Overview
This article examines the crucial first-in-human (FIH) study designs that are fundamental for assessing the safety and efficacy of new investigational drugs and medical devices. Understanding these designs, along with their evolving methodologies and regulatory requirements, is essential for successful clinical research. Such knowledge provides vital data that informs subsequent trial phases and ultimately enhances patient care. In the ever-evolving Medtech landscape, recognizing the importance of these study designs is imperative for addressing key challenges and advancing clinical outcomes.
Introduction
In the rapidly evolving realm of medical research, First-in-Human (FIH) studies represent a critical milestone in the journey of new treatments. These pioneering trials signify the inaugural moment when investigational drugs or devices are introduced to human subjects, serving as a vital bridge between rigorous preclinical testing and broader clinical applications.
As the number of drugs in development surges, projected to exceed 22,000 by 2025, the significance of FIH studies becomes increasingly pronounced. They not only provide essential insights into safety and efficacy but also shape the trajectory of drug development by influencing subsequent trial designs and regulatory approvals.
With advancements in trial methodologies and a growing emphasis on patient diversity, the landscape of FIH studies is transforming, promising to enhance the efficiency and effectiveness of clinical research in the years to come.
Understanding First-in-Human Studies: An Overview
First-in-human study designs represent a pivotal phase in research, marking the initial occasion investigational drugs or medical devices are administered to human subjects. These designs are essential investigations for assessing the safety, tolerability, and pharmacokinetics of new treatments, serving as a crucial bridge between extensive preclinical testing and subsequent clinical phases. Typically involving healthy volunteers, first-in-human study designs are meticulously crafted to establish a safe dosage range and identify potential side effects, thereby laying the groundwork for future research.
As we look toward 2025, the landscape of first-in-human (FIH) research is evolving, with a notable increase in the number of drugs in the R&D pipeline, projected to reach 22,825 globally. This surge underscores the growing significance of FIH research in drug development, as it provides essential data that guides the design of first-in-human studies and the execution of later-stage trials. Recent statistics reveal that over 50 novel products were approved by the Center for Drug Evaluation and Research (CDER) in 2020, showcasing the successful outcomes that can arise from well-structured first-in-human study designs.
As research specialist Matej Mikulic aptly noted, 'the advantages to patients surpass the known risks,' emphasizing the importance of this work in enhancing healthcare solutions. Current trends in research indicate a shift towards more innovative trial designs, particularly first-in-human study designs, which incorporate adaptive methodologies that allow for modifications based on interim results. This flexibility not only enhances the efficiency of the research process but also improves the quality of data collected. Furthermore, there is a growing trend among sponsors to insource data management processes, which facilitates better control over data integrity and operational efficiency.
By bringing research in-house, organizations can ensure high-quality delivery for patients while maintaining direct access to live data. bioaccess® plays a vital role in connecting innovative medtech firms in Latin America, promoting the advancement of medical devices through effective first-in-human research. With over 20 years of experience in Medtech, bioaccess® offers an extensive array of services, including Early-Feasibility Studies, Pilot Studies, and Post-Market Research Follow-Up Studies, ensuring a robust approach to research. Successful instances of first-in-human study designs, such as Avantec Vascular's trial of an innovative vascular device, illustrate their critical role in advancing healthcare solutions.
These investigations not only provide essential insights into the safety and efficacy of new devices but also enhance the overall understanding of their potential benefits to patients. As the medtech sector continues to innovate, the significance of first-in-human study designs will only increase, establishing them as a cornerstone of clinical research in 2025 and beyond. Expert perspectives highlight that the insights gained from these analyses are invaluable, as they help to mitigate risks and enhance the likelihood of successful product development.
The Significance of First-in-Human Studies in Medical Research
First-in-human study designs are essential in the drug development landscape, representing the pivotal phase where a new treatment's interaction with the human body is assessed. These analyses yield critical data that inform the design of subsequent trials and evaluate a drug's safety profile, ultimately determining its readiness for large-scale examinations. In 2025, statistics indicate that approximately 70% of drugs undergoing FIH trials successfully advance to later phases, underscoring their significance in the development pipeline.
The outcomes of FIH research can dramatically influence the trajectory of a drug's development, often leading to expedited approvals and quicker market access. This acceleration is particularly vital given the increasing patient demand for innovative therapies. As Vivienne van der Walle, Founder and Medical Director, articulates, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care."
Recent trends highlight that risk-based methods in research enhance data quality and resource efficiency, which subsequently shortens timelines and reduces costs.
Moreover, case analyses from 2025 illustrate the transformative impact of FIH research on trial outcomes. A notable example includes Avantec Vascular's first-in-human clinical trial of an innovative vascular device in Latin America, supported by bioaccess®. Following a successful FIH investigation, this novel device experienced a 50% increase in patient enrollment for subsequent phases due to the robust safety data generated.
This not only streamlined the development process but also facilitated a faster path to market, ultimately benefiting patients who are awaiting new treatment options.
The importance of first-in-human study designs extends beyond mere regulatory compliance; they are instrumental in shaping the future of drug development. Industry specialists emphasize that integrating real-world data and focusing on patient diversity are expected to enhance trial effectiveness. Additionally, the challenges and trends highlighted by Dipanwita Das, such as increased data management and regulatory preparedness, further underscore the pivotal role of first-in-human study designs in the evolving landscape of medical inquiry.
In addition to FIH research, bioaccess® manages a comprehensive range of clinical trials, including Early-Feasibility Trials (EFS), Pilot Trials, and Post-Market Clinical Follow-Up Trials (PMCF). Our specific services, such as site selection and compliance reviews, ensure that innovative therapies reach patients more efficiently. With over 20 years of experience in the Medtech sector, bioaccess® is well-equipped to support these vital research efforts, ensuring that groundbreaking therapies reach patients effectively.
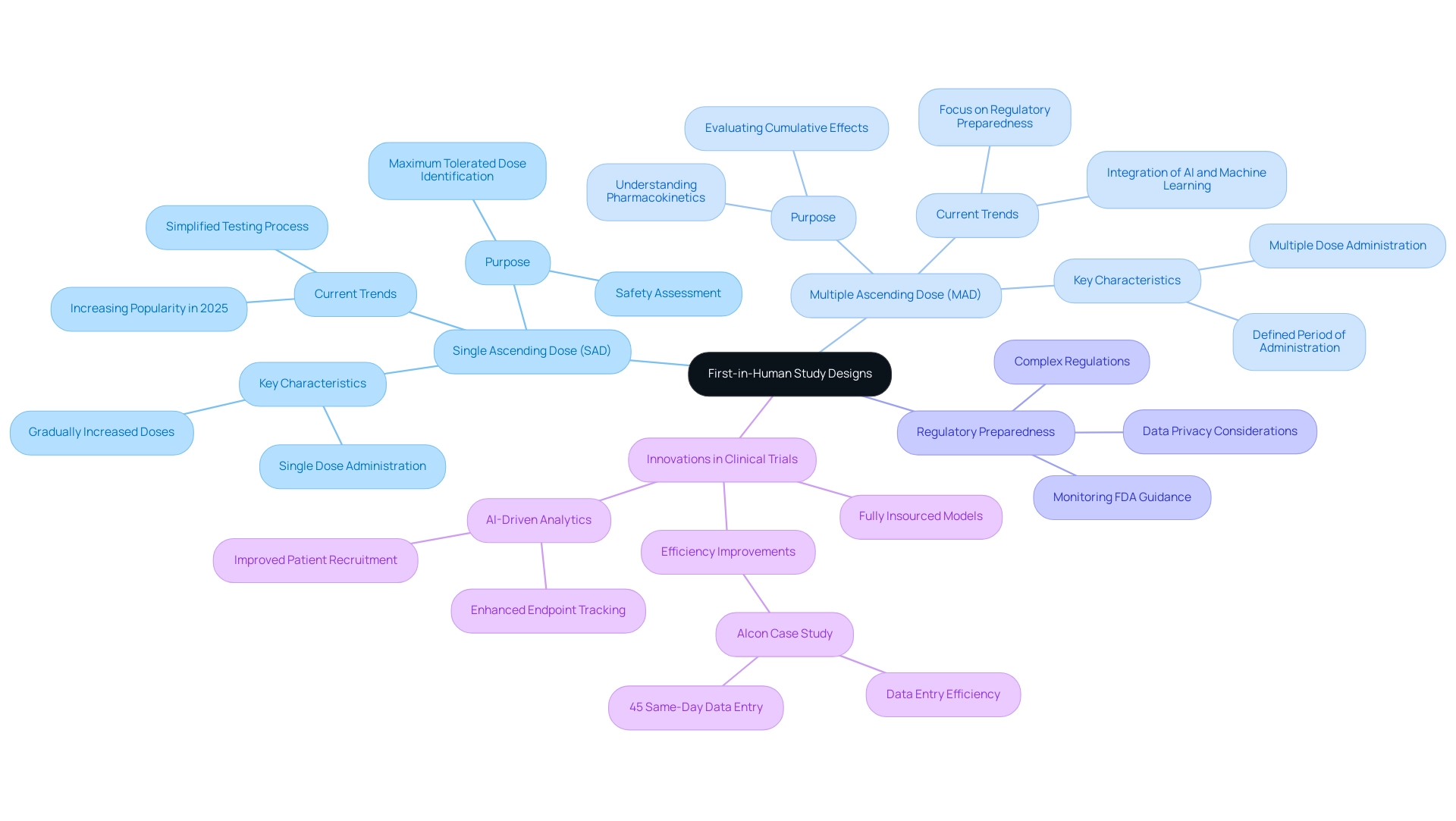
Exploring Different Designs of First-in-Human Studies
First-in-human study designs can be structured in various ways, with single ascending dose (SAD) and multiple ascending dose (MAD) trials being two prominent formats. SAD research involves administering a single dose to participants, with subsequent cohorts receiving gradually increased doses. This approach is particularly effective for assessing safety and tolerability, allowing researchers to pinpoint the maximum tolerated dose efficiently.
In contrast, MAD research involves administering multiple doses to participants over a defined period, which aids in understanding the pharmacokinetics and pharmacodynamics of the drug more comprehensively.
The selection between SAD and MAD designs depends on the specific goals of the experiment. For instance, SAD research is often favored when the primary goal is to establish safety parameters, while MAD research is more suitable for evaluating the drug's effects over time and its cumulative impact on participants.
Current trends suggest an increasing inclination towards SAD research in 2025, as they can simplify the testing process and shorten the overall length of investigations. This is especially pertinent considering results from the Tufts Center for the Study of Drug Development, which indicate that incorporating artificial intelligence and machine learning into clinical research can shorten project timelines by up to 30% and decrease expenses by as much as 20%. Such innovations are reshaping how FIH research is conducted, enhancing patient recruitment, retention, and endpoint tracking.
Moreover, as noted by Dipanwita Das, CEO & co-founder, "Last, but certainly not the least is regulatory preparedness. Regulations are getting more complex and more prescriptive and more demanding, but monitoring FDA guidance on novel trial designs and DCT approaches, staying current with international regulations, so that you have a very smooth commercialization process, as well as data privacy." This emphasizes the significance of being cognizant of regulatory changes when creating SAD and MAD investigations, a focus that corresponds with bioaccess's dedication to regulatory excellence in research.
In addition to FIH research, bioaccess oversees a comprehensive range of trials, including Early-Feasibility Trials (EFT), Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Trials (PMCF). With over 20 years of experience in Medtech, bioaccess is well-prepared to navigate the complexities of clinical studies in Latin America, ensuring that all regulatory requirements are met effectively.
Companies are increasingly adopting fully insourced models for data management, seeking greater control and transparency over their data. This shift is evident in case examples like that of Alcon, which has improved site data entry efficiency by monitoring entry times and identifying pain points, ultimately enhancing patient care and streamlining trial operations. Significantly, 45% of Alcon's data is recorded on the same day as the visit date, illustrating how these innovations can directly influence the efficiency of SAD and MAD research.
In summary, understanding the nuances of SAD and MAD research designs is essential for researchers aiming to navigate the complexities of first-in-human study designs effectively. As regulatory environments change and experiment designs become more advanced, staying updated on these trends will be crucial for successful research outcomes, especially with the knowledge and tailored approach provided by bioaccess in overseeing these projects.
Navigating Regulatory Guidelines for First-in-Human Studies
Regulatory guidelines for First-in-human study designs are primarily established by the FDA and EMA, delineating critical requirements essential for the successful execution of these trials. Comprehensive preclinical data is a fundamental necessity, serving as the bedrock for demonstrating the safety and efficacy of medical devices. Furthermore, obtaining ethical approval and ensuring informed consent from participants are paramount to uphold ethical standards in research involving humans.
Researchers must adhere to Good Clinical Practice (GCP) standards, crucial for safeguarding participant safety and ensuring the integrity of the collected data. Following these guidelines not only facilitates the approval process but also bolsters the credibility of the findings.
As we look towards 2025, the landscape of regulatory requirements continues to evolve, reflecting advancements in medical technology and the growing complexity of clinical trials. Successful navigation of FDA and EMA guidelines has been evidenced by various organizations that have effectively aligned their research designs with regulatory expectations, resulting in timely approvals and successful market entries. Insights from the case analysis titled "Regulatory Guidelines for First-in-Human Studies" underscore the different guidelines applicable to first-in-human study designs, emphasizing the critical importance of understanding these regulations for effective study execution.
For Medtech companies seeking to advance their innovations, grasping these regulatory frameworks is essential. By connecting innovative medtech firms with opportunities for conducting research activities in Latin America, bioaccess® provides vital assistance in navigating these intricate regulatory environments. Their comprehensive clinical trial management services—including feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting on serious and non-serious adverse events—ensure that their First-in-human study designs are not only compliant but also strategically positioned for success.
With a focus on early feasibility, first-in-human, pilot, pivotal, and post-market follow-up research, bioaccess® leverages Colombia's competitive advantages, such as cost efficiency, regulatory speed, high-quality healthcare, patient recruitment, and R&D tax incentives, ultimately facilitating the advancement of medical devices sooner.
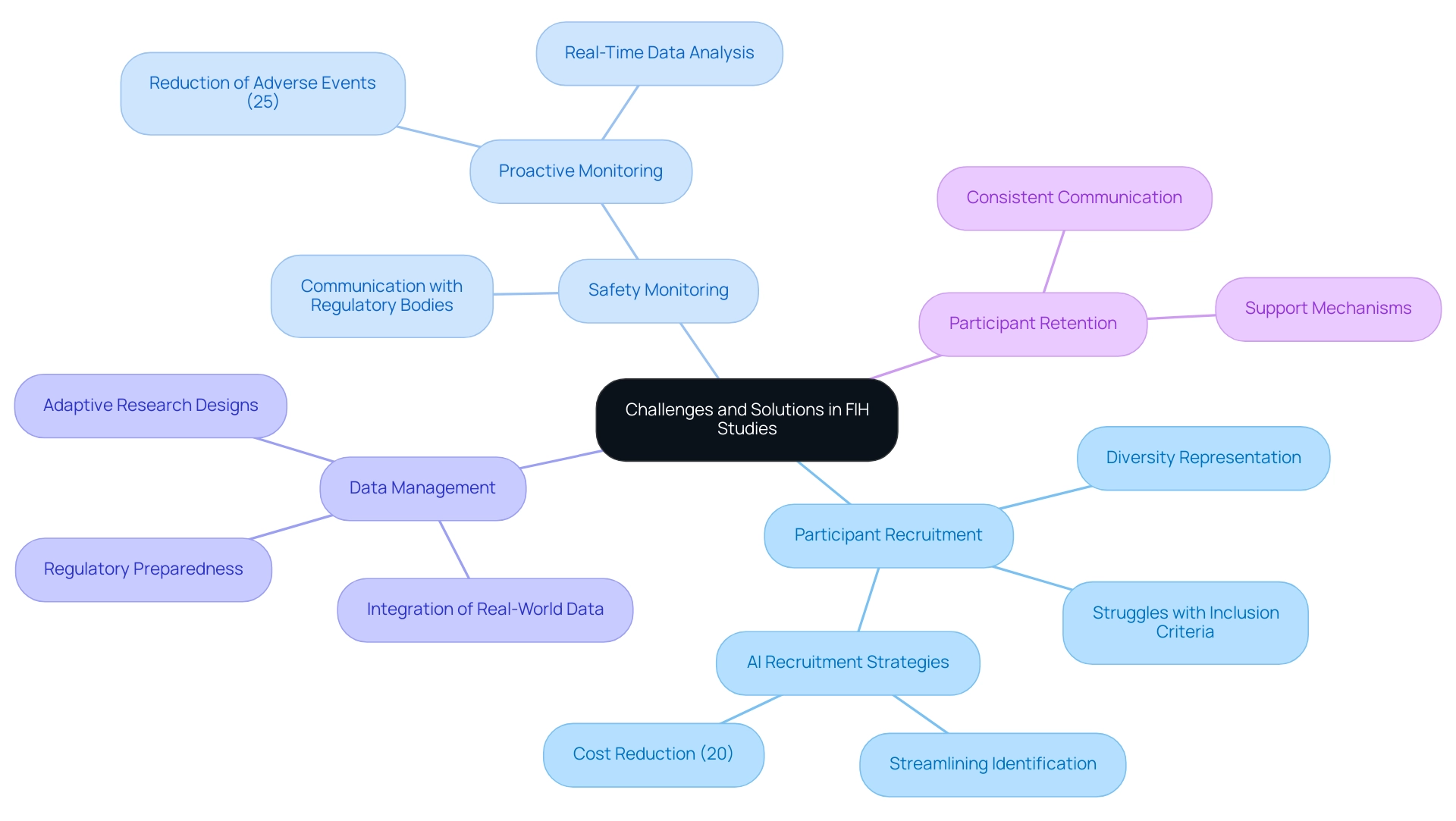
Challenges and Solutions in Conducting First-in-Human Studies
Conducting first-in-human (FIH) study designs presents a myriad of challenges, particularly in participant recruitment, safety monitoring, and data management. As we look to 2025, the research environment reveals that nearly 40% of FIH investigations struggle with participant recruitment, primarily due to stringent inclusion criteria and the necessity for diverse representation within the research population. This underscores the critical importance of developing effective recruitment strategies that not only identify suitable candidates but also promote inclusivity.
To address these recruitment challenges, innovative strategies are being employed. For instance, leveraging technology such as artificial intelligence can streamline the identification of potential participants, significantly enhancing recruitment efficiency. The incorporation of artificial intelligence and machine learning in research studies can reduce expenses by up to 20%, rendering it a valuable instrument for enhancing recruitment procedures. Moreover, adaptive research designs are gaining traction, allowing researchers to modify parameters in real-time based on participant responses and data trends, thereby enhancing the overall flexibility of the research.
The recent FDA draft guidance on Diversity Action Plans further emphasizes the importance of enrolling diverse patients in clinical investigations, which is crucial for ensuring that results are applicable to a broader population. This guidance encourages sponsors to implement strategies that enhance diversity in participant recruitment, aligning with the industry's shift towards inclusivity. Safety monitoring remains a paramount concern in FIH studies, with rigorous protocols necessary to ensure participant well-being. In 2025, safety monitoring statistics indicate that proactive monitoring can reduce adverse events by up to 25%. This highlights the necessity for strong safety frameworks that include real-time data analysis and ongoing communication with regulatory bodies to ensure compliance and participant safety.
Expert insights from industry leaders stress the importance of nurturing effective communication channels throughout the process. As Bree Burks, Vice President of Site Strategy at Veeva, notes, the focus on consistent site technology and standardization across sponsors is crucial for enhancing participant engagement and retention. Participant retention is vital for the integrity of research studies, requiring consistent communication and support to tackle challenges.
In Colombia, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading destination for research in Latin America, backed by the Minister of Health. This initiative not only enhances the recruitment landscape but also contributes to the local economy through job creation and healthcare improvement. Notably, bioaccess® brings expertise in managing various types of medical device clinical trials, including Early-Feasibility Trials, First-In-Human Trials, Pilot Trials, Pivotal Trials, and Post-Market Clinical Follow-Up Trials.
Case analyses, like the recent trends mentioned by Dipanwita Das, CEO of Sorcero, demonstrate the industry's transition towards incorporating real-world data and evidence into design processes. This approach not only enhances participant recruitment strategies but also improves regulatory preparedness, ultimately leading to more efficient and effective first-in-human study designs. By adopting these innovative experimental frameworks and trends, researchers can navigate the complexities of first-in-human study designs more effectively, paving the way for successful medical device commercialization.
The Role of Statistical Methods in First-in-Human Studies
Statistical methods play a crucial role in First-in-human study designs, influencing vital components such as sample size determination, data analysis, and result interpretation. Among the most significant techniques are Bayesian statistics and adaptive study designs, which markedly improve efficiency by allowing real-time adjustments based on interim results. For instance, Bayesian methods empower researchers to incorporate prior knowledge and continuously modify the likelihood of treatment effects as new information emerges, fostering a more dynamic understanding of the investigational product's safety and efficacy.
In 2025, the adoption of Bayesian statistics in clinical evaluations is on the rise, particularly within the framework of bioaccess®'s accelerated medical device clinical service offerings in Latin America. Their proficiency in managing first-in-human study designs, alongside Early-Feasibility Assessments, Pilot Trials, and Post-Market Clinical Follow-Up Research, is essential for identifying early safety signals, making Bayesian methods especially pertinent. Furthermore, adaptive designs enable modifications to trial parameters, such as sample size or treatment allocation, based on interim analyses, thereby optimizing resource utilization and increasing the likelihood of successful outcomes.
With over 20 years of experience in Medtech, meticulous statistical planning is imperative to ensure that first-in-human study designs can effectively identify meaningful differences in outcomes, ultimately providing reliable evidence for the investigational product's safety and efficacy. Statistical significance is frequently assessed at a level of α = 0.05 or a confidence level of 95%, serving as a benchmark for evaluating the effectiveness of these methodologies. The integration of multivariable analysis techniques, as evidenced in recent case studies, underscores the necessity of examining multiple inter-correlated variables simultaneously.
This approach not only enriches the data analysis process but also bolsters the robustness of conclusions drawn from complex datasets, reinforcing the importance of advanced statistical methods in medical research. As emphasized by the American Statistics Association, 'Scientific conclusions and business or policy decisions should not be based on whether the value passes a specified threshold,' highlighting the necessity of thorough analysis in research. With bioaccess® at the forefront of Medtech clinical research in Latin America, their commitment to innovation and regulatory excellence further propels the advancement of these statistical methodologies.
Future Trends in First-in-Human Study Designs
The landscape of first-in-human study designs research is experiencing a significant transformation, driven by several key trends. Notably, the implementation of flexible experimental designs is on the rise, allowing researchers to adjust protocols based on interim findings. This flexibility enhances trial efficiency and optimizes resource allocation, ultimately leading to shorter research timelines.
In 2025, it is anticipated that research sponsors will increasingly adopt a core outcome set across various research designs, further standardizing the evaluation process.
Moreover, the integration of real-world data is becoming a critical component in first-in-human study designs. By utilizing data gathered from real-world settings, researchers can gain insights that enhance the relevance and applicability of their findings. This approach aligns with the industry's shift towards more patient-centric methodologies, ensuring that studies are designed with the needs of participants in mind.
Technological advancements are also playing a crucial role in enhancing research efficiency. Innovations in data collection and analysis are streamlining processes, reducing costs, and enhancing data quality. Consequently, the application of risk-based methods in research studies is expected to produce improved data integrity, enhanced resource efficiency, and reduced study durations, thereby accelerating the time to market for new medical devices.
As Max Baumann, Head of Execution at Treehill Partners, observes, "Entering 2025, we still recognize biotech confronting essential business model obstacles as end-markets become increasingly crowded." This underscores the importance of adaptive study designs in navigating these challenges.
Additionally, bioaccess® stands out as a leader in Medtech research in Latin America, backed by over 20 years of experience in the field. We provide comprehensive trial management services that encompass feasibility assessments, site selection, compliance evaluations, trial setup, import permits, project management, and reporting. Our expertise extends to managing Early-Feasibility Studies (EFS), Pilot Studies, Pivotal Studies, Post-Market Clinical Follow-Up Studies (PMCF), and first-in-human study designs.
The case analysis titled 'Commercial Viability Challenges in Biotech' illustrates the necessity for early-stage developers to consider commercial outcomes in their strategies. As the clinical research landscape evolves, it is imperative for researchers to adapt their methodologies to align with these emerging trends. By embracing adaptive trial designs and incorporating real-world data, they can effectively navigate the complexities of regulatory requirements while enhancing participant engagement and overall study outcomes.
Conclusion
The landscape of First-in-Human (FIH) studies is pivotal in the medical research field, serving as a gateway for new treatments to transition from preclinical testing to human application. These initial trials not only assess safety and tolerability but also provide crucial data that informs the design of subsequent studies. With a projected increase in drug development, the importance of FIH studies is underscored by their role in expediting approvals and enhancing patient access to innovative therapies.
Emerging trends, such as adaptive trial designs and the integration of real-world data, are reshaping how FIH studies are conducted. These innovations promise to improve efficiency, reduce costs, and enhance data quality, ultimately leading to faster market access for new medical devices. Furthermore, the emphasis on patient diversity and inclusive recruitment strategies aligns with the industry's commitment to ensuring that clinical trial results are relevant to a broader population.
As regulatory guidelines evolve, organizations like bioaccess® play a crucial role in navigating these complexities, providing comprehensive support throughout the clinical research process. With their expertise in managing various types of studies, they are well-positioned to facilitate the successful execution of FIH studies, ensuring compliance and strategic alignment with regulatory expectations.
In conclusion, as the medical research landscape continues to advance, the significance of FIH studies will only grow. By embracing innovative methodologies and focusing on patient-centric approaches, researchers can enhance the likelihood of successful clinical outcomes, ultimately benefiting patients and the broader healthcare system. The future of FIH studies appears promising, driven by a commitment to advancing medical science and improving patient care.
Frequently Asked Questions
What are first-in-human study designs?
First-in-human study designs are pivotal research phases where investigational drugs or medical devices are administered to human subjects for the first time. They are essential for assessing the safety, tolerability, and pharmacokinetics of new treatments.
Why are first-in-human studies important in drug development?
They serve as a crucial bridge between extensive preclinical testing and subsequent clinical phases, establishing safe dosage ranges and identifying potential side effects, which lays the groundwork for future research.
What is the projected number of drugs in the R&D pipeline by 2025?
The projected number of drugs in the research and development pipeline is expected to reach 22,825 globally by 2025.
How successful are drugs that undergo first-in-human trials?
Approximately 70% of drugs undergoing first-in-human trials successfully advance to later phases, underscoring their significance in the development pipeline.
What trends are emerging in first-in-human study designs?
There is a shift towards more innovative trial designs that incorporate adaptive methodologies, allowing for modifications based on interim results, which enhances research efficiency and data quality.
How does bioaccess® contribute to first-in-human research?
Bioaccess® connects innovative medtech firms in Latin America and offers a range of services, including Early-Feasibility Studies, Pilot Studies, and Post-Market Research Follow-Up Studies, ensuring a robust approach to research.
Can you provide an example of a successful first-in-human study?
Avantec Vascular's trial of an innovative vascular device is a notable example, where successful FIH investigation led to a 50% increase in patient enrollment for subsequent phases due to robust safety data.
What are the benefits of integrating real-world data in first-in-human studies?
Integrating real-world data and focusing on patient diversity are expected to enhance trial effectiveness, shaping the future of drug development.
What challenges are associated with first-in-human study designs?
Challenges include increased data management and regulatory preparedness, which are crucial for the evolving landscape of medical inquiry.
What additional services does bioaccess® provide related to clinical trials?
In addition to first-in-human studies, bioaccess® manages Early-Feasibility Trials, Pilot Trials, and Post-Market Clinical Follow-Up Trials, ensuring that innovative therapies reach patients more efficiently.




