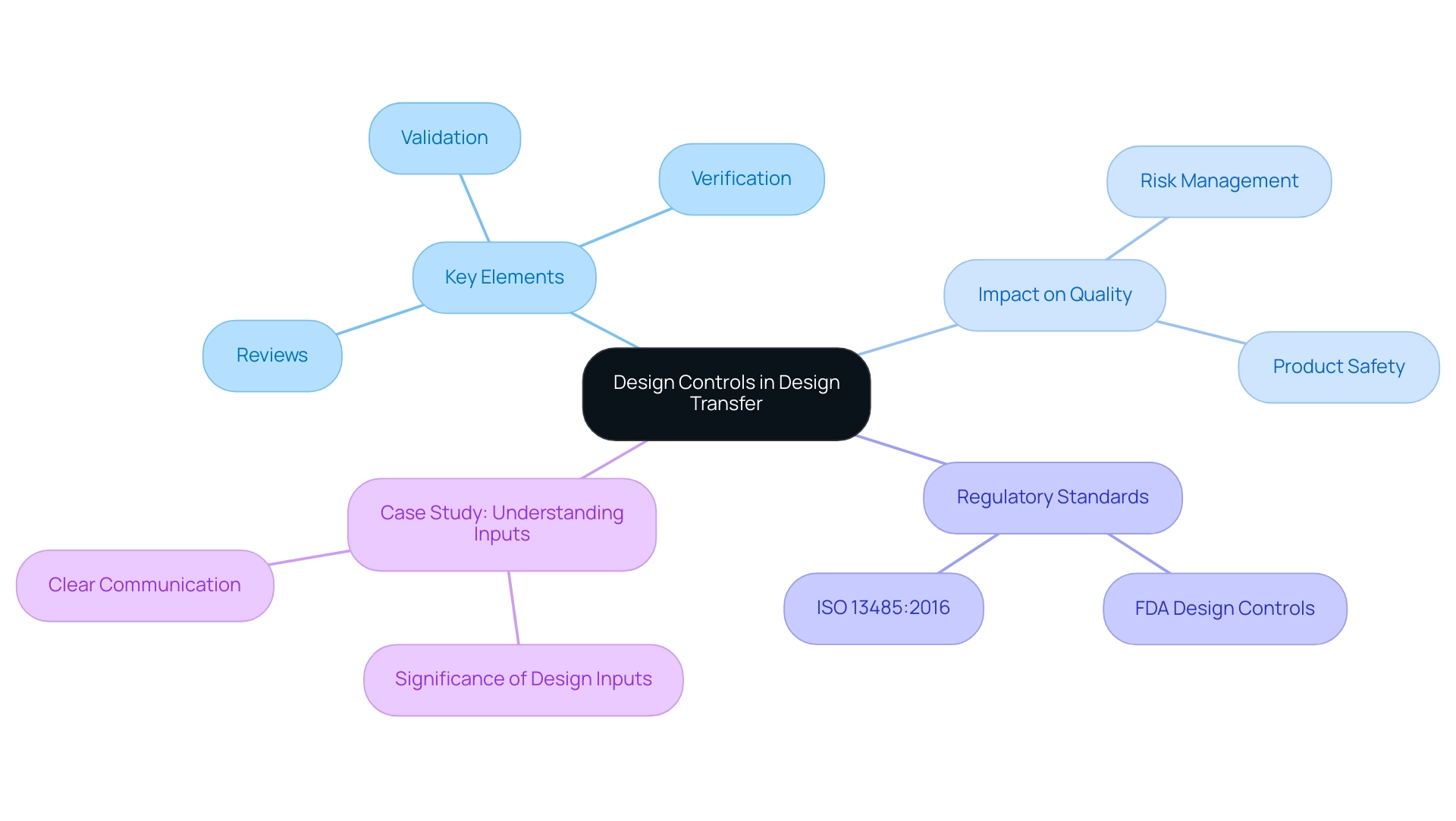
The Role of Design Controls in Successful Design Transfer
Design controls are integral systematic measures that guarantee medical devices are developed and manufactured in alignment with their intended use. Key elements of these controls include reviews, verification, and validation of the design. Implementing robust control measures during the design transfer medical device process is crucial for sustaining quality and compliance.
Statistics indicate that effective controls improve risk management by implementing appropriate measures to enhance product quality and safety. By facilitating early identification and mitigation of potential risks, these controls ensure that issues are addressed before production commences. Furthermore, adherence to development controls not only bolsters the safety and efficacy of products throughout their lifecycle but also aligns with the latest regulatory standards.
For instance, both FDA Design Controls and ISO 13485:2016 underscore the necessity of thorough documentation and record-keeping across the product development continuum. As Mike Drues aptly states, 'They think they understand what intended use means, but they really don’t… Intended use is all about what we say this device is to be used for, and indications for use is under what circumstances or under what conditions you would use that particular product.' This emphasizes the critical need for clarity in input specifications.
Furthermore, the case study titled 'Understanding Inputs' emphasizes the significance of clear communication among team members regarding inputs to avoid miscommunication and ensure compliance during audits. This structured approach ultimately enhances product quality and fosters a culture of compliance, which is critical in the highly regulated medical equipment industry, particularly during the design transfer of medical devices.
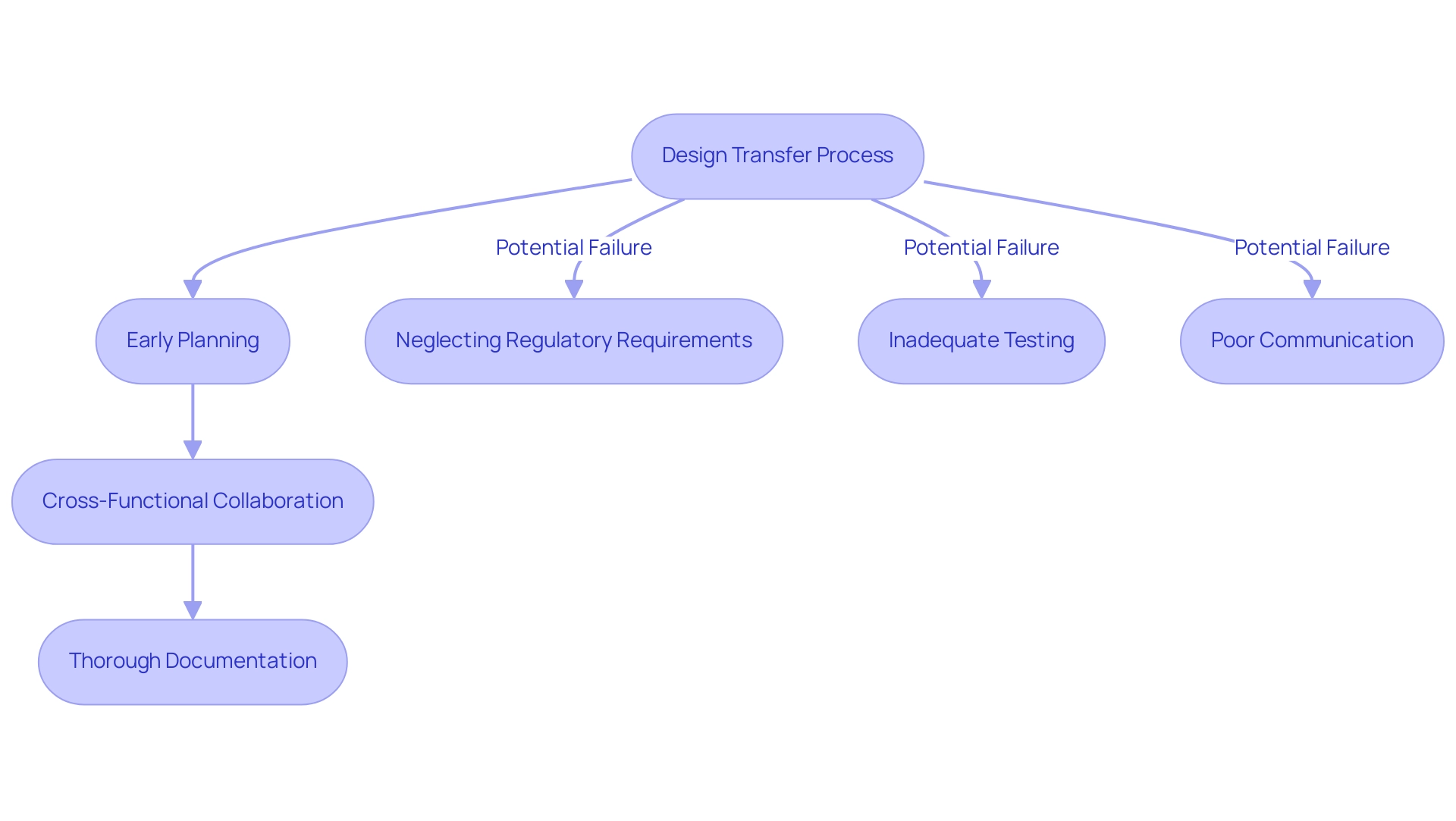
Best Practices and Common Pitfalls in Design Transfer
Optimal methods for effective design transfer of medical devices are essential for guaranteeing a smooth shift from concept to production. Key practices include:
- Early Planning: Starting the transition of concepts at the very beginning of the development cycle is essential. This proactive approach helps mitigate last-minute complications that can arise when manufacturing considerations are not adequately integrated from the outset. According to industry statistics, early engagement in product transition can reduce production delays by up to 30%.
- Cross-Functional Collaboration: Encouraging cooperation among all stakeholders—including development, manufacturing, and quality assurance teams—facilitates a smoother transition. As emphasized in recent insights, incorporating varied viewpoints early on diminishes the chances of oversights and mistakes. For example, converting device creation into component and assembly illustrations and specifications early in the workflow can greatly simplify operations and improve clarity.
- Thorough Documentation: Keeping detailed records throughout the creation transition is essential. Documenting each step not only aids adherence to regulatory requirements but also simplifies the review procedure during audits, ensuring that all development decisions are traceable.
Common pitfalls to avoid in the transition include:
-
Neglecting Regulatory Requirements: Overlooking regulatory standards can result in significant delays and necessitate extensive rework, which can be costly and time-consuming.
-
Inadequate Testing: Skipping crucial verification and validation steps can lead to safety issues in the final product, jeopardizing patient outcomes and regulatory compliance.
-
Poor Communication: A lack of effective communication among team members can foster misunderstandings and errors during the transition. As industry expert Czuba emphasizes,
Along the way, you should gather data that explains how you transitioned from one version to another, resolved a problem, or addressed a question about the earlier iteration. This data-focused method can greatly improve the transition of concepts and reduce risks. Citing a case study on the timing of the development handover process, it is clear that beginning early helps prevent expensive delays and alterations when increasing production. By adhering to these best practices and steering clear of common pitfalls, manufacturers can significantly improve the success rates of their design transfer medical device processes, ensuring that devices meet both regulatory standards and user needs.
Conclusion
Mastering the design transfer process in medical device development is essential for ensuring that innovative concepts are translated into safe and effective products. This intricate journey encompasses critical components such as:
- Thorough documentation
- Quality control measures
- Cross-functional collaboration
All of which play a vital role in facilitating a smooth transition from design to production. Regulatory compliance, particularly with standards set by INVIMA and the FDA, is paramount to safeguarding patient safety and enhancing device efficacy.
The article highlights the importance of early planning and cross-functional teamwork, which significantly reduces the likelihood of production delays and errors. By fostering an environment of collaboration and maintaining meticulous records throughout the design transfer process, manufacturers can avoid common pitfalls that jeopardize compliance and patient outcomes. Moreover, adherence to design controls is crucial for risk management and ensuring that devices meet their intended use.
Ultimately, a deep understanding of the design transfer process not only aligns with regulatory requirements but also enhances the overall quality and safety of medical devices. As the landscape of medical technology continues to evolve, manufacturers must commit to excellence in design transfer to meet the growing demands of patient care and regulatory scrutiny. Emphasizing these practices will not only lead to successful product launches but also contribute to improved health outcomes for patients globally.
Frequently Asked Questions
What is the design transfer medical device process?
The design transfer medical device process involves converting design inputs into effective production workflows, ensuring that products are manufactured according to intended specifications and regulatory requirements.
Why is compliance with INVIMA important?
Compliance with INVIMA is essential for patient safety and efficacy, as it oversees the marketing and manufacturing of health items, including medical devices.
What are the key components of the design transfer process?
Key components include thorough documentation of specifications, formulation of detailed manufacturing procedures, and implementation of stringent quality control measures.
How does the ICD-9 and ICD-10 code list contribute to the design transfer process?
A comprehensive list of 365 ICD-9 and ICD-10 codes helps in cross-referencing patient profiles, which improves the accuracy of medical device development and aligns equipment with specific patient needs.
What statistical significance is noted in the design transfer process?
A statistically significant p-value of < .05 indicates that careful selection of parameters during the design transfer process positively influences patient outcomes, enhancing efficacy and safety.
What role do engineers play in the design transfer process?
Engineers must consider risk factors introduced by human use or error, highlighting the importance of human interactions during the development phase.
What is the significance of a well-defined vision and value proposition in medical technology?
A well-defined vision and robust value proposition are crucial for the scrutiny and adoption of innovative medical technologies, ensuring they meet regulatory standards.
How do regulatory requirements affect medical device manufacturers?
Compliance with FDA Quality System Regulation (QSR) is crucial, especially with upcoming updates effective February 2, 2026, which enhance clarity and interpretation of quality management system regulations.
What is the importance of adhering to ISO standards?
Adhering to ISO 13485 ensures that quality management systems are effectively implemented, which is vital for regulatory review and market approval.
What should manufacturers document during the development process?
Manufacturers must document input, output, changes, and validation and verification activities to demonstrate compliance with safety and effectiveness standards.
Who are some experts highlighting the importance of regulatory compliance?
Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for medical devices in Colombia, emphasize staying informed on evolving regulations.

