Overview
Expanding clinical research into Latin America is increasingly viable due to significant funding increases, diverse patient populations, and improved healthcare infrastructures in key countries like Brazil, Mexico, and Argentina. The article supports this by detailing how these factors, alongside streamlined regulatory processes and cost-effectiveness, create an attractive environment for pharmaceutical firms, while also emphasizing the importance of overcoming cultural and logistical challenges to maximize research potential.
Introduction
Latin America is on the brink of a clinical research revolution, with a surge in investment and a growing interest from global pharmaceutical companies reshaping the landscape. Countries like Brazil, Mexico, and Argentina are emerging as key players, thanks to their diverse patient populations and robust healthcare infrastructures. This transformation is fueled by the urgent demand for innovative treatments and the necessity for inclusive clinical trial representation.
As investments in clinical trials soar—particularly in the Andean Region—the region is positioning itself as a vital hub for Medtech advancements. However, unlocking its full potential requires navigating linguistic, cultural, and regulatory challenges.
By collaborating with specialized clinical research organizations, stakeholders can bridge these gaps, enhancing the quality and efficiency of clinical trials while significantly improving patient outcomes.
The Evolving Landscape of Clinical Research in Latin America
Latin America is undergoing a notable change in the medical investigation environment, which is crucial for expanding clinical research into Latin America, as evidenced by a substantial rise in funding and growing interest from international pharmaceutical firms. Key players such as Brazil, Mexico, and Argentina are emerging as significant contributors to expanding clinical research into Latin America, thanks to their diverse patient populations and established healthcare infrastructures. This evolution is propelled by the pressing need for innovative therapies and a growing demand for expanding clinical research into Latin America for inclusive research representation.
Significantly, funding for research studies throughout the Andean Region has soared from $3-4 million to over $50 million each year, indicating a strong dedication to improving research capabilities. This growth is significantly connected to expanding clinical research into Latin America, as seen in the increasing number of outsourced trials in Peru, Colombia, and Chile. However, to truly unlock Latin America's potential for Medtech startups, expanding clinical research into Latin America is essential to address the linguistic, cultural, and socio-economic barriers, as well as the regulatory hurdles and financial constraints that impact the industry.
Working together with a prominent CRO such as bioaccess®, which focuses on supporting studies for Medtech firms, can assist in closing these gaps. Bioaccess® not only provides rapid, affordable, and high-quality medical data services but also actively partners with local hospitals and stakeholders in expanding clinical research into Latin America by navigating the complexities of research studies. With a focus on advancing medical devices through various study types—including EFS, FIH, pilot, pivotal, and PMCF studies—bioaccess® plays a crucial role in enhancing research efforts.
This alignment not only facilitates the advancement of medical knowledge but also greatly enhances patient outcomes, making South America an attractive location for expanding clinical research into Latin America.
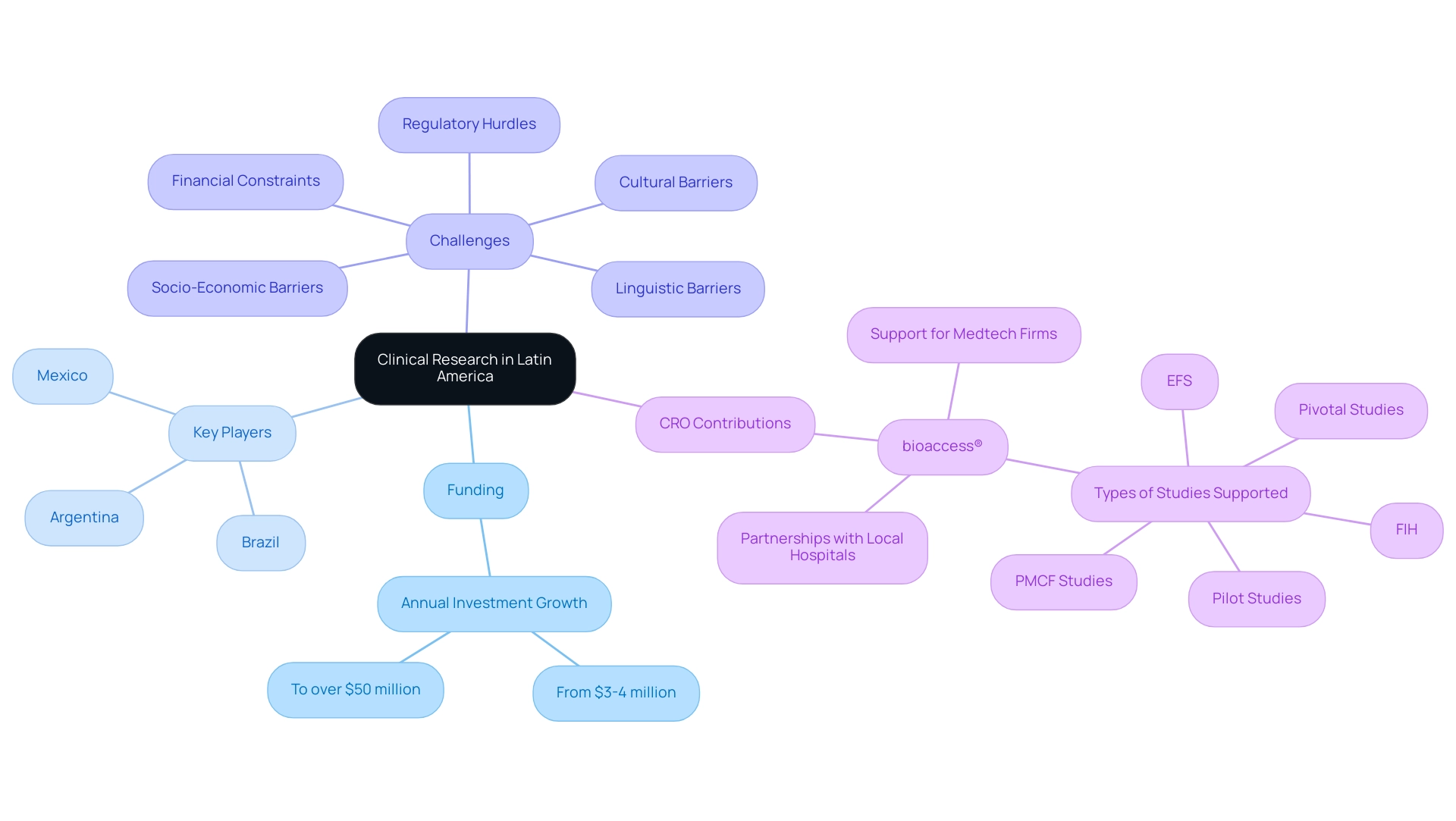
Why Latin America is an Attractive Location for Clinical Trials
Expanding clinical research into Latin America provides various appealing benefits for the execution of research studies, making it a desirable location for pharmaceutical firms. Key benefits include:
- Diverse Patient Populations: The region is characterized by a rich tapestry of ethnic backgrounds and health conditions, facilitating comprehensive data collection. Participants were notably recruited from community-based clinics providing services primarily to uninsured Latinos.
Research suggests that individuals who view themselves as at risk, including those susceptible to breast cancer, demonstrate a considerably greater probability of signing up for research studies with more flexible requirements (p<.01). To further enhance recruitment, Ajay Singh, a senior associate dean at Harvard Medical School, emphasizes the importance of providing appropriate incentives to encourage a diverse participant pool, highlighting that understanding the dynamics of participation among diverse populations is crucial, as reflected in the case study titled 'Limitations of Self-Reported Data in Research Studies.' This study acknowledges the limitations of self-reported data and suggests the need for larger studies to better understand participation dynamics.
- Regulatory Benefits: Many Latin American countries have made strides in streamlining their regulatory processes, which allows for quicker approvals and faster initiation of studies.
This aspect is essential as it corresponds with the increasing need for quality and patient safety specialists, ensuring that studies meet both ethical and scientific standards. Significantly, Barranquilla, with the partnership between bioaccess™ and Caribbean Health Group, is becoming a leading center for medical studies, backed by Colombia's Minister of Health. The recent meeting held in Miami emphasized this partnership, where specific commitments were made to improve the research landscape in Barranquilla.
- Cost-Effectiveness: Expanding clinical research into Latin America can result in significantly more affordable research studies compared to their North American and European counterparts, leading to substantial operational cost savings. This cost-effectiveness is especially attractive given the continuous demand for more accessible healthcare solutions.
- Growing Infrastructure: The area is experiencing significant investments in healthcare infrastructure and technology, which improve its ability for high-quality medical studies. Such enhancements not only strengthen the efficiency of the process but also add to the overall dependability of findings. Moreover, thorough management services for medical studies provided by bioaccess™ and Caribbean Health Group—including feasibility assessments, site selection, compliance evaluations, setup, monitoring, and reporting—are essential for ensuring successful outcomes.
These combined factors, especially the strategic initiatives in Barranquilla, position the region favorably for pharmaceutical companies aiming to conduct effective and efficient studies, which is key for expanding clinical research into Latin America as the environment for scientific exploration evolves into 2024.
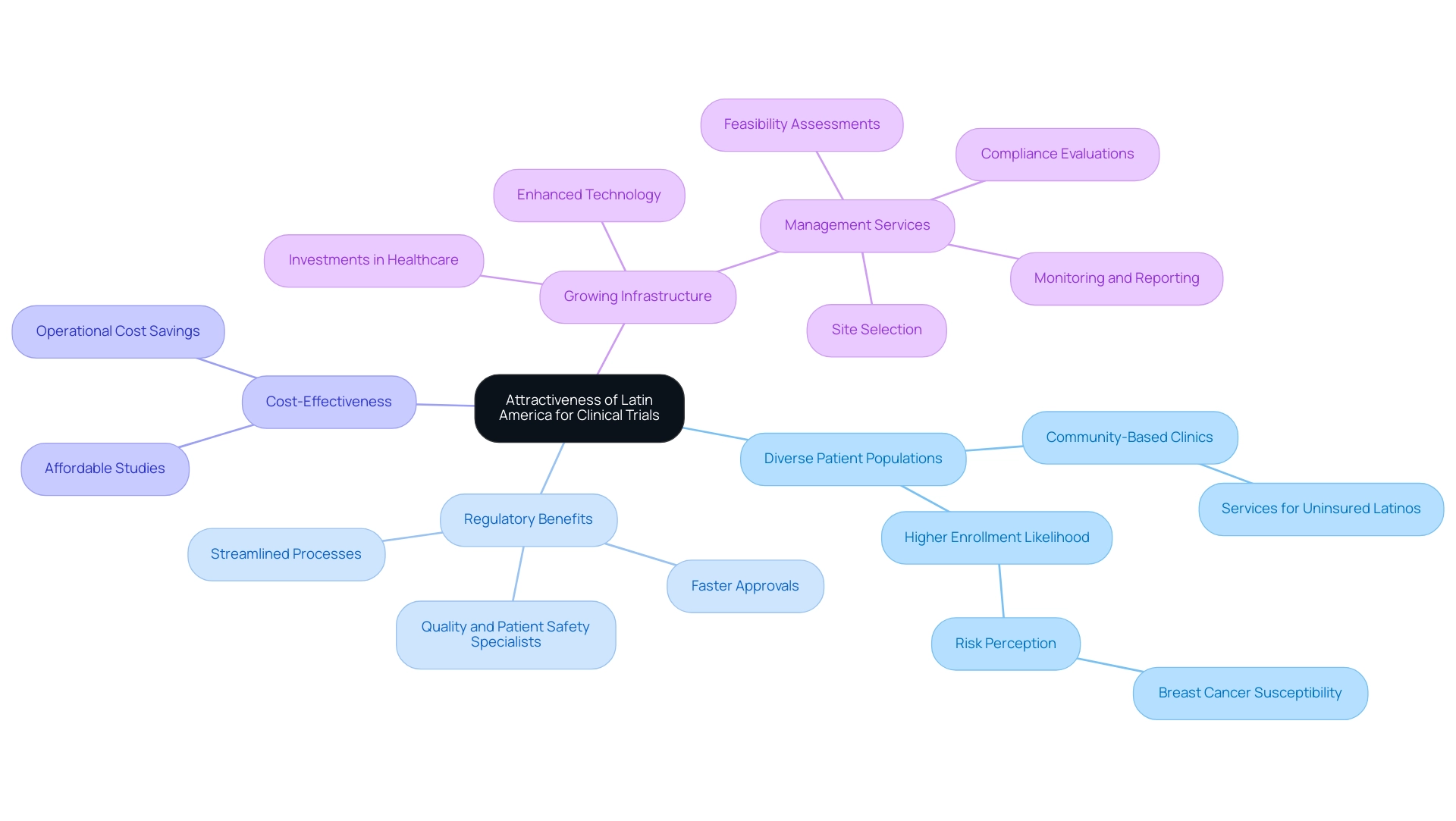
Navigating Challenges in Latin American Clinical Research
The region of America provides substantial prospects for medical research and medtech innovations, and bioaccess® distinguishes itself as your reliable CRO for expanding clinical research into Latin America while speeding up medical device evaluations in this dynamic area. However, it also presents a range of formidable challenges that must be navigated effectively. Key hurdles include:
- Regulatory Variability: The diverse regulatory frameworks across Latin American countries can lead to inconsistencies and delays in the approval process, complicating the execution of clinical trials. Comprehending regional regulations is crucial for promoting smoother collaboration and improving efficiency. As noted by industry leaders, this relationship significantly helps to increase subject enrollment and retention rates, and improves patient compliance.
- Logistical Issues: Coordinating studies across multiple countries complicates logistics, particularly in patient recruitment and data management. This is a crucial aspect to consider, as inefficient logistics can hinder the progress of research studies and impact timelines.
- Cultural Differences: A thorough understanding of local customs and healthcare practices is essential for effective communication and patient engagement. Engaging with local communities is not just beneficial; it’s vital for the success of research trials, ensuring that protocols resonate with participants and meet their needs.
- Limited Access to Resources: In certain areas, access to advanced medical technologies and facilities can be restricted, which may impact the quality and reliability of study outcomes. Developing countries often face challenges such as deficient health systems and a lack of skilled workforce, further complicating participant recruitment. For example, the high occurrence of gallbladder cancer in Peru highlights the pressing need for medical investigations to tackle specific health concerns in the area.
- Ethical Concerns: Offering additional care to study participants can raise ethical issues regarding inducement, which must be carefully managed to uphold the integrity of medical studies. Additionally, it is crucial to ensure compliance with data protection regulations and to have clear grievance handling processes in place, as outlined by our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"). Participants should be informed of their rights regarding data protection and how to address any concerns they may have.
Addressing these challenges necessitates meticulous planning, fostering local partnerships, and cultivating a deep understanding of the regional landscape, particularly in the context of expanding clinical research into Latin America. Bioaccess® is focused on expanding clinical research into Latin America by improving the efficiency of medical studies, demonstrating how partnerships with local communities and stakeholders are essential for addressing the challenges encountered in conducting medical evaluations. Moreover, following the best practices detailed in our user manuals will guarantee operational efficiency and compliance throughout the research process.
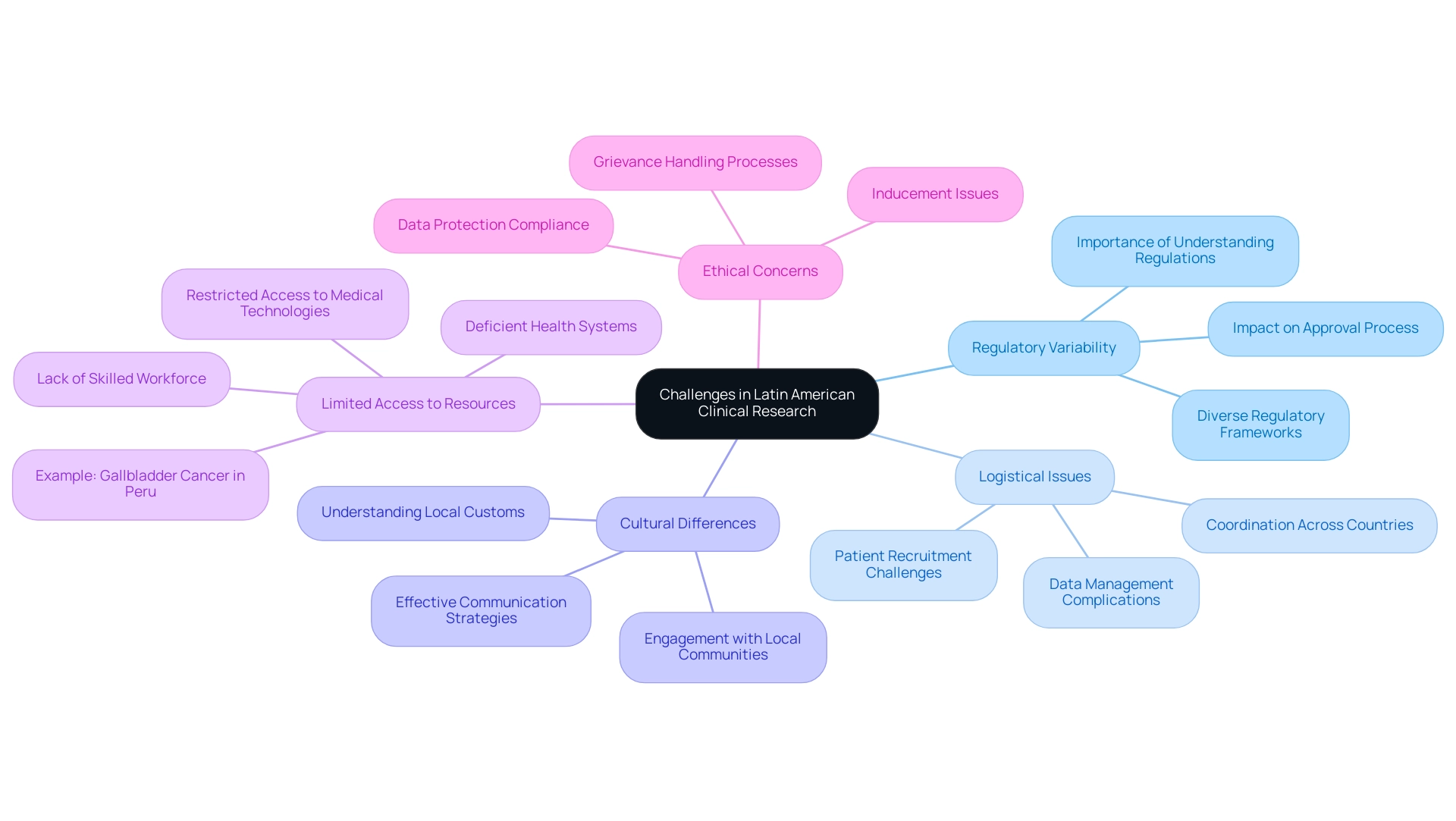
Embracing Patient-Centric Strategies in Clinical Trials
Expanding clinical research into Latin America is vital, as applying patient-focused strategies is essential for the success of research studies in countries like Uruguay, which conducts only 16 studies, Cuba with 25, and Paraguay with merely 1, where cancer occurrence and death rates are disturbingly high. This disparity highlights the urgent need for effective strategies to enhance participation and engagement, including:
- Community Engagement: Building partnerships with local healthcare providers and community leaders is essential for establishing trust and increasing awareness about clinical trials. Gustavo Gössling emphasizes, 'LACOG has developed a great work regarding fostering and improving access to cancer studies,' underscoring the importance of community involvement in these efforts.
- Tailored Communication: Using culturally appropriate and accessible language in all communications is vital to ensure understanding and encourage participation among diverse populations.
- Flexible Participation Options: Providing multiple ways to participate, including telehealth choices, can meet the diverse needs of patients, making it easier for them to engage in research.
- Feedback Mechanisms: Establishing continuous feedback channels from participants allows researchers to promptly address concerns and enhance the experience.
By prioritizing the needs and viewpoints of patients, researchers can not only encourage greater involvement but also improve the overall quality of medical studies. This patient-centric approach is necessary, especially in light of recent survey findings that identified significant awareness and education gaps among both physicians and patients, as highlighted in the case study titled 'Awareness and Education Gaps.' These gaps obstruct recruitment rates for medical studies.
Furthermore, the media attention on research studies in South America, especially by Clinical Leader, plays a crucial role in expanding clinical research into Latin America, increasing awareness and emphasizing the influence of Medtech studies on local economies—creating jobs, promoting economic growth, and enhancing healthcare access. For example, Clinical Leader's recent article discusses how improved research management services, including feasibility studies, site selection, and compliance reviews, are essential for successful study implementation. With focused measures to enhance information, training, and education, the effectiveness of community engagement strategies can be significantly improved, paving the way for more successful health studies in America.
A Step-by-Step Approach to Launching Clinical Trials in Latin America
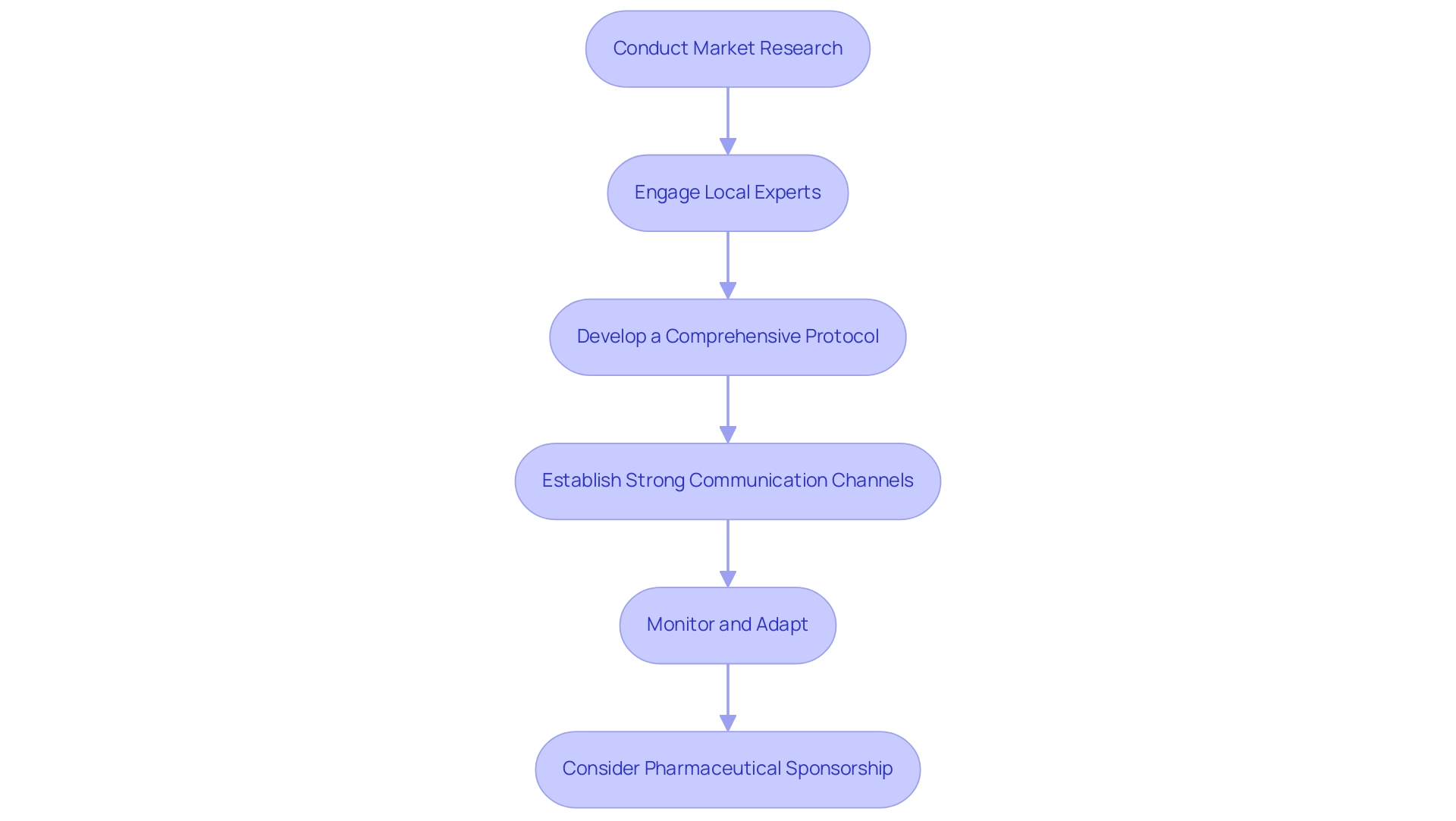
Initiating medical studies in South America demands a strategic method to maneuver through the distinct healthcare environment, especially in Colombia, which offers notable competitive benefits. Organizations should consider the following steps:
-
Conduct Market Research: Begin by analyzing the specific needs and characteristics of the target population in selected countries, such as Colombia, where the healthcare system is ranked among the best globally.
Grasping local health challenges is essential for customizing initiatives. Recent statistics show that the market for medical trials in Latin America is growing, which is a result of expanding clinical research into Latin America, with heightened interest from pharmaceutical firms aiming to broaden their development efforts in the region.
-
Engage Local Experts: Working with local trial organizations (CROs) is essential.
These partnerships not only provide insights into regulatory requirements but also enhance patient recruitment strategies. As Gustavo Werutsky observes,
The academic Cancer Research Groups operating in this area have a crucial role in training medical oncologists and study specialists in proposing investigator-initiated studies assessing topics with epidemiological significance for this region.
Furthermore, local CROs are instrumental in navigating the complexities of the local healthcare system, including the rigorous ICH/GCP certification process that hospitals must pass to conduct clinical research with pharmaceutical drugs, which significantly contributes to the success of clinical trials.
-
Develop a Comprehensive Protocol: Craft a protocol that is adaptable to local regulations and cultural contexts, ensuring relevance and compliance throughout the process. In Colombia, acquiring IRB/EC approval requires around 90-120 days, a factor that can accelerate study initiation.
-
Establish Strong Communication Channels: Effective communication among all stakeholders—including sponsors, investigators, and patients—is crucial for study integrity and participant engagement.
-
Monitor and Adapt: Continuously observe progress and be prepared to adjust strategies based on real-time feedback and the challenges encountered. This flexibility will enhance the likelihood of successful execution.
-
Consider Pharmaceutical Sponsorship: It is essential to acknowledge that a substantial majority of research studies in South America and the Caribbean are funded by pharmaceutical firms.
While this sponsorship enhances access to new cancer treatments, it emphasizes the necessity for more independent study initiatives to meet local health requirements. Moreover, media coverage has emphasized the rising interest and investment in experimental procedures within Colombia, further establishing its reputation as a viable location for exploration.
By following these steps and utilizing Colombia's advantageous circumstances—such as a population surpassing 50 million, universal healthcare coverage, and strong R&D tax incentives—organizations can greatly enhance their chances for successful research initiatives in South America, especially through expanding clinical research into Latin America, with a focus on developing markets like Chile, which provides opportunities for research tourism and rare diseases.
Leveraging Technology to Enhance Clinical Trials in Latin America
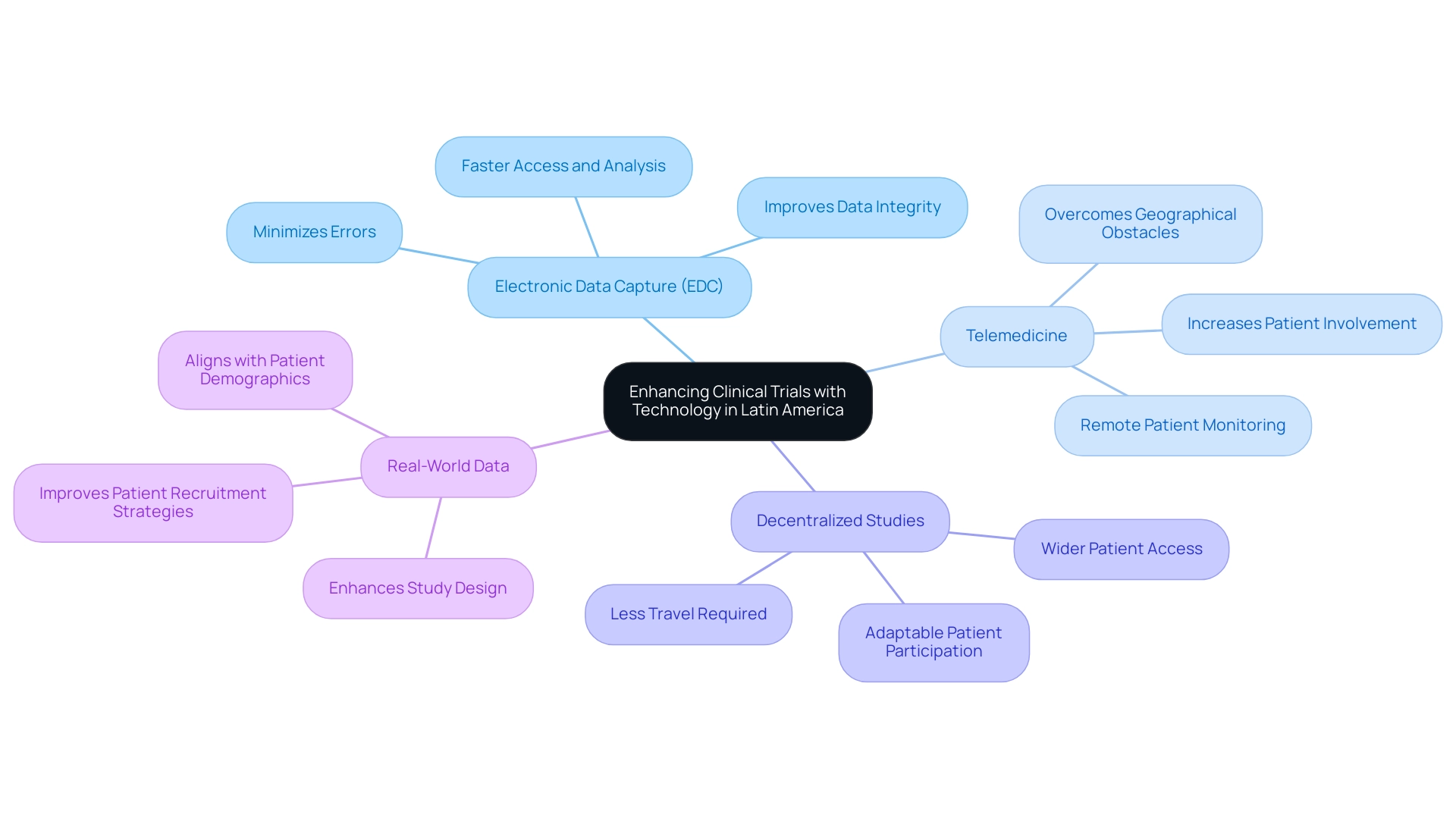
Incorporating technology into medical studies provides a revolutionary method for enhancing efficiency and data quality, which is essential when expanding clinical research into Latin America. With over 20 years of experience in Medtech, bioaccess® is well-equipped to leverage key technologies, including:
- Electronic Data Capture (EDC): Implementing EDC systems can streamline data collection processes, significantly minimizing the errors commonly associated with traditional paper-based methods. This not only improves data integrity but also promotes faster data access and analysis.
- Telemedicine: The adoption of telehealth solutions enables remote patient monitoring and consultations, effectively increasing patient involvement and adherence to study protocols. This is particularly vital in overcoming geographical obstacles common in the area.
- Decentralized Studies: Adopting decentralized study models allows for more adaptable patient participation, lessening the need for travel and thus improving access to research for a wider patient population.
- Real-World Data: Leveraging real-world evidence can enhance study design and improve patient recruitment strategies, ensuring that investigations are better aligned with the actual patient demographic.
bioaccess® specializes in comprehensive research management services, including feasibility studies, regulatory approvals, project management, and patient recruitment, ensuring that startups receive the expert guidance needed to navigate the regulatory landscape effectively. The influence of Medtech research extends beyond the studies themselves, contributing to local economies through job creation, economic growth, and healthcare enhancements. Recent statistics indicate that only 1/3 of acute treatment studies achieve their targeted number of patients within the planned time frame, highlighting the urgent need for innovative methodologies in recruitment.
The findings from Stanton et al. suggest a compelling use case for machine learning and data mining, combined with virtual communication, to optimize these processes further. As Rentschler Biopharma demonstrates with its expanded global cGMP capacity and enhanced cell & gene therapy offerings, expanding clinical research into Latin America can significantly bolster operational capabilities and improve patient outcomes.
Additionally, a comparative analysis titled 'Digital Methods to Recruit Stroke Survivors' highlighted the challenges of recruitment, where digital promotion resulted in fewer participants compared to traditional methods. Expanding clinical research into Latin America by addressing the linguistic, cultural, and socio-economic barriers identified in nations like Peru, Colombia, and Chile can help clinical research organizations elevate their effectiveness and ensure more successful trial executions.
Conclusion
Latin America is on the verge of a clinical research revolution, driven by increased investments and the interest of global pharmaceutical companies. With its diverse patient populations in countries like Brazil, Mexico, and Argentina, the region is well-positioned to generate valuable clinical data. The dramatic rise in trial investments, especially in the Andean Region, highlights the urgent demand for innovative treatments and inclusive trial representation.
To unlock its full potential, the region must navigate challenges such as:
- Complex regulatory frameworks
- Logistical issues
- Cultural differences
Collaborating with specialized clinical research organizations like bioaccess® can help overcome these barriers, ensuring high-quality data collection and better patient outcomes.
Implementing patient-centric strategies is also vital. Engaging communities, tailoring communication, and providing flexible participation options can enhance patient recruitment and involvement. This approach addresses critical awareness gaps and improves the overall trial experience.
Additionally, leveraging technology will be key to enhancing efficiency and data quality. The adoption of electronic data capture, telemedicine, and decentralized trial models can broaden patient access and streamline processes.
In conclusion, the future of clinical research in Latin America is promising. By fostering collaboration, prioritizing patient needs, and embracing innovative technologies, stakeholders can transform the clinical trial landscape, improve health outcomes, and position the region as a leader in medical research.
Frequently Asked Questions
What changes are occurring in the medical investigation environment in Latin America?
Latin America is experiencing a notable change in its medical investigation environment, marked by a significant increase in funding and growing interest from international pharmaceutical firms, particularly in countries like Brazil, Mexico, and Argentina.
Why are Brazil, Mexico, and Argentina important for clinical research in Latin America?
These countries are emerging as key contributors to clinical research due to their diverse patient populations and established healthcare infrastructures, which are essential for inclusive research representation.
How has funding for research studies in the Andean Region changed recently?
Funding for research studies in the Andean Region has dramatically increased from $3-4 million to over $50 million annually, reflecting a strong commitment to enhancing research capabilities.
What challenges does Latin America face in expanding clinical research?
Latin America needs to address linguistic, cultural, and socio-economic barriers, as well as regulatory hurdles and financial constraints, to fully unlock its potential for Medtech startups and clinical research.
How can collaboration with a Contract Research Organization (CRO) like bioaccess® benefit clinical research in Latin America?
Bioaccess® supports studies for Medtech firms by providing rapid, affordable, and high-quality medical data services, and by partnering with local hospitals and stakeholders to navigate the complexities of research studies.
What types of studies does bioaccess® focus on to enhance research efforts?
Bioaccess® focuses on advancing medical devices through various study types, including Early Feasibility Studies (EFS), First-in-Human (FIH) studies, pilot studies, pivotal studies, and Post-Market Clinical Follow-up (PMCF) studies.
What are the benefits of conducting clinical research in Latin America?
The benefits include diverse patient populations for comprehensive data collection, streamlined regulatory processes for quicker approvals, cost-effectiveness compared to North America and Europe, and significant investments in healthcare infrastructure and technology.
How does the partnership between bioaccess™ and Caribbean Health Group impact research in Barranquilla?
This partnership is positioning Barranquilla as a leading center for medical studies in Colombia, with commitments made to enhance the research landscape, supported by the Minister of Health.
Why is cost-effectiveness an important factor for pharmaceutical firms in Latin America?
Conducting research studies in Latin America can lead to significant operational cost savings, making it an attractive option for firms looking to provide more accessible healthcare solutions.
What strategic initiatives are being taken to improve the research environment in Latin America as of 2024?
Strategic initiatives, especially in Barranquilla, aim to strengthen the region's capabilities for conducting effective and efficient clinical studies, enhancing the overall landscape for scientific exploration.

