Overview
The article outlines a comprehensive step-by-step guide for implementing clinical trial innovation in Medtech in Mexico. It emphasizes the significance of:
- Understanding the regulatory landscape
- Leveraging local partnerships for patient recruitment
- Adopting innovative methodologies to enhance efficiency and outcomes in clinical trials
These strategies position Mexico as an attractive destination for Medtech research, ultimately driving advancements in clinical practices.
Introduction
In recent years, Mexico has emerged as a key player in the global clinical trial landscape, particularly within the Medtech sector. With a vast and diverse patient population, the country offers unparalleled opportunities for clinical research that can yield valuable insights and robust data. As the number of active clinical research sites continues to rise, so too does the favorable regulatory environment, which has been streamlined to facilitate quicker market access for innovative medical devices.
However, navigating this evolving landscape requires a deep understanding of local healthcare infrastructure, cultural attitudes, and the competitive dynamics at play. This article delves into the multifaceted aspects of conducting clinical trials in Mexico, exploring best practices, innovative methodologies, and the strategic partnerships that can drive success in this burgeoning market.
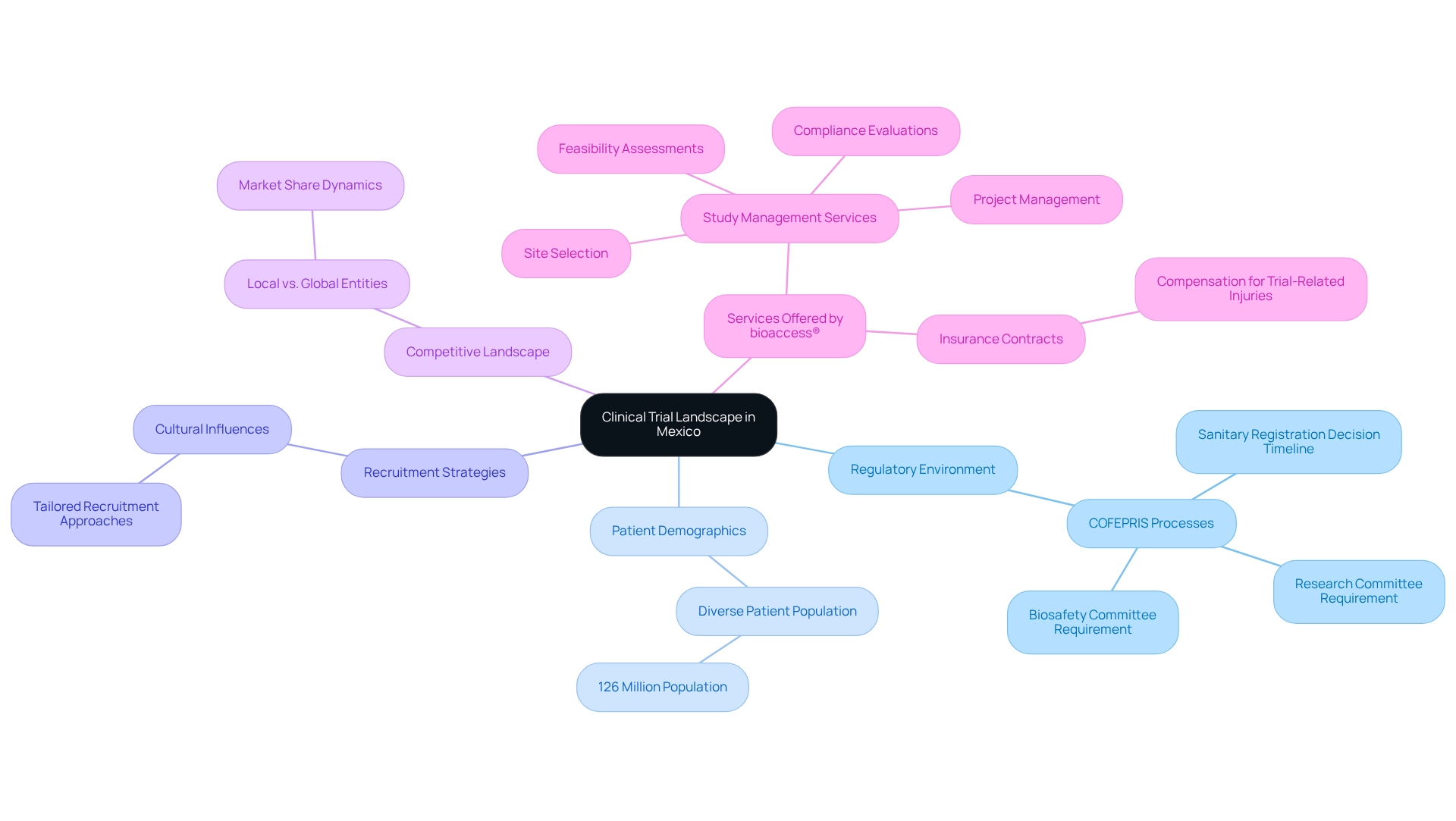
Understanding the Clinical Trial Landscape in Mexico
Mexico has established itself as a pivotal center in the global research trial landscape, particularly through its emphasis on clinical trial innovation for medtech. With a diverse patient population exceeding 126 million, the country offers a rich demographic for medical studies, which is vital for obtaining reliable data. By 2025, there are over 300 active medical study sites across Mexico, reflecting a significant increase in infrastructure dedicated to trials.
The regulatory environment in Mexico is evolving to support medical investigations more effectively. The Federal Commission for the Protection against Sanitary Risk (COFEPRIS) has streamlined processes, allowing a maximum of 60 business days for decisions on sanitary registration, thus facilitating faster market access for new medical devices. Insights from Kindle, a CRO specializing in study management, further underscore this efficiency, noting that average approval durations typically range from 14 to 16 weeks.
Moreover, every health facility involved in research is now mandated to establish a Research Committee and a Biosafety Committee, ensuring that proposed projects adhere to high technical and scientific standards. For Medtech companies aiming to conduct successful tests, understanding the local healthcare framework, which includes both public and private hospitals, is crucial. Additionally, cultural perspectives surrounding clinical studies significantly influence patient recruitment and retention.
Tailoring recruitment strategies to align with local views can enhance participation rates and improve outcomes.
In tandem with these factors, the competitive landscape is shifting, with both local and global entities vying for market share. Companies must remain cognizant of this dynamic environment to effectively position their studies and capitalize on Mexico's advantages, such as cost-effectiveness and access to a large patient pool. Recent trends indicate a growing interest in early-feasibility studies and post-market research follow-up studies, highlighting clinical trial innovation for medtech in Mexico that can accelerate the development of Medtech solutions.
bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting. Their expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies equips Medtech companies to navigate the complexities of research effectively. Furthermore, it is essential for Medtech companies to ensure that insurance contracts are established to address any study-related injuries to research participants, thereby addressing ethical considerations and enhancing participant protection.
bioaccess® is committed to safeguarding information security and fostering client confidence, with established grievance and data protection procedures to address any concerns transparently.
Overall, Mexico's research landscape presents a distinctive opportunity for Medtech companies to leverage clinical trial innovation to advance their innovations while navigating a supportive regulatory framework and a diverse patient demographic.
Navigating Regulatory Requirements for Medtech Trials
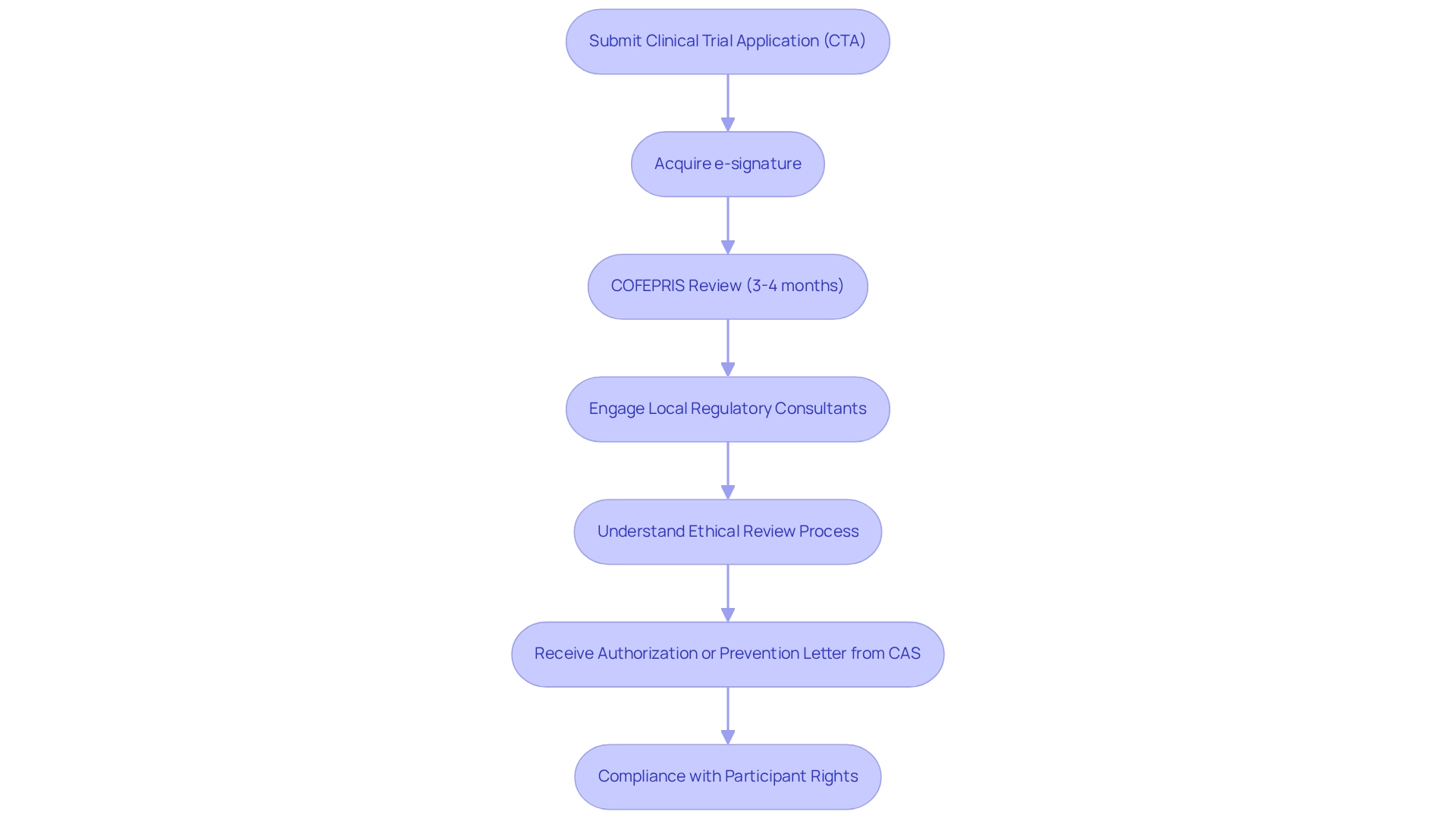
Clinical trial innovation for medtech in Mexico necessitates that companies conduct trials successfully while adhering to the regulations set forth by the Federal Commission for Protection Against Sanitary Risks (COFEPRIS). This process commences with the submission of a Clinical Trial Application (CTA), detailing the study's objectives, methodology, and ethical considerations. A crucial step in this process is acquiring an e-signature to register and submit requests for protocol authorization via DIGIPRiS.
Companies should prepare for a rigorous review process, typically lasting between 3 to 4 months, during which COFEPRIS assesses the application for compliance with Mexican legislation. Engaging local regulatory consultants is highly advisable, as they offer invaluable guidance on specific documentation requirements and facilitate effective communication with COFEPRIS. Moreover, understanding the ethical review process is essential; this involves securing approval from independent ethics committees to ensure compliance and protect participant rights.
In 2025, the regulatory landscape continues to evolve, with COFEPRIS's Sanitary Authorization Commission (CAS) playing a pivotal role in evaluating protocol authorization requests. The commission issues resolutions of authorization or prevention letters based on adherence to established regulations.
Significantly, one of Mexico’s largest hospital networks recently completed a $700 million expansion, resulting in 15 new hospital facilities. This development underscores the growing infrastructure that supports medical research and the potential for economic development in the region.
Best practices for submitting Clinical Trial Applications in Mexico include:
- Thorough preparation of all required documents
- Proactive engagement with regulatory bodies
- A clear understanding of participant rights
This is particularly crucial considering case analyses of biobank regulations, which indicate that participants retain substantial rights over their biological materials, including the ability to revoke consent at any time. This principle must be respected by researchers and biobank operators.
By adhering to these guidelines, Medtech companies can navigate the application process for clinical trial innovation in Mexico more efficiently, facilitating advancements in medical technology while promoting job creation and global collaboration. bioaccess® offers extensive management services for research, encompassing feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting. This ensures that companies are well-prepared to meet regulatory requirements and achieve successful outcomes in their research. The impact of these medical studies on local economies is significant, as they not only create jobs but also enhance healthcare infrastructure and foster international collaboration.
Leveraging Latin America's Advantages for Medtech Clinical Trials
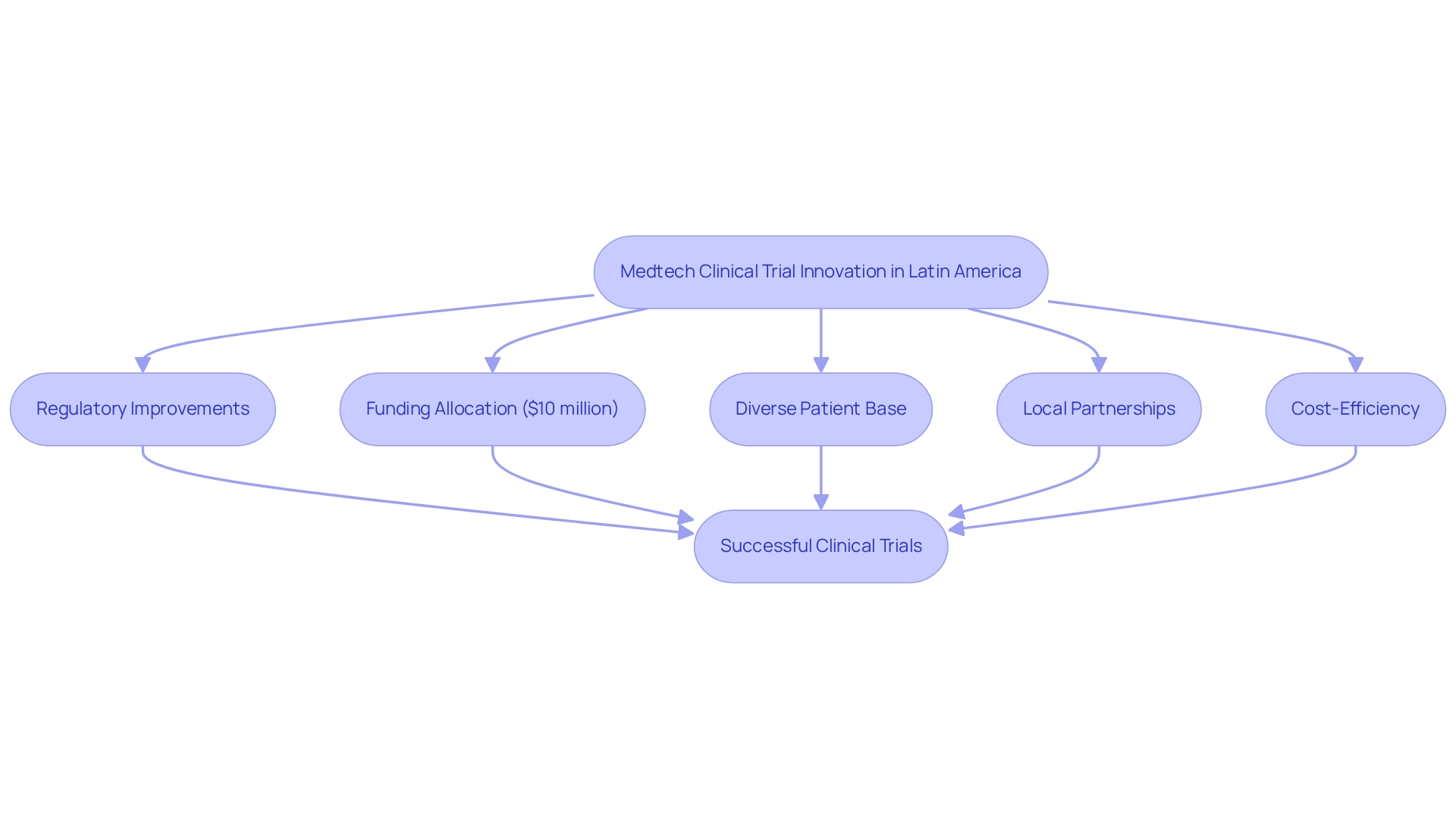
Latin America presents a compelling environment for Medtech studies, particularly with the clinical trial innovation for medtech in Mexico, characterized by significantly reduced operational expenses and expedited patient enrollment. By 2025, patient recruitment rates in the region are projected to surpass those in many other global markets, driven by a diverse patient base and an expanding network of trial organizations (CROs). Mexico stands out due to its robust healthcare infrastructure, which not only facilitates clinical trial innovation for medtech but also enhances the overall scientific environment.
The region has undergone substantial regulatory improvements aimed at fostering clinical trial innovation for medtech in Mexico, rendering it a more attractive destination for Medtech firms. For instance, the government allocates approximately $10 million in funding for development projects, further stimulating innovation within the sector. Additionally, as Julio G. Martinez-Clark, CEO, notes, "The government of Colombia appears to be the sole nation in Latin America with a proactive effort to draw more research studies as part of its strategy to transform into a knowledge economy by 2031," underscoring the regional commitment to advancing medical research.
A prominent example of this dedication is ReGelTec's Early Feasibility Study on HYDRAFIL™, which successfully treated eleven patients suffering from chronic low back pain in Barranquilla, Colombia. This study exemplifies the innovative approaches being adopted in the region, with processes managed remotely, showcasing the integration of technology in medical assessments.
Effective patient recruitment strategies in Latin America often leverage local partnerships and community engagement to foster trust and awareness among potential participants. Collaborating with local healthcare providers can enhance outreach and educational initiatives, resulting in improved recruitment rates. Case studies indicate that clinical trial innovation for medtech in Mexico offers a cost-efficient alternative, with study expenses significantly lower than those in the United States.
This cost analysis highlights the financial advantages of conducting research in Mexico, where the cost-efficiency of Medtech studies is bolstered by the region's favorable economic conditions.
Moreover, expert perspectives emphasize the benefits of conducting clinical studies in Mexico, reinforcing the significance of clinical trial innovation for medtech in Mexico as the country emerges as a key player in the global clinical investigation landscape. The emphasis on personalized medicine and technological advancement positions Latin America as a noteworthy contributor to medical innovation trends, with projections indicating that the application of medicine in the region will grow more rapidly than in other areas over the next five years. Addressing data gaps for reliable insights into medical funding in Latin America is crucial, as it supports effective decision-making and resource allocation.
By conducting feasibility analyses to identify suitable locations and patient demographics, companies can ensure that their evaluations are both cost-effective and efficient, ultimately accelerating the market introduction of medical devices.
Types of Clinical Studies: From First-in-Human to Post-Market Follow-Up
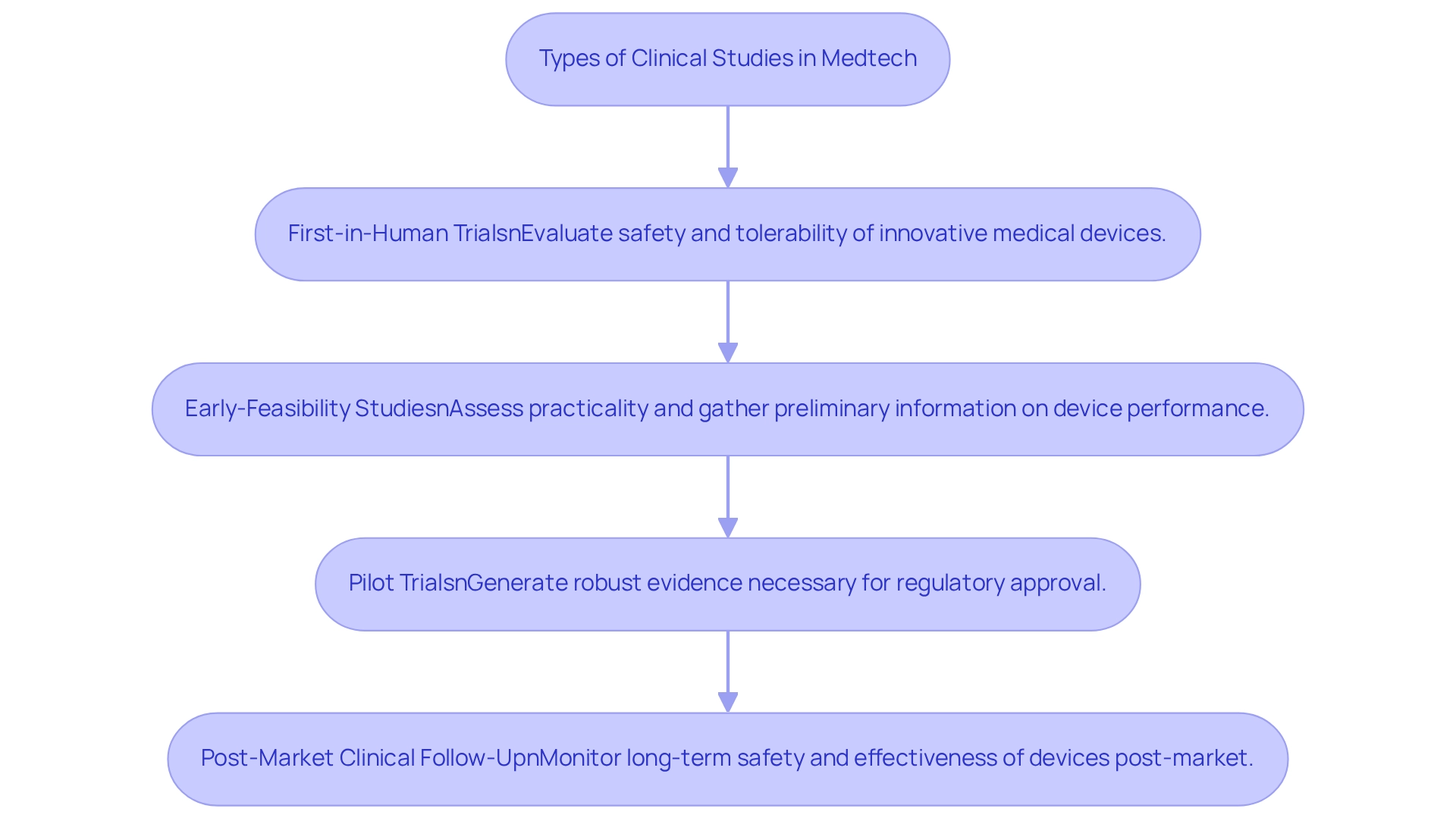
Medtech trials, as integral components of clinical trial innovation for medtech in Mexico, encompass a variety of trial types, each tailored to achieve specific objectives within the investigative process. First-in-Human (FIH) trials are pivotal for evaluating the safety and tolerability of innovative medical devices, underscoring the significance of clinical trial innovation for medtech in Mexico as the foundational phase in medical assessment. These investigations yield essential insights into how a device interacts with human physiology, thereby guiding subsequent research phases.
For instance, Avantec Vascular has chosen bioaccess™ to facilitate its first-in-human trial of a groundbreaking vascular device in Latin America, emphasizing the critical role of expert guidance during this vital phase.
Early-Feasibility Studies (EFS) complement the framework of clinical trial innovation for medtech in Mexico by assessing the practicality of a device within a medical context. These evaluations aim to gather preliminary information on device performance and usability, which is crucial for refining product design and ensuring alignment with healthcare standards. The success rates of EFS have shown promising trends, with numerous research initiatives yielding valuable insights that inform further development.
Bioaccess® is dedicated to overseeing these evaluations, leveraging over 20 years of Medtech expertise to navigate the complexities of clinical trial innovation for medtech in Mexico.
In conjunction with EFS and FIH investigations, bioaccess® also manages Pilot Trials and Post-Market Clinical Follow-Up (PMCF) evaluations, which contribute significantly to the clinical trial innovation landscape for medtech in Mexico. Pivotal research is focused on generating robust evidence necessary for regulatory approval, while PMCF studies monitor the long-term safety and effectiveness of devices post-market introduction, ensuring ongoing compliance with regulatory standards.
Understanding these diverse study types is essential for Medtech companies to effectively develop their protocols and navigate the regulatory landscape, particularly concerning clinical trial innovation for medtech in Mexico. As the sector evolves, the integration of innovative approaches and technologies, such as artificial intelligence, is expected to enhance the efficacy and outcomes of these studies. Notably, 64% of clinicians in South America express optimism regarding AI's future influence on healthcare decision-making, signaling a transition towards more innovative methodologies in clinical research.
Moreover, it is crucial to acknowledge that randomized controlled trials (RCTs) exhibited the lowest percentage of research demonstrating a positive impact of health IT on safety outcomes at 28%. This statistic highlights the necessity for high-quality, large-scale studies to identify effective health IT tools and their optimal implementation contexts, as discussed in the case study titled "Implications for Stakeholders in Healthcare." Additionally, as Julio G. Martinez-Clark emphasizes, identifying a reliable Contract Research Organization (CRO) in Latin America is vital, particularly in understanding the regulatory requirements for the authorization of first-in-human medical device studies.
Overcoming Challenges in Clinical Research Management
Clinical research management in Medtech studies faces significant challenges, particularly in patient recruitment, regulatory compliance, and data management. As we look to 2025, the complexities of patient recruitment persist, with statistics revealing that recruitment sites typically require around 30.5 days to schedule the first patient visit. This delay often results in a notable drop-off in interested patients, underscoring the urgent need for effective strategies to mitigate this issue.
To address these obstacles, companies must adopt robust project management practices that encompass clear timelines and effective resource allocation. Engaging local investigators who possess a deep understanding of the patient population can significantly bolster recruitment efforts. This localized approach not only fosters trust but also enhances the likelihood of participation among underrepresented groups. For instance, GlobalCare Clinical Studies' collaboration with bioaccess™ has demonstrated the effectiveness of this strategy, achieving more than a 50% reduction in subject recruitment time and an impressive retention rate exceeding 95%.
Such results underscore the necessity for clinical trial innovation in Medtech within Mexico, aimed at enhancing accessibility and inclusivity in medical trials. Furthermore, leveraging technology for data collection and monitoring can streamline processes and improve data integrity. The implementation of electronic data capture systems and real-time monitoring tools can alleviate the common data management challenges faced in medical studies. Consistent training and transparent communication with the project team are crucial for maintaining compliance and ensuring that all team members are aligned with the project's goals.
As emphasized by bioaccess®, which specializes in comprehensive research study management services—including feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting—partnering with a vetted CRO is essential for U.S. medical device companies operating in Colombia. Additionally, bioaccess® offers a 'no cure – no pay' solution to ensure budget adherence and timely target achievement, further enhancing its innovative approach to research study management. By concentrating on these strategies, Medtech firms can achieve clinical trial innovation in Mexico, enabling them to navigate the intricacies of study management more efficiently and ultimately leading to successful outcomes.
Best Practices for Recruiting and Managing Clinical Research Teams
Assembling and overseeing a high-performing medical study team is essential for the successful execution of studies in the Medtech sector. Organizations must prioritize hiring individuals who possess relevant experience and a thorough understanding of research protocols. This foundational expertise is crucial for navigating the complexities of research effectively, particularly in the context of bioaccess's comprehensive management services for medical experiments, which encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Furthermore, bioaccess boasts over 20 years of experience in the Medtech field, specializing in managing:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
This specialization enhances our capability to deliver successful outcomes.
Ongoing training is vital for maintaining team competency and adapting to evolving industry standards. Training programs that cover critical topics such as trial endpoints, sample size calculations, and advanced methodologies for phase III trials can significantly enhance team performance. For instance, an examination of British orthopedic journals revealed that only 3 out of 49 articles (6.1%) included a statistical power analysis with sufficient sample size, underscoring the necessity for strong training in statistical methods among medical investigation teams.
Moreover, the case study titled "Methodological Weaknesses in Clinical Investigations" advocates for conducting pilot studies to enhance study designs and improve the robustness of evidence.
Fostering a collaborative environment is equally important. Regular meetings to discuss progress, address challenges, and share insights can help maintain momentum and encourage open communication. Additionally, leveraging technology for communication and project management can streamline operations, improve coordination, and ultimately lead to better trial outcomes.
Optimal strategies for recruiting medical study teams in 2025 highlight the significance of diversity and inclusion, which can boost creativity and problem-solving within groups. Case studies have shown that organizations implementing structured recruitment processes and prioritizing cultural fit tend to build more effective teams. As Howard Barkan, an affiliated researcher and consulting statistician, observes, "Statistics in medical research: Important considerations" emphasize the importance of training in statistical methodologies.
By concentrating on these strategies, Medtech firms can ensure they have the right talent to drive clinical trial innovation for Medtech in Mexico and achieve success in research studies. Moreover, continuous training programs addressing study endpoints, sample size calculations, and advanced techniques for phase III studies are crucial to ensure teams are in sync with current industry standards. The influence of Medtech research studies on local economies, encompassing job creation and healthcare enhancement, further emphasizes the significance of efficient research management.
Implementing Innovative Methodologies in Clinical Trials
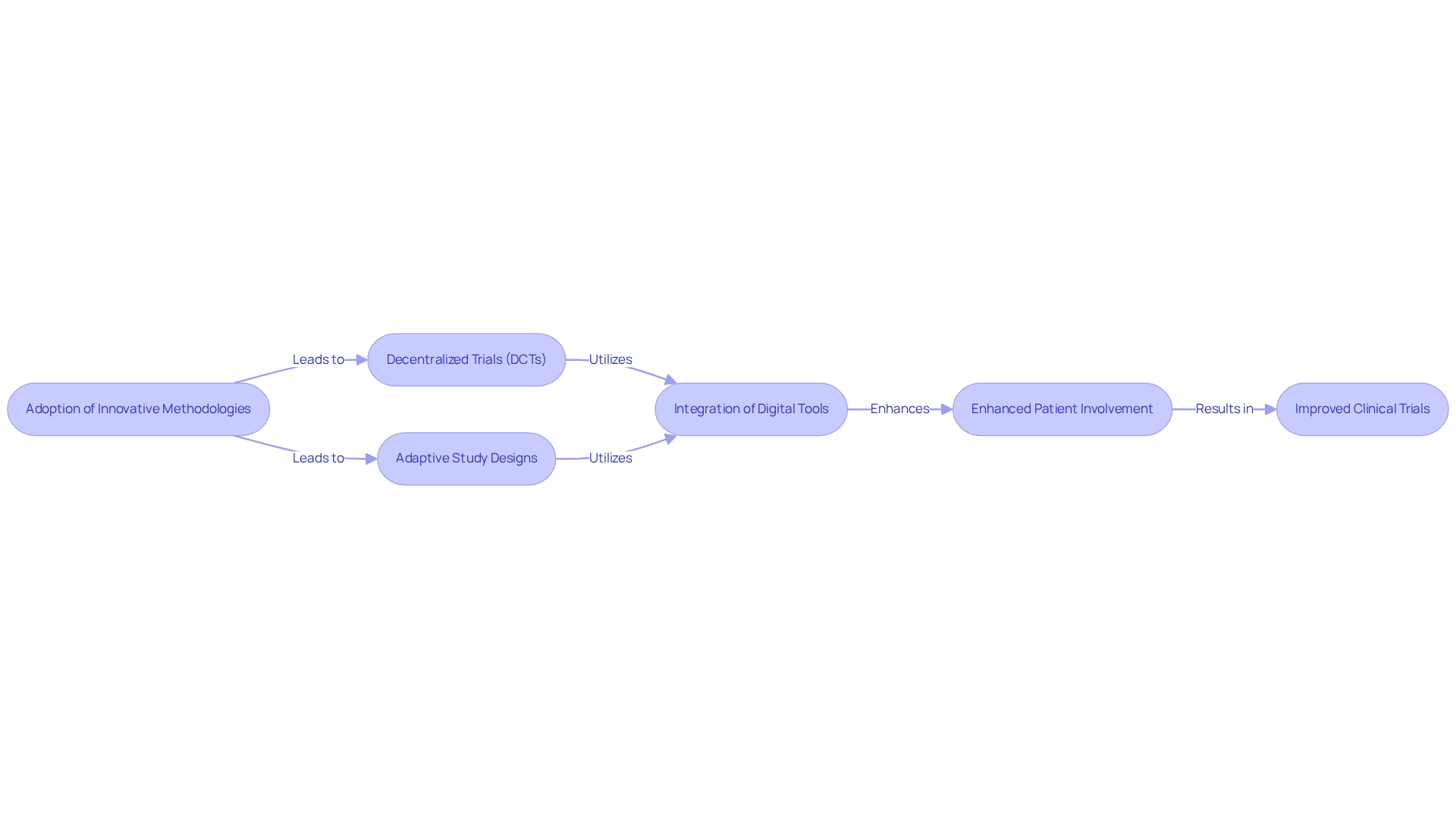
Innovative approaches in research studies, such as clinical trial innovation for medtech in Mexico, are essential for sustaining a competitive advantage in the Medtech industry. Methods like decentralized research (DCTs) and adaptive designs are at the forefront of clinical trial innovation for medtech in Mexico, significantly improving patient involvement and simplifying data gathering processes. DCTs, for instance, are anticipated to decrease study costs by 10-25% due to fewer necessary locations, reduced researcher fees, and minimized patient travel expenses, as emphasized in the case study titled "Cost and Time Savings from DCTs."
A recent survey by Byron et al. indicated that 94% of patients would likely use a mobile application for involvement in clinical studies, underscoring the increasing acceptance of digital health technologies. Moreover, integrating tools like wearables and mobile applications facilitates remote monitoring and data capture, making participation more convenient for patients. Adaptive study designs represent a key aspect of clinical trial innovation for medtech in Mexico, further enhancing efficiency by permitting adjustments based on interim outcomes, which can lead to quicker decision-making and resource distribution. As bioaccess® emphasizes, their expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) ensures that these innovative methodologies are effectively implemented.
As Ibrahim Kamstrup-Akkaoui, Vice President of Data Systems Innovation, noted, "We did a small AI initiative to see if we can generate meaningful test data for setting up and validating our systems. It turns out we can. An algorithm that we created examines previous research we conducted, learns from the actual data gathered, and applies it to produce something we can utilize for new investigations."
The influence of Medtech research extends beyond single experiments; they support local economies through job creation, economic development, and healthcare enhancement. Companies such as bioaccess® play a crucial role in promoting global cooperation, ensuring that clinical trial innovation for medtech in Mexico not only advances medical technology but also enhances community health outcomes. With over 20 years of experience in the Medtech field, bioaccess® is committed to data protection and client trust, ensuring that all processes are compliant and transparent.
As the Medtech landscape continues to evolve, staying informed about technological advancements and regulatory changes is essential for successful implementation. Organizations that adopt these innovative approaches will not only boost their operational efficiency but also enhance patient outcomes, ultimately accelerating the transition from development to market.
Building Strategic Partnerships for Successful Clinical Trials
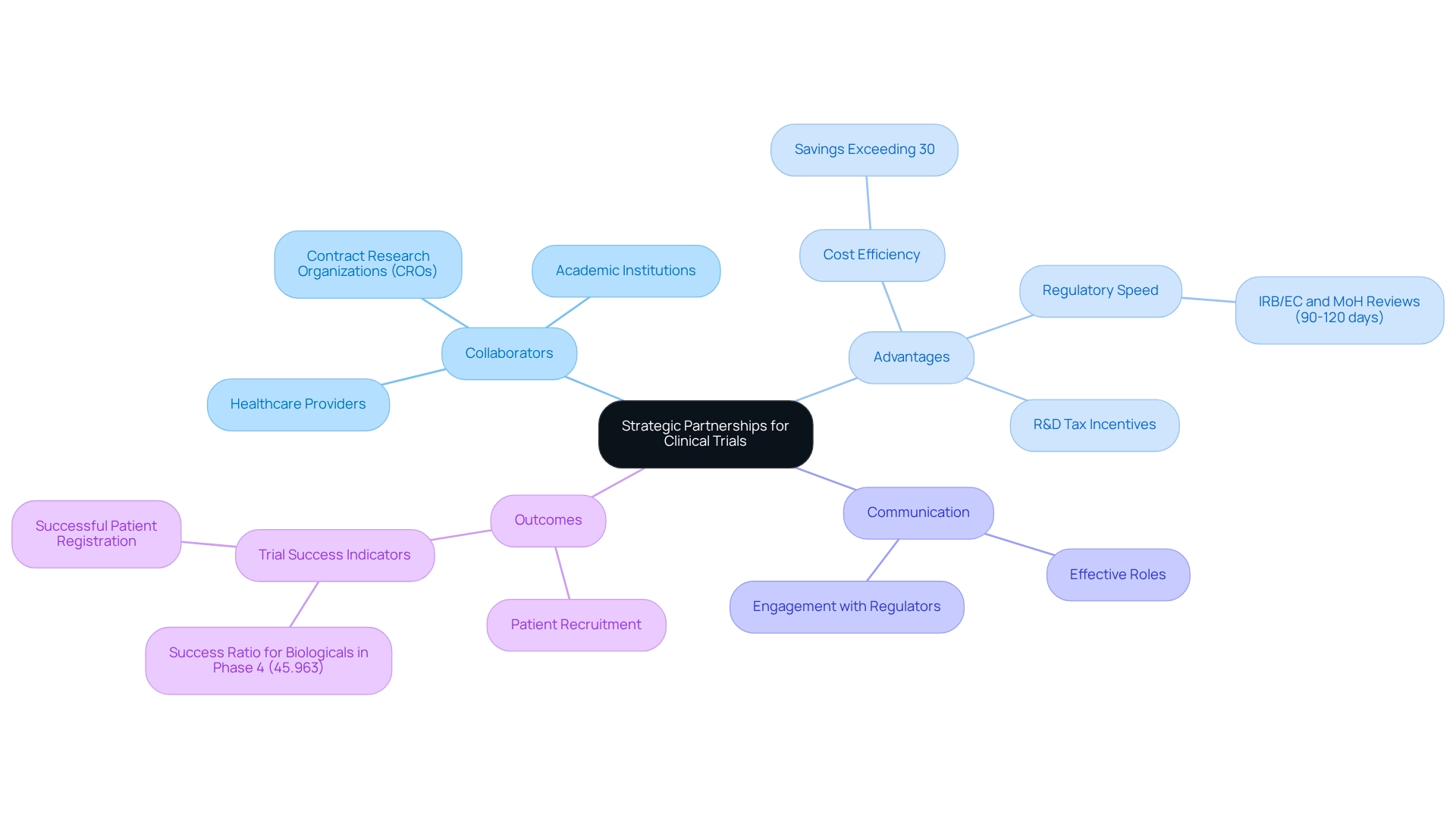
Strategic collaborations are essential for the success of clinical trial innovation in medtech within Mexico and across other research studies in the sector, particularly throughout Latin America. Collaborating with local contract study organizations (CROs), academic institutions, and healthcare providers significantly enhances clinical trial innovation for medtech in Mexico by improving resource availability and patient recruitment efforts. For instance, bioaccess™ and Caribbean Health Group (CHG) have partnered to establish Barranquilla as a premier location for medical studies in Latin America, a decision supported by Colombia's Minister of Health during their meeting on March 29, 2019.
This partnership aims to leverage the strengths of both organizations to enhance the examination process and improve recruitment strategies.
Colombia offers numerous competitive advantages for conducting studies, including:
- Cost efficiency, with savings exceeding 30% compared to studies in North America or Western Europe
- Regulatory speed, with IRB/EC and MoH (INVIMA) reviews taking only 90-120 days
- R&D tax incentives that provide substantial financial benefits for investment
Research indicates that partnerships can significantly enhance clinical trial innovation for medtech in Mexico, resulting in improved outcomes and expedited timelines through effective collaborations. The success ratio for Biologicals in Phase 4 is approximately 45.963, underscoring the potential advantages of strategic partnerships in achieving favorable results, particularly through clinical trial innovation for medtech in Mexico. To fully capitalize on these partnerships, companies should align their research objectives with those of their partners, leveraging the unique strengths of each entity.
Local CROs bring invaluable insights into regional regulatory landscapes and patient demographics, which can streamline the study process and bolster recruitment efforts. However, as noted by Reus & Lamont, regional distance between collaborating parties can exacerbate cultural differences, leading to communication challenges that must be addressed proactively. Effective communication and clearly defined roles are vital for maintaining productive collaborations, ensuring that all parties remain aligned and focused on shared goals. Engaging with regulatory agencies early in the process is another critical element that can facilitate smoother approvals and enhance the overall experience.
By fostering open communication with regulators, Medtech firms can navigate the complexities of compliance more efficiently, which is crucial for achieving clinical trial innovation for medtech in Mexico and ultimately yielding more favorable testing outcomes. As the Medtech landscape evolves in 2025, the importance of strategic collaborations will be paramount for clinical trial innovation in Mexico. Companies that prioritize collaboration with local CROs and other stakeholders will be better positioned to achieve clinical trial innovation for medtech in Mexico, enabling them to bring their medical devices to market efficiently.
Moreover, the introduction of successful patient registration as a new measure of clinical trial success provides a fresh perspective on evaluating the effectiveness of these partnerships, thereby enhancing patient outcomes and advancing healthcare solutions in the region.
Conclusion
Mexico emerges as a burgeoning hub for clinical trials within the Medtech sector, presenting a unique blend of a diverse patient population, an increasingly favorable regulatory environment, and cost-effective research opportunities. The insights shared throughout this article illuminate the key factors contributing to the success of clinical trials in Mexico, from understanding local healthcare infrastructure to leveraging strategic partnerships and innovative methodologies.
The streamlined processes established by COFEPRIS facilitate quicker market access for new medical devices, while a robust network of clinical research organizations enhances patient recruitment and retention. By adopting best practices in regulatory compliance and engaging local investigators, Medtech companies can significantly improve their trial outcomes and contribute to the region's economic growth.
Moreover, the integration of innovative methodologies, such as decentralized clinical trials and adaptive designs, proves vital for maintaining competitiveness in the rapidly evolving Medtech landscape. Companies that embrace these advancements, alongside strategic collaboration with local stakeholders, are positioned to maximize their impact and accelerate the development of groundbreaking medical technologies.
As the clinical trial landscape in Mexico continues to evolve, the opportunities for Medtech companies are vast. By navigating this dynamic environment with a well-informed approach, organizations can position themselves for success, ultimately enhancing patient care and contributing to global medical advancements. The future of clinical trials in Mexico is bright, and seizing these opportunities will be crucial for driving innovation and improving healthcare outcomes in the region.
Frequently Asked Questions
What role does Mexico play in the global research trial landscape?
Mexico has established itself as a pivotal center for clinical trial innovation, particularly in medtech, due to its diverse patient population of over 126 million, which provides a rich demographic for medical studies.
How many medical study sites are expected to be active in Mexico by 2025?
By 2025, there are expected to be over 300 active medical study sites across Mexico, indicating a significant increase in infrastructure dedicated to clinical trials.
What regulatory body oversees clinical trials in Mexico?
The Federal Commission for the Protection against Sanitary Risk (COFEPRIS) oversees clinical trials in Mexico and has streamlined processes to facilitate faster market access for new medical devices.
What is the typical duration for COFEPRIS to make decisions on sanitary registration?
COFEPRIS allows a maximum of 60 business days for decisions on sanitary registration, with average approval durations typically ranging from 14 to 16 weeks.
What committees must health facilities involved in research establish?
Every health facility involved in research is required to establish a Research Committee and a Biosafety Committee to ensure adherence to high technical and scientific standards.
Why is it important for Medtech companies to understand the local healthcare framework in Mexico?
Understanding the local healthcare framework, which includes both public and private hospitals, is crucial for Medtech companies to conduct successful tests and navigate cultural perspectives that influence patient recruitment and retention.
What recent trends are noted in the clinical trial landscape in Mexico?
Recent trends indicate a growing interest in early-feasibility studies and post-market research follow-up studies, highlighting innovation in clinical trials for medtech.
What services does bioaccess® provide for clinical studies?
bioaccess® offers comprehensive management services including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting for various types of studies.
What ethical considerations must Medtech companies address during clinical trials?
Medtech companies must ensure that insurance contracts are established to address any study-related injuries to research participants, thereby enhancing participant protection and addressing ethical considerations.
What best practices should companies follow when submitting Clinical Trial Applications in Mexico?
Best practices include thorough preparation of all required documents, proactive engagement with regulatory bodies, and a clear understanding of participant rights, including their ability to revoke consent over their biological materials.




