Introduction
In the intricate landscape of medical device development, the significance of design control cannot be overstated. As manufacturers navigate the stringent regulatory frameworks set by authorities like INVIMA in Colombia, a systematic approach to design control emerges as a critical foundation for ensuring product efficacy and patient safety.
This article delves into the essential phases of the design control process, highlighting the importance of meticulous planning, rigorous validation, and comprehensive documentation. It also examines the evolving regulatory compliance landscape, the challenges faced by industry professionals, and the strategies necessary for overcoming these hurdles.
By understanding and implementing effective design control practices, stakeholders can not only meet regulatory demands but also foster innovation and enhance the overall quality of medical devices.
Understanding Design Control: A Foundation for Medical Device Development
The design control process for medical devices serves as a structured framework essential for the development and production of healthcare instruments, particularly within the context of INVIMA's regulatory landscape in Colombia. This structured approach, which is part of the design control process for medical devices, encompasses a series of meticulously planned and documented activities designed to align products with user needs and regulatory mandates set forth by INVIMA, the Colombia National Food and Drug Surveillance Institute. INVIMA is responsible for inspecting and supervising the marketing and manufacturing of health products, and its classification as a Level 4 health authority by PAHO/WHO underscores the significance of stringent oversight.
The importance of oversight resides in its capacity to reduce risks associated with equipment failures, which is crucial for improving patient safety and guaranteeing the effectiveness and dependability of healthcare products. Particular development oversight procedures, such as the design control process for medical devices, along with risk management, verification and validation, and reviews, are crucial in ensuring adherence to INVIMA's regulations. Statistics show that healthcare equipment failures continue to present significant dangers, with an urgent requirement for strict supervision as the sector encounters a competitive talent environment approaching 2024.
The 18th annual Pulse of the MedTech Industry report reveals that the healthcare technology sector continues to advance at new frontiers, as ongoing breakthroughs in MedTech AI and renewed optimism for MedTech M&A present the potential for creating smarter, smaller, and more personalized solutions, thereby highlighting the significance of strong development management processes. Furthermore, as regulatory requirements evolve, maintaining compliance with INVIMA's standards is essential to ensure the design control process for medical devices upholds patient safety standards. Instances like Providence Health & Services, which attained a settlement for HIPAA breaches in 2008 totaling $100,000, demonstrate the essential importance of oversight in protecting both patient information and equipment integrity.
In conclusion, efficient oversight is not merely a regulatory requirement; it is a fundamental element of patient safety in the healthcare equipment sector, a belief shared by specialists in Regulatory Affairs such as Katherine Ruiz.
The Phases of the Design Control Process: From Planning to Validation
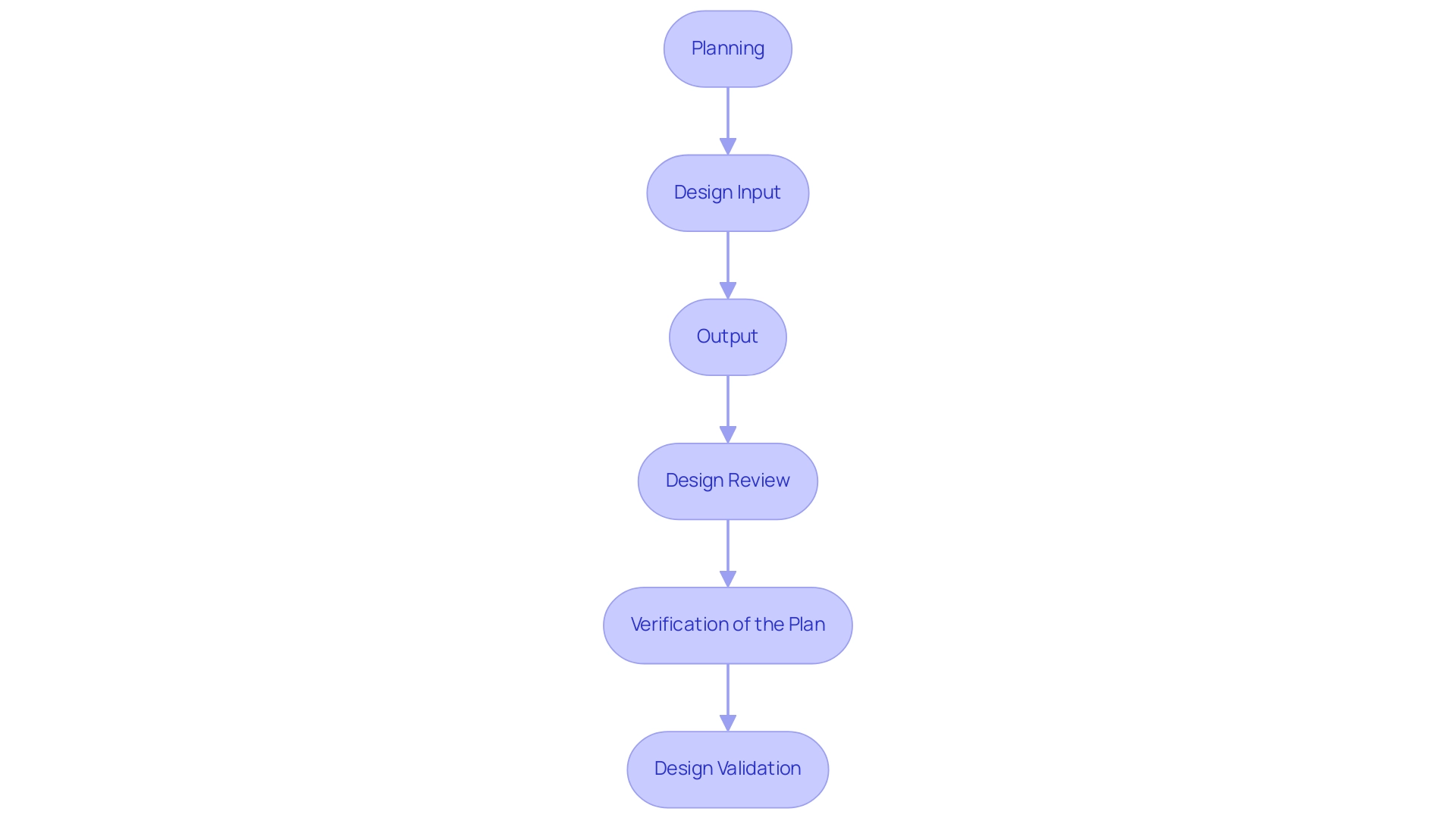
The design control process for medical devices includes several vital stages that together guarantee a systematic method to product development, particularly when combined with extensive clinical trial management services. These phases are:
-
Planning - This foundational stage involves establishing clear objectives, identifying user needs, and defining the overall scope of the project.
Effective planning is crucial as it sets the direction for subsequent phases, especially in the context of managing Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
-
Design Input - In this phase, comprehensive requirements and specifications are gathered, which are essential for guiding the layout. According to 21 CFR 820.3(a), these inputs include both physical and performance requirements that the device must satisfy, ensuring compliance with regulatory standards during trials.
-
Output - This involves documenting the results of the creation process, which includes detailed drawings and specifications. These outputs act as benchmarks for ensuring that the framework meets the established inputs and aligns with the objectives of Pilot and Pivotal Studies.
Design Review - Regular assessments are performed at different phases to ensure that the layout conforms to the established requirements. This phase is vital for early identification of potential issues, allowing for timely adjustments, particularly relevant in Post-Market Clinical Follow-Up Studies (PMCF).
-
Verification of the Plan - Testing is conducted to ensure that the outputs align with the specified inputs. This verification is essential to ascertain that the product will function as intended, which is critical for successful clinical trial outcomes.
-
Design Validation - The final product undergoes thorough evaluations, including clinical assessments and usability testing, to ensure it meets user needs and intended uses. This stage is crucial in confirming the product's effectiveness in practical applications, supported by bioaccess®’s expertise in clinical study management, backed by over 20 years of experience in the Medtech field.
Each of these stages is interdependent, forming a robust framework within the design control process for medical devices. A structured approach, as illustrated in the case study titled 'Key Stages of Product Design and Development,' emphasizes the importance of research and discovery, followed by ideation and concept development. This methodology not only enhances product management but also increases the likelihood of project success.
As highlighted by QA/RA specialist Sumatha Kondabolu, who possesses various certifications including ISO 13485 and IATF 16949, following these development oversight stages is crucial for attaining compliance and quality in medical device production. Furthermore, the management team may project a 50% increase in sales over five years; however, without a structured approach, they may only achieve a 25% increase, underscoring the critical nature of these processes. Furthermore, alterations can occur at any time and must adhere to established procedures, including risk assessments related to modifications, to ensure that the integrity of the structure is maintained.
'bioaccess®'s adaptable and personalized method further aids the development oversight process, ensuring that each stage is customized to fulfill specific project requirements.
Regulatory Compliance: Navigating FDA and ISO Design Control Requirements
Regulatory adherence acts as a key foundation in the development oversight process for healthcare instruments. The FDA provides explicit guidelines under 21 CFR Part 820, requiring manufacturers to establish and maintain a robust quality system that incorporates a design control process for medical devices. This is further supported by the International Organization for Standardization (ISO), especially through ISO 13485, which outlines the framework for quality management systems within the healthcare equipment sector.
Compliance with these regulations is not just a formality; it protects patient safety and the effectiveness of instruments, thereby fostering trust in the design control process for medical devices and healthcare innovations. As James Riddle, SVP of Global Review Services, articulates,
The FDA recognizes the utility of these independent oversight committees and, with this draft guidance, is reinforcing their importance within the continuum of research oversight.
This underscores the essential nature of adherence to the design control process for medical devices and the necessity for continuous vigilance in upholding high standards of quality and integrity.
Furthermore, the independence of the Data Monitoring Committee (DMC) from the sponsor is crucial in ensuring unbiased oversight in the regulatory compliance process. Furthermore, the extensive services provided by specialists like Ana Criado—Director of Regulatory Affairs, a professor, and CEO of Mahu Pharma—assist in tackling the difficulties encountered by healthcare startups in clinical trials, including regulatory obstacles, competition, recruitment challenges, and financial limitations. Moreover, Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, provides additional insights and support in navigating the complex regulatory landscape.
The significant increase in the FDA's issuance of 24 Warning Letters concerning healthcare instruments in fiscal year 2023—up from earlier years—indicates a changing regulatory environment that requires heightened focus on adherence to standards. Notably, prior years showed relatively low numbers of Warning Letters, with the highest being 21 in 2020, indicating a shift in regulatory scrutiny. For further insights and assistance, manufacturers can utilize a resource library that includes podcasts, blogs, white papers, and various educational materials to improve their comprehension of compliance and development processes.
Specific services, such as feasibility and selection of research sites and principal investigators, trial set-up, start-up, and project management, are critical components that ensure a successful clinical trial process.
Overcoming Challenges in the Design Control Process: Strategies for Success
The design control process for medical devices frequently faces considerable obstacles, such as unclear requirements, restricted resources, and the intricacies of managing cross-functional teams. These obstacles can hinder progress and compromise product quality. To address these issues effectively, fostering open communication among all stakeholders is paramount.
Establishing clear project timelines and allocating resources judiciously are critical steps in ensuring that teams remain aligned and focused. Regular reviews of concepts and iterative testing methodologies can proactively identify issues early in the process, facilitating timely adjustments and minimizing setbacks. Moreover, investing in training and development for team members improves their understanding of regulatory principles, leading to more efficient project execution.
Additionally, it is worth noting that:
- One fifth of researchers currently utilize AI in their research.
- An extra 38% are planning to incorporate it in the future.
This trend emphasizes the changing environment of medical device creation, where technological advancements can simplify the design control process for medical devices. The significance of efficient communication among cross-functional teams cannot be emphasized enough; as per recent findings:
- 94% of consumers prioritize easy navigation as a vital feature in digital platforms.
- 83% also regard an attractive and modern user experience as essential.
A case study on UX revealed that enhancing customer retention by just 5% through effective UX strategies can yield a remarkable 25% increase in profits. This correlation highlights the importance of creating strong communication strategies that not only facilitate clearer project objectives but also promote a collaborative atmosphere where team members can exchange insights and solutions, ultimately driving innovation and success in the design control process for medical devices.
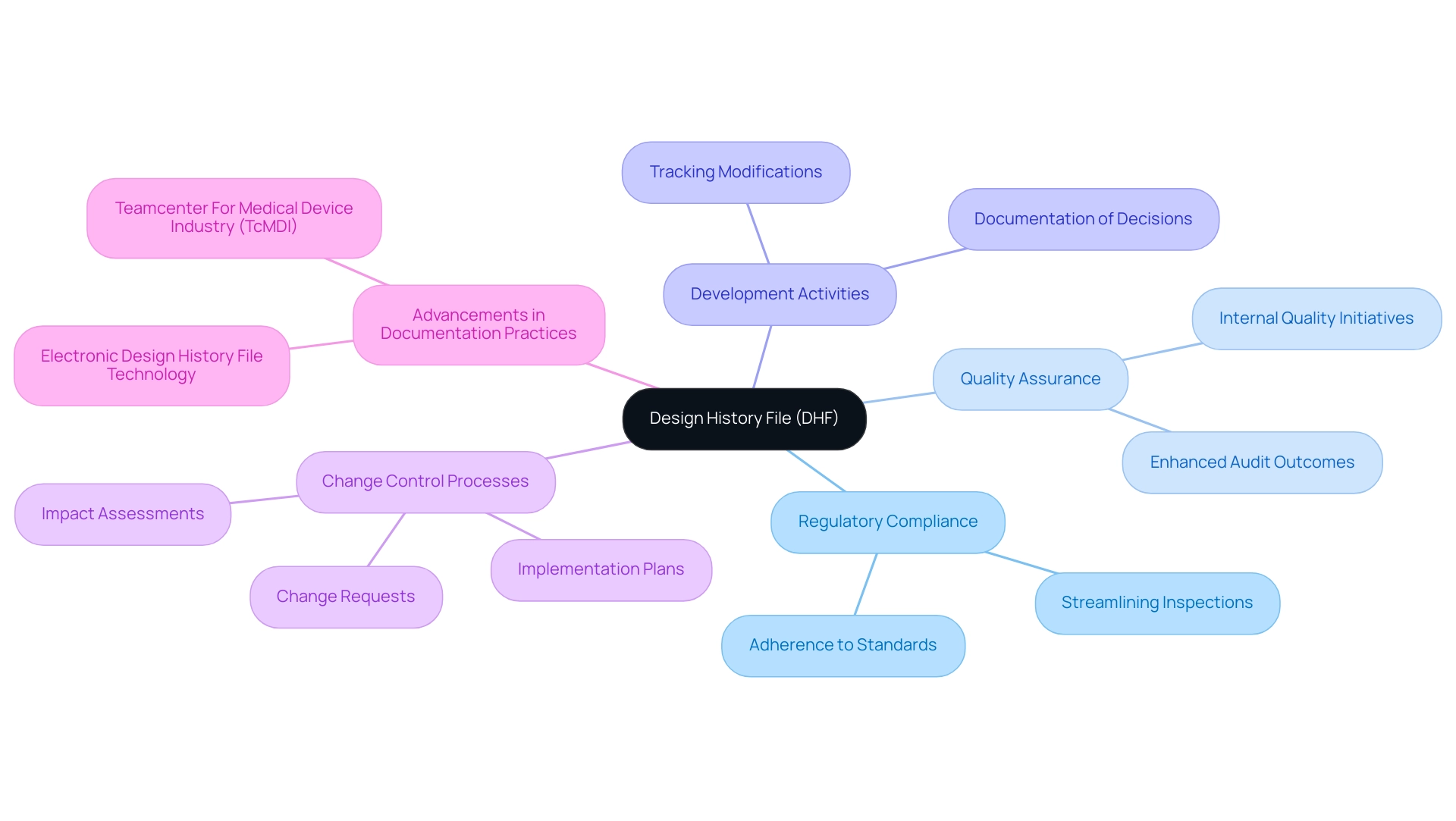
The Role of Documentation: Importance of the Design History File (DHF)
The Design History File (DHF) is a crucial component in the design control process for medical devices, acting as a comprehensive repository that tracks all development activities, decisions, and modifications throughout a product's lifecycle. This documentation is essential for compliance with stringent regulatory requirements in the design control process for medical devices, ensuring that every aspect of the development process remains traceable and transparent. A meticulously maintained DHF not only streamlines regulatory inspections but also bolsters internal quality assurance initiatives.
By providing a clear record of the development evolution, it serves as an invaluable resource for future projects, enabling teams to draw insights from past experiences and implement continuous improvements. As highlighted in various expert analyses, the maintenance of a robust DHF is crucial for navigating the complexities of regulatory inspections, with studies indicating that organizations with well-documented DHFs experience enhanced audit outcomes and quicker time-to-market. Furthermore, the DHF supports robust change control processes by documenting change requests, impact assessments, and implementation plans within the design control process for medical devices.
Recent advancements in documentation practices, particularly with integrated solutions like Teamcenter For Medical Device Industry (TcMDI), further underscore the importance of a thoroughly structured DHF in the design control process for medical devices, which is essential for achieving regulatory compliance and optimizing quality management systems. As Sahida Khatun notes, 'This white paper presents an overview of how Teamcenter For Healthcare Product Industry (TcMDI), a fully integrated PLM solution for the healthcare product sector, provides Electronic Design History File technology to help manufacturers improve the performance of several critical business processes including regulatory compliance, quality systems management, detailed design, change management, and requirements management.' Ana Criado, with her extensive experience in Regulatory Affairs and her role as a consultant for various global companies, emphasizes the importance of a well-maintained DHF in the design control process for medical devices to ensure adherence to regulatory standards and best practices in medical device development.
Conclusion
Design control is an indispensable element in the development of medical devices, serving as a structured framework that ensures compliance with regulatory standards while prioritizing patient safety. The article has highlighted the critical phases of the design control process, from planning and design input to verification and validation. Each phase plays a vital role in mitigating risks associated with device failures, thereby enhancing the overall efficacy and reliability of medical products.
Moreover, navigating the complexities of regulatory compliance, particularly with authorities such as INVIMA and the FDA, underscores the importance of maintaining a rigorous design control process. The challenges faced by industry professionals, including vague requirements and resource limitations, necessitate effective strategies such as fostering communication and investing in team training to ensure successful project execution.
Ultimately, a thorough understanding and implementation of design control practices not only facilitate adherence to regulatory demands but also promote innovation within the medical device sector. As the landscape continues to evolve, embracing these best practices will be crucial for stakeholders aiming to enhance product quality and drive advancements in patient care. The commitment to robust design control processes is essential for safeguarding the future of medical device development.
Frequently Asked Questions
What is the design control process for medical devices?
The design control process for medical devices is a structured framework that guides the development and production of healthcare instruments, ensuring alignment with user needs and regulatory mandates, particularly those set by INVIMA in Colombia.
Why is oversight important in the design control process?
Oversight is crucial as it reduces risks associated with equipment failures, enhancing patient safety and ensuring the effectiveness and reliability of healthcare products.
What role does INVIMA play in the regulation of medical devices?
INVIMA, the Colombia National Food and Drug Surveillance Institute, is responsible for inspecting and supervising the marketing and manufacturing of health products, ensuring compliance with regulatory standards.
What are the key stages in the design control process for medical devices?
The key stages include: 1. Planning 2. Design Input 3. Output 4. Design Review 5. Verification of the Plan 6. Design Validation.
What is the purpose of the Planning stage in the design control process?
The Planning stage establishes clear objectives, identifies user needs, and defines the overall project scope, setting the direction for subsequent phases.
How does the Design Input phase contribute to the development process?
The Design Input phase involves gathering comprehensive requirements and specifications that guide the device's layout, ensuring compliance with regulatory standards during trials.
What is involved in the Output phase of the design control process?
The Output phase documents the results of the creation process, including detailed drawings and specifications that serve as benchmarks for ensuring alignment with established inputs.
Why are Design Reviews conducted during the process?
Design Reviews are conducted to assess whether the layout conforms to established requirements, allowing for early identification of potential issues and timely adjustments.
What is the significance of Verification of the Plan?
Verification of the Plan involves testing to ensure that the outputs align with specified inputs, which is critical for confirming that the product functions as intended.
What does the Design Validation stage entail?
The Design Validation stage includes thorough evaluations, such as clinical assessments and usability testing, to ensure the product meets user needs and intended uses.
How are changes managed during the design control process?
Changes must adhere to established procedures, including risk assessments related to modifications, to maintain the integrity of the design control framework.
What is the impact of a structured approach in the design control process?
A structured approach enhances product management and increases the likelihood of project success, as highlighted by industry specialists.




