Introduction
In the realm of medical devices, the intersection of technology and human interaction is more critical than ever. Human Factors Engineering (HFE) emerges as a vital discipline aimed at enhancing usability and safety by meticulously understanding user behavior and capabilities. As healthcare continues to evolve, the design of medical devices must prioritize intuitive interfaces and error prevention to mitigate risks that can compromise patient safety.
The implications of HFE extend beyond mere compliance with regulatory standards; they encompass a commitment to fostering trust and satisfaction among users. This article delves into the essential principles of HFE, the regulatory frameworks guiding its implementation, and the future challenges and opportunities that lie ahead in the quest for safer, more effective medical technologies.
Understanding Human Factors Engineering in Medical Devices
Human factors engineering medical devices is a critical discipline that focuses on optimizing user interaction to enhance both usability and safety. It involves a thorough understanding of human behavior, capabilities, and limitations in relation to design. By applying human factors engineering medical devices principles, manufacturers can create products that are not only effective but also intuitive and user-friendly, which is particularly vital in the healthcare sector.
This complexity can lead to user errors, which are significant contributors to software errors. As Yi Zhang observes, 'Errors in these two components represent 80.16% and 12.63% of the observed UI software errors, respectively,' emphasizing the significant effect of these errors on functionality. For instance, in examining audio/visual notifications, six identified errors resulted in incorrect timing or absence of critical device state notifications, emphasizing the need for meticulous design.
Such errors can directly compromise patient safety, underscoring the importance of human factors engineering medical devices in minimizing risks and enhancing patient outcomes. A relevant case study titled 'Accommodating a Physically Encumbered Individual' illustrates the challenges encountered by individuals in sterile environments, where the requirements for data input can conflict with practical usage scenarios, such as when individuals are wearing gloves or managing multiple tasks. Furthermore, detecting UI software errors related to human factors necessitates comprehensive hazard analysis and participant studies, as highlighted by recent developments in the field.
Addressing these interactions through human factors engineering medical devices not only reduces risks but also promotes enhanced patient outcomes and satisfaction, thereby strengthening the essential role of usability in the design of healthcare products.
Regulatory Framework and Guidelines for Human Factors Engineering
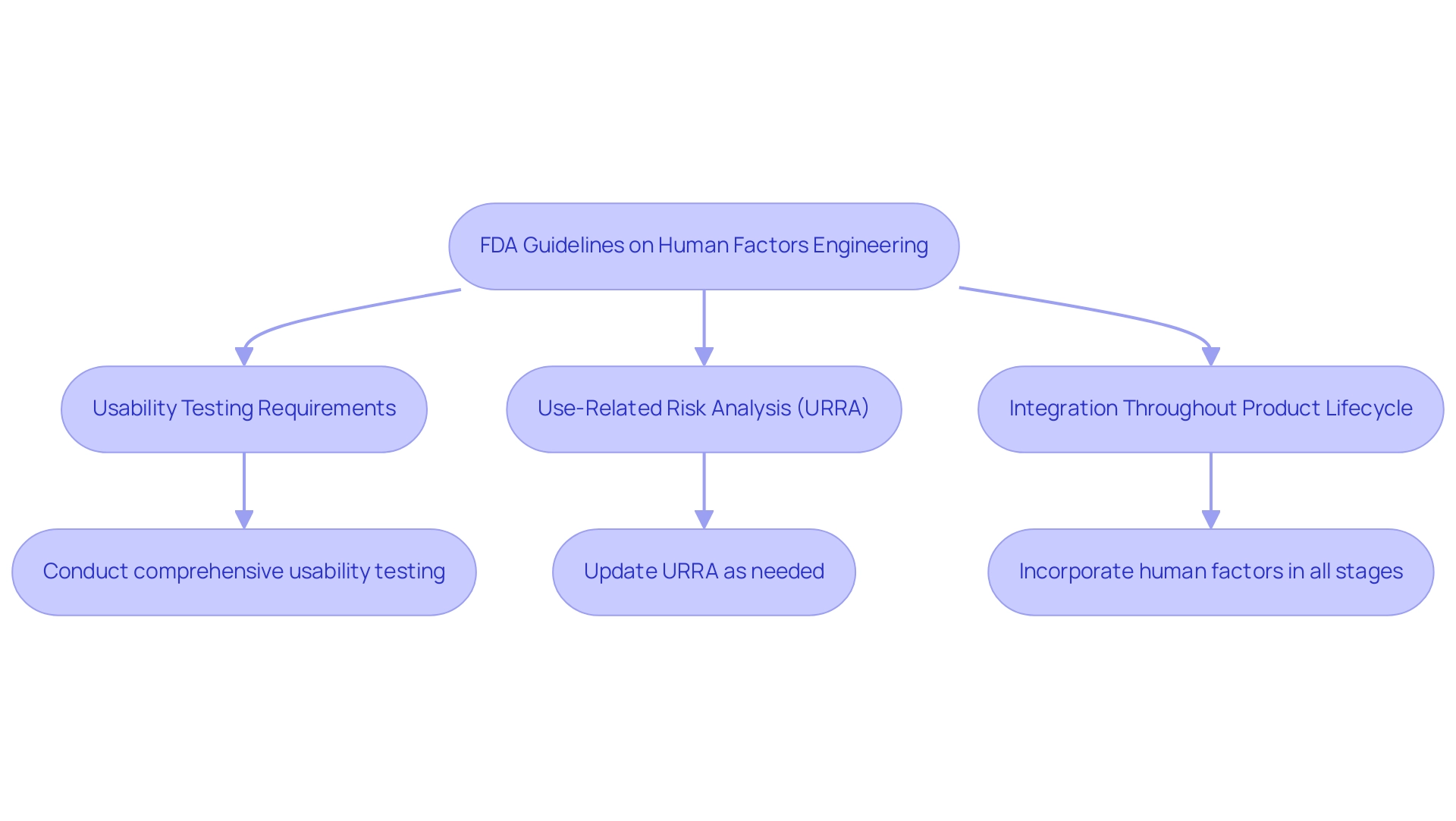
The regulatory framework governing human factors engineering medical devices is predominantly shaped by the guidelines issued by the FDA, which recognize the critical role of usability in device design. Effective immediately, manufacturers are mandated to conduct comprehensive usability testing to identify potential consumer errors and mitigate associated risks. The FDA's 'Human Factors and Usability Engineering Guidance' (Docket Number: FDA-2011-D-0469) outlines essential recommendations for integrating human factors engineering medical devices into the design process, ensuring that usability considerations are woven throughout all development stages. In particular, the upcoming revision of HE75 in 2024 is anticipated to provide enhanced clarity and expectations regarding usability testing requirements, with the FDA poised to acknowledge this revision in its entirety.
Moreover, the guidance emphasizes the necessity for the Use-Related Risk Analysis (URRA) to be regarded as a 'living document', requiring updates whenever interfaces undergo changes or new risks arise. This dynamic approach ensures the URRA remains relevant and effectively addresses potential risks, ultimately enhancing both patient safety and product usability, and emphasizes that human factors engineering medical devices must be integrated throughout the entire product lifecycle—from initial concept through to post-market surveillance. As stated by James Myers from the Center for Biologics Evaluation and Research, for more detailed inquiries regarding these guidelines, he can be contacted at the FDA.
Furthermore, industry leaders such as Ana Criado, who held several executive positions at Colombia’s regulatory agency INVIMA, play a crucial role in shaping regulations that affect usability and safety in healthcare products. With her extensive background in Regulatory Affairs, biomedical engineering, and as a professor at leading Colombian universities, Ana guides manufacturers through these complexities, ensuring compliance with evolving regulations and the promotion of user-centered design in healthcare products. Her experience in the cannabis sector further informs her understanding of regulatory challenges, making her insights invaluable in the context of usability and safety.
Applying Human Factors Engineering: Usability Testing and Design Principles
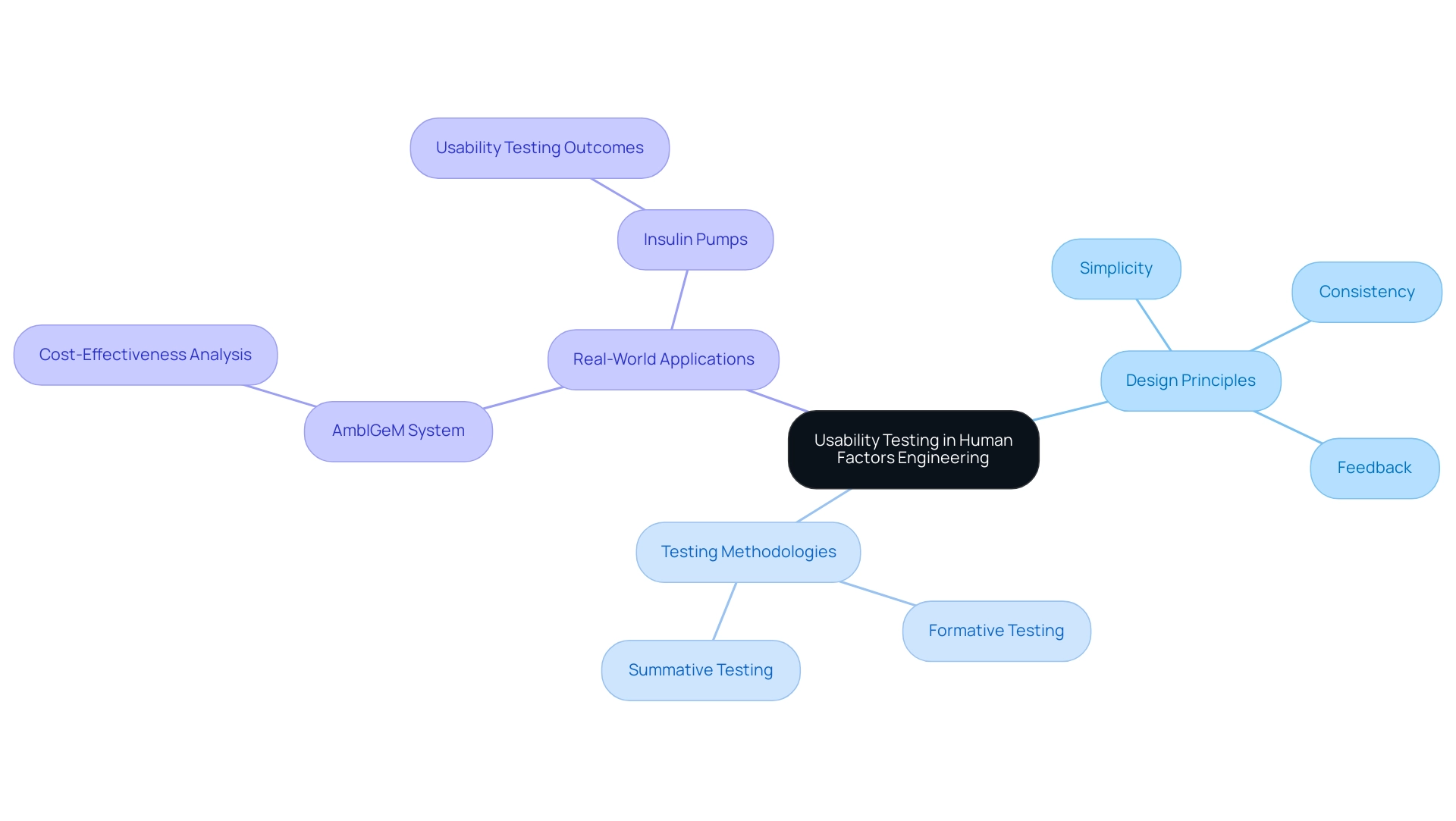
Usability testing serves as a crucial element of human factors engineering medical devices in healthcare product design, offering essential insights into interactions through structured scenarios. Essential design principles—simplicity, consistency, and feedback—are pivotal in ensuring that users can operate equipment both effectively and safely. Employing methodologies such as formative and summative testing enables designers to identify usability issues early in the development process, facilitating iterative enhancements.
For instance, the AmbIGeM system has shown promise in preventing falls among older patients in hospitals, demonstrating the importance of usability testing in various healthcare contexts. Additionally, insulin pumps have undergone rigorous usability testing to ensure that patients can intuitively understand and operate these tools, thereby significantly reducing the risk of medication delivery errors. Recent statistics reveal that the average coverage probabilities for usability testing methods were 17.9%, 31.5%, and 33.7% for naive, GT, and double-deflation methods, respectively, highlighting the effectiveness of these methodologies.
Vincent Vandewalle highlights that 'Alexandre Caron and Vincent Vandewalle contributed equally to the manuscript and should both be regarded as first author,' reinforcing the collaborative efforts in enhancing usability testing in healthcare technologies. These discussions emphasize that usability testing is not only advantageous but crucial for developing products, particularly human factors engineering medical devices, that are user-friendly, effective, and safe, reinforcing the need for adherence to robust design principles in the field.
Enhancing Patient Safety Through Human Factors Engineering
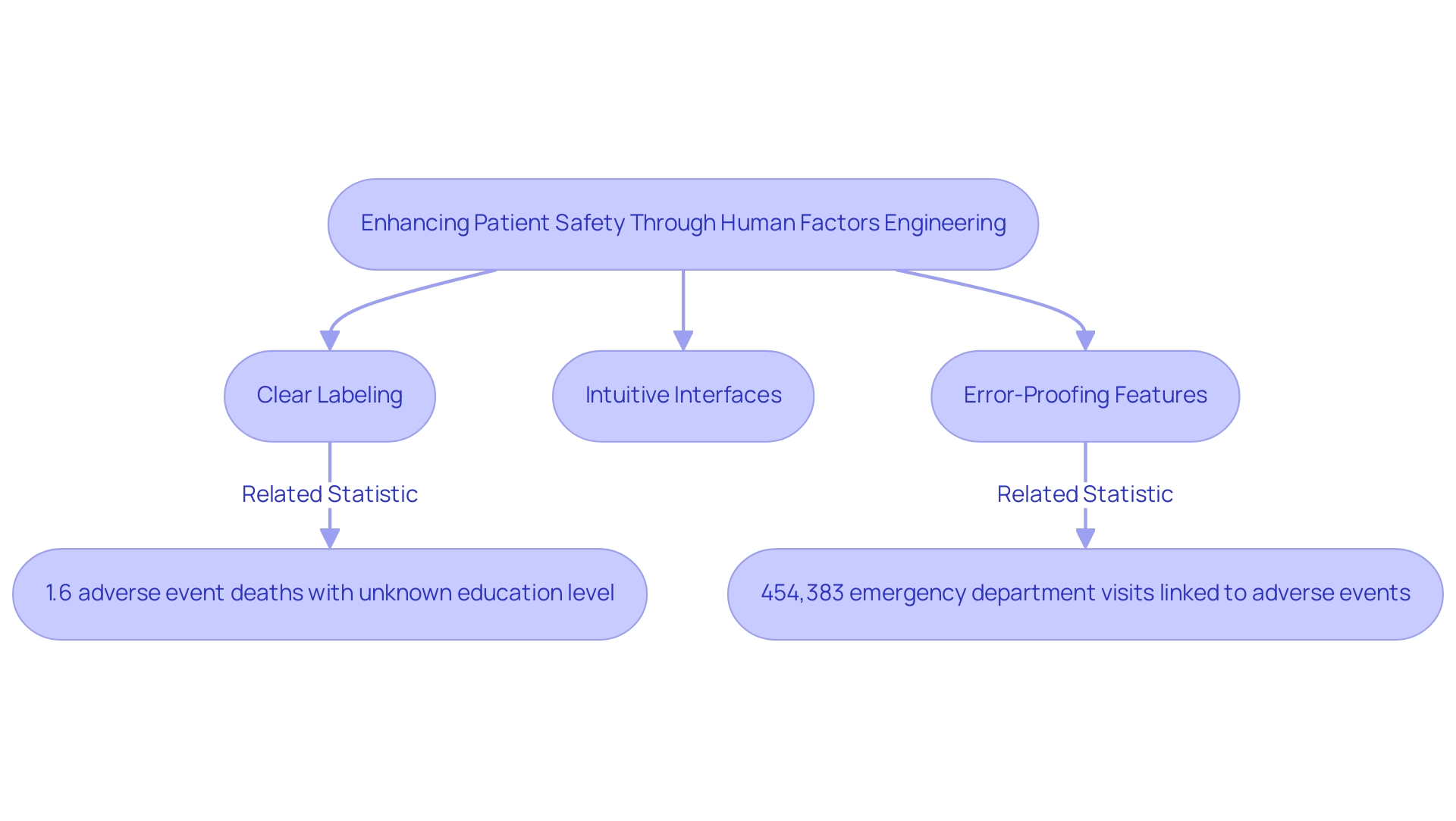
The role of human factors engineering medical devices is crucial in enhancing patient safety by systematically addressing potential mistakes related to healthcare instruments. By gaining insights into user behavior and designing products that align with human limitations, manufacturers significantly decrease the likelihood of misuse. For instance, statistics indicate that 1.6% of adverse event deaths had an unknown education level compared to 1.8% of non-adverse event deaths, emphasizing the critical need for effective HFE in healthcare product design.
Effective strategies include:
- The implementation of clear labeling
- Intuitive interfaces
- Specific error-proofing features that mitigate common operational mistakes
A recent case study estimated that around 454,383 emergency department visits within a year were associated with adverse events related to healthcare apparatus, highlighting the urgent need for improved safety measures. As Wu A.W. noted in 'The Impact of Adverse Events on Clinicians: What's in a Name?', addressing these issues is essential for improving patient safety. Research consistently shows that products developed using human factors engineering medical devices principles result in a significant reduction in adverse events and enhance patient outcomes.
Such findings highlight that prioritizing human factors engineering medical devices in the development of healthcare products not only protects patients but also builds trust in healthcare technologies, ultimately nurturing a more dependable and efficient healthcare environment.
Future Trends and Challenges in Human Factors Engineering
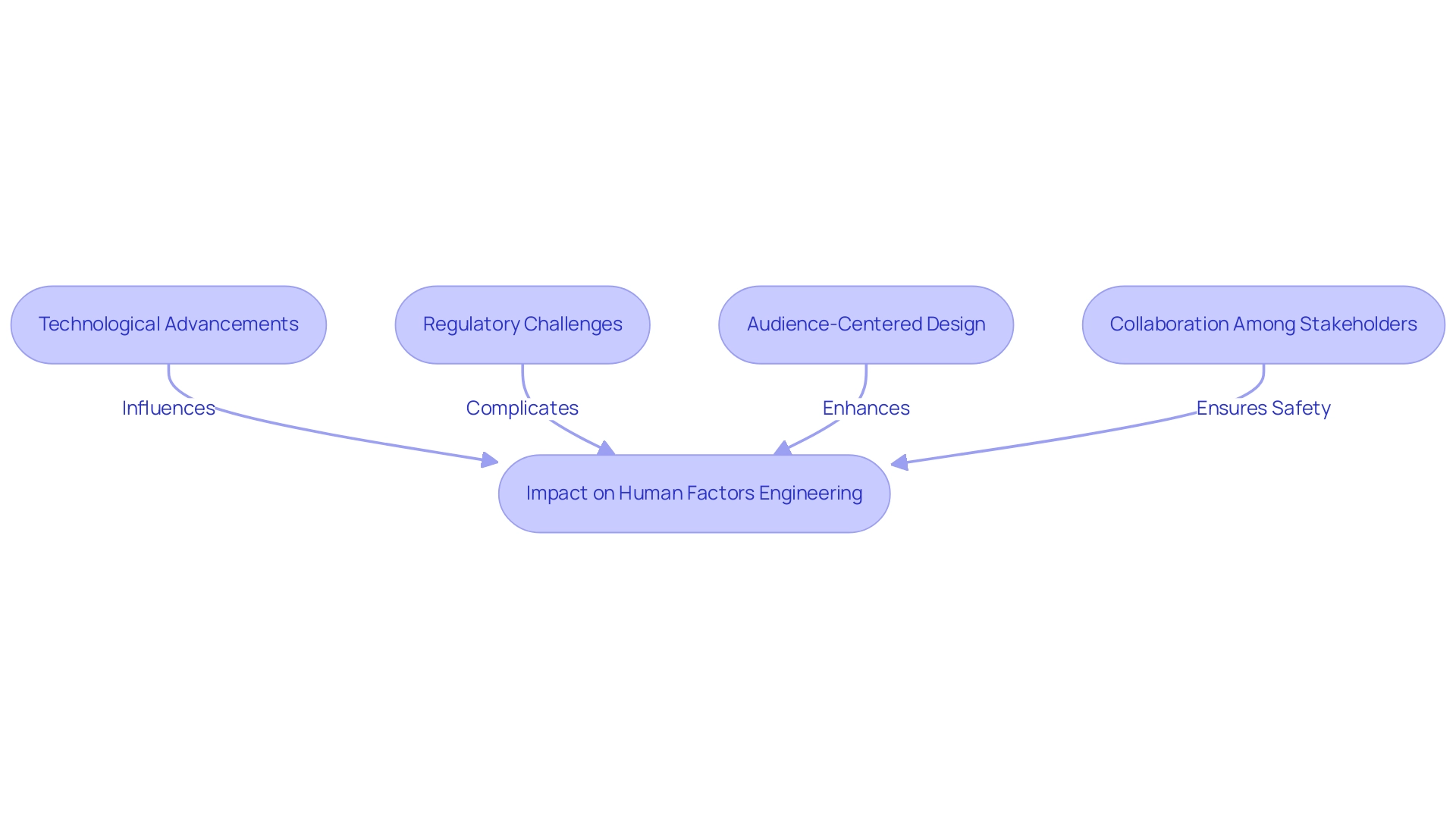
As technological advancements transform the field of healthcare equipment design, human factors engineering medical devices faces both extraordinary opportunities and significant challenges. The integration of emerging technologies, particularly artificial intelligence and telemedicine, necessitates innovative approaches in human factors engineering medical devices to guarantee both usability and safety. Bainbridge L. discusses the ironies of automation in man-machine systems, highlighting the complexities that arise when integrating automated solutions into healthcare environments.
Moreover, the evolving regulatory landscape complicates this integration, requiring manufacturers to adapt swiftly to new compliance guidelines, especially in light of the recent increase in the U.S. Import Price Index by 0.1% in November 2024, which may impact manufacturing costs. A prominent trend is the increasing focus on audience-centered design, which emphasizes understanding and addressing the needs and preferences of individuals throughout the development process.
However, the integration of human factors engineering medical devices into rapid technological innovations demands continuous collaboration among stakeholders—including designers, engineers, and regulatory bodies—to ensure that patient safety remains the primary concern in the development of these devices. For instance, a case study evaluating a deep learning-based eye-screening system revealed that nurses faced challenges due to added workload and low image quality, underscoring the importance of addressing experience in HFE. This collaborative effort is crucial not only for meeting regulatory expectations but also for enhancing the overall user experience, ultimately improving health outcomes.
Conclusion
The exploration of Human Factors Engineering (HFE) in medical devices underscores its critical role in enhancing usability and patient safety. By prioritizing an understanding of user behavior, capabilities, and limitations, manufacturers can design intuitive devices that significantly mitigate risks associated with user errors. The evidence presented highlights the substantial impact of usability on device functionality, demonstrating that effective HFE practices can lead to improved patient outcomes and satisfaction.
Regulatory frameworks, particularly those established by the FDA, guide the implementation of HFE principles, mandating comprehensive usability testing and ongoing risk analysis. These guidelines not only ensure compliance but also foster trust and acceptance among users, reinforcing the necessity of integrating HFE throughout the product lifecycle. As the landscape of medical technology evolves, it becomes increasingly imperative for manufacturers to adapt to these regulatory demands while maintaining a focus on user-centered design.
The future of HFE in medical device development is rife with both challenges and opportunities, particularly with the rise of artificial intelligence and telemedicine. The need for innovative approaches that prioritize user experience, alongside effective collaboration among designers, engineers, and regulatory bodies, is essential for addressing the complexities introduced by new technologies. As the industry moves forward, a steadfast commitment to HFE will be vital in ensuring that medical devices not only meet regulatory standards but also truly serve the needs of users, thereby enhancing patient safety and overall healthcare quality.
Frequently Asked Questions
What is human factors engineering in medical devices?
Human factors engineering in medical devices is a discipline that focuses on optimizing user interaction to enhance usability and safety. It involves understanding human behavior, capabilities, and limitations in relation to design to create intuitive and user-friendly products.
Why is human factors engineering important in healthcare?
It is crucial in healthcare because it helps minimize user errors, which can significantly compromise patient safety and lead to software errors. By applying human factors principles, manufacturers can improve patient outcomes and satisfaction.
What are some common errors associated with medical device user interfaces?
Common errors include incorrect timing or absence of critical device state notifications, which can arise from complex user interactions. For example, six identified errors in audio/visual notifications highlighted these issues.
How does the FDA influence human factors engineering in medical devices?
The FDA provides guidelines that mandate manufacturers to conduct comprehensive usability testing to identify potential consumer errors and mitigate risks. The FDA’s guidance ensures that usability considerations are integrated throughout all stages of the design process.
What is the Use-Related Risk Analysis (URRA)?
The URRA is a dynamic document that requires updates whenever interfaces change or new risks arise. It is essential for enhancing patient safety and product usability by addressing potential risks effectively.
What upcoming changes are expected in the FDA guidelines regarding usability testing?
The upcoming revision of HE75 in 2024 is anticipated to provide enhanced clarity and expectations regarding usability testing requirements, ensuring that human factors engineering is integrated throughout the entire product lifecycle.
Who are some industry leaders influencing regulations in human factors engineering?
Industry leaders like Ana Criado, who has held executive positions at Colombia’s regulatory agency INVIMA, play a crucial role in shaping regulations that affect usability and safety in healthcare products, guiding manufacturers through regulatory complexities.




