Introduction
The De Novo application process stands as a pivotal regulatory pathway for medical devices classified as low to moderate risk, particularly those lacking a predicate device. As the demand for innovative healthcare solutions escalates, understanding this process becomes essential for manufacturers aiming to introduce new technologies that enhance patient care.
This article delves into the intricacies of the De Novo application, exploring its critical components, submission procedures, and the review process, while also addressing the challenges faced by applicants. Through real-world examples and expert insights, it highlights the significance of rigorous preparation and compliance in navigating the evolving landscape of medical device regulation, ultimately paving the way for advancements in medical technology.
Understanding the De Novo Application Process
The de novo application represents a crucial regulatory pathway established by the FDA specifically for instruments classified as low to moderate risk that lack a predicate. This process empowers manufacturers to seek a new classification for their product based on demonstrated safety and effectiveness, thereby facilitating its entry into the market. Gaining a thorough understanding of the de novo application procedure is vital, as it not only ensures compliance with regulatory standards but also fosters advancements in medical technology.
The process begins with a comprehensive assessment of the item's intended use alongside its associated risks, which subsequently informs the preparation steps for the application. As of September 30, 2024, the FDA has granted a total of 1,041 Breakthrough Designations, indicating a growing recognition of innovative products that may benefit from expedited regulatory pathways. This is particularly relevant in light of the 37 rare disease treatments that were approved by the FDA in 2023, showcasing the agency's commitment to facilitating access to innovative therapies.
Takeda emphasizes this significance, stating, 'Eohilia becomes the first oral treatment for EoE, a chronic allergic condition of the esophagus,' highlighting the impact of innovative treatments on patient care. Furthermore, the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, is paramount in overseeing medical device regulation, ensuring compliance and safety, especially as classified by PAHO/WHO as a Level 4 health authority. Katherine Ruiz, a Regulatory Affairs expert with extensive experience advising foreign manufacturers on obtaining market clearance in Colombia, exemplifies the expertise necessary for navigating such complex regulatory landscapes.
Her background, including prior work at INVIMA, positions her as a key resource for manufacturers looking to understand the Colombian market. Additionally, bioaccess® offers comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services are essential for navigating the regulatory landscape. The case study of Annalise Enterprise CXR Triage Trauma, created by Annalise-AI Pty Ltd and authorized on 03/28/2023, serves as a real-world example of a successful de novo application, illustrating the practical implications of this regulatory pathway.
Overall, these elements emphasize the significance of the de novo application within the broader context of medical product regulation and innovation.
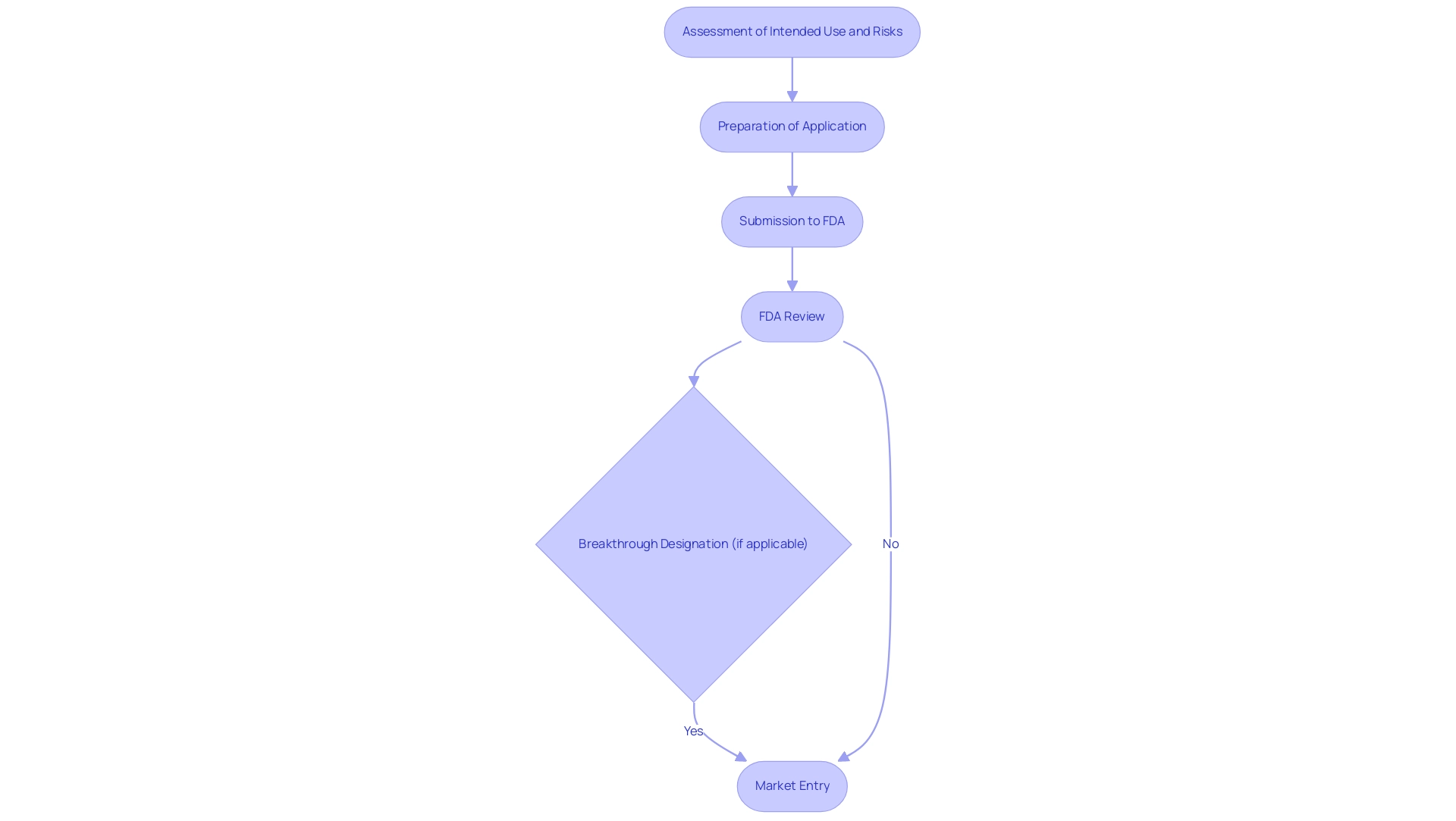
Essential Components of a De Novo Request
A well-prepared de novo application is crucial for navigating the regulatory landscape and must encompass several essential components:
- Equipment Description: This section requires a comprehensive overview of the equipment, detailing its intended use, design, and underlying technology. Clarity and precision are vital to ensure regulatory reviewers fully understand the product's function and purpose.
- Risk Assessment: Applicants must conduct a thorough analysis of potential risks linked to the equipment. This includes identifying risks and outlining strategies to mitigate them, which is critical for demonstrating the device's safety.
- Clinical Data: Robust evidence from clinical studies is necessary to substantiate claims of safety and effectiveness. This data should be meticulously gathered and presented to align with the latest FDA expectations for 2024. Our services include feasibility studies and site selection to ensure high-quality clinical data collection.
- Trial Setup and Approval: Our team assists in the trial setup and startup processes, ensuring compliance with ethics committee requirements and health ministry approvals. This is essential for the legal and ethical conduct of clinical trials.
- Reporting Information: We provide comprehensive reporting on study status, inventory, and both serious and non-serious adverse events, which are critical for ongoing compliance and safety monitoring.
- Labeling Information: Proposed labeling must adhere to regulatory requirements, providing clear instructions for use and safety information. Proper labeling is essential for guiding healthcare providers and ensuring patient safety.
- Manufacturing Information: This component involves detailing the manufacturing processes, quality control measures, and compliance with Good Manufacturing Practices (GMP). Such transparency is vital for establishing credibility with the FDA, and it is important to note that the FDA will review the de novo application within 120 days after receipt or after additional information that results in acceptance. Applicants should also be aware that a De Novo request may be considered withdrawn if the requester submits a written notice of withdrawal, fails to respond to information requests, or does not allow FDA inspection of facilities.
By ensuring these components are rigorously addressed, applicants can significantly enhance their chances of success in the de novo application process. A relevant case study, such as the examination of de novo gene disruptions in children diagnosed with autism spectrum disorder, emphasizes the importance of the de novo application in identifying genetic risk factors. As Rob MacCuspie aptly stated, don't be afraid of the de novo application process.
It’s actually a really great tool. Following these guidelines not only simplifies the process but also corresponds with the recent changes in FDA requirements, which will become effective 90 days after publication in the Federal Register. With expertise from leaders like Katherine Ruiz in Regulatory Affairs for medical products and in vitro diagnostics, and support from bioaccess®—a company renowned for its innovation and regulatory excellence in Latin America—applicants can navigate this complex landscape with confidence.
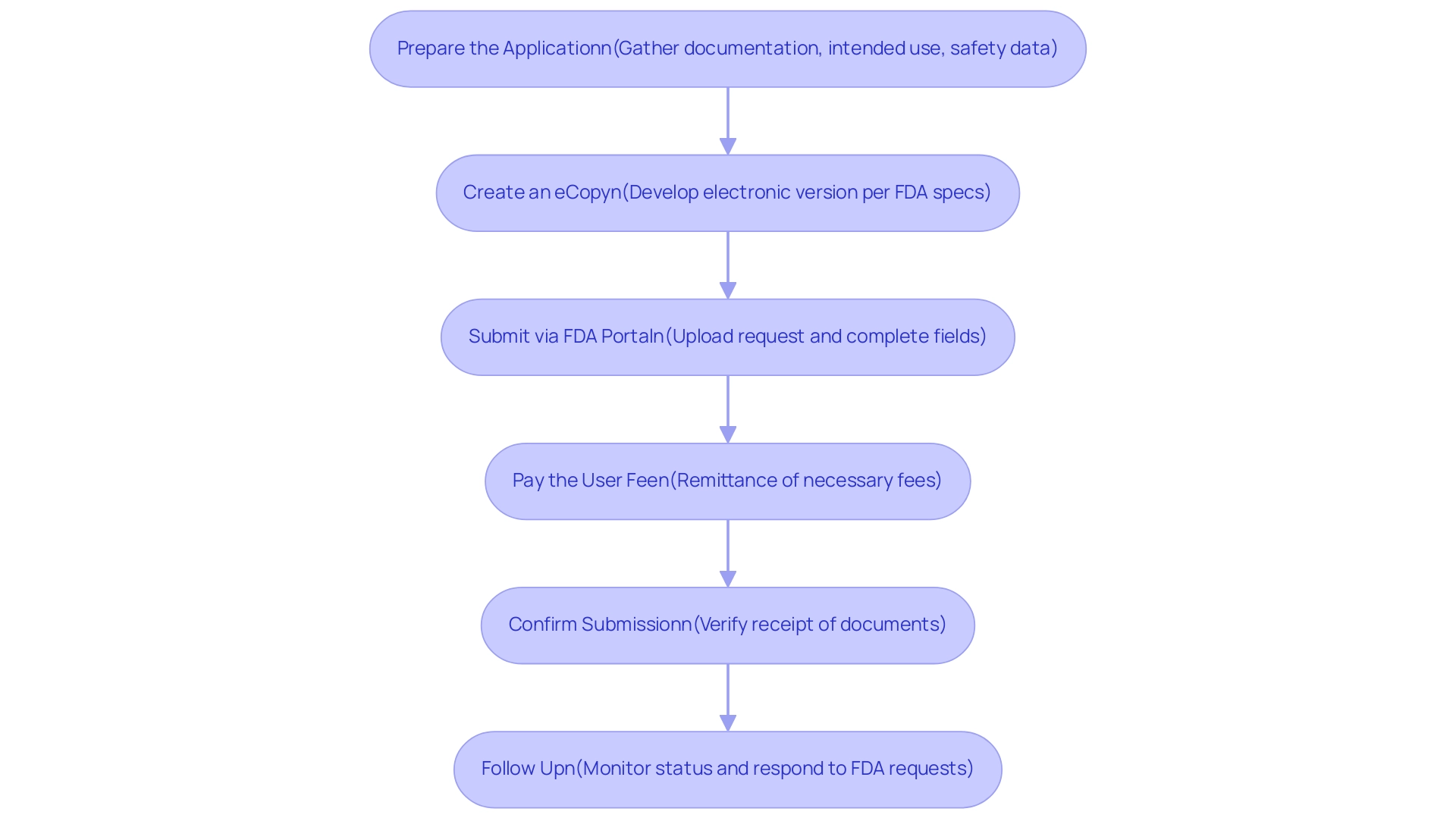
Step-by-Step Submission Procedures for De Novo Applications
Submitting a de novo application involves several critical steps to ensure compliance with FDA regulations and facilitate a streamlined review process. Here is a comprehensive guide to navigating this procedure effectively:
-
Prepare the Application: Gather all necessary documentation that clearly details the device’s intended use, technological characteristics, and safety and effectiveness data.
According to the FDA, this guide is intended to provide additional clarifications regarding the de novo application process, highlighting key considerations for submission. It’s crucial to align your submission with section 513(f)(2) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) to ensure compliance.
Create an eCopy: Develop an electronic version of the document that adheres to the FDA’s specifications.
Ensure that all formats and technical requirements are met to prevent delays in processing.
-
Submit via FDA Portal: Access the FDA's submission portal to upload your request. Complete all required fields accurately to avoid any complications during review.
-
Pay the User Fee: If relevant, remit the necessary user fee for the submission. For 2024, it is essential to be aware of the latest FDA user fee statistics, which can impact budget planning for manufacturers.
-
Confirm Submission: After submitting, verify receipt of your documents through the portal.
This step is vital to ensure that your submission is officially logged and in process.
-
Follow Up: Actively monitor the status of your submission and be prepared to respond promptly to any requests for additional information from the FDA. The FDA generally assesses de novo application submissions for completeness within 15 calendar days.
During the substantive evaluation, which can take longer than the 510(k) assessment process due to the absence of a precedent, applicants should be ready for potential inquiries regarding safety and effectiveness. If authorized, the apparatus can be promoted promptly, and the FDA releases a notice in the Federal Register with the new classification designation.
By diligently following these steps and utilizing comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, import permits, project management, and reporting on study status, inventory, and adverse events—you can significantly enhance the efficiency of the assessment process. Engaging experts like Katherine Ruiz in Regulatory Affairs for medical devices and in vitro diagnostics can provide additional support in determining eligibility and preparing submissions, ensuring alignment with the FDA’s evolving guidelines, which saw updates as recently as October 2021.
Navigating the Review Process of De Novo Applications
Upon submission of a De Novo request, the evaluation process unfolds through several critical stages:
- Initial Assessment: The FDA begins a preliminary evaluation to verify the completeness of the application and ensure it meets all submission requirements. This step is essential for identifying any deficiencies early in the process. Pre-submission requirements can significantly reduce assessment time and enhance investor confidence in De Novo projects.
- Technical Review: This stage involves a comprehensive evaluation of the apparatus's safety and effectiveness, relying on data from clinical studies, including those managed by experts like Katherine Ruiz at bioaccess®, who has over 20 years of experience in Medtech. Their proven track record in conducting Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF) ensures rigorous risk assessments are conducted. The evaluation team rigorously assesses whether the device meets the necessary standards for market entry.
- Communication: During the assessment, the FDA may request additional information or clarification from the applicant. Timely and comprehensive replies are essential, as they assist in keeping the evaluation timeline and enabling a smoother assessment process. As noted by industry experts, the new Estar templates were first utilized in 2021, and our firm has observed shorter overall assessment timelines and fewer deficiencies identified by FDA evaluators when they submit an 'Additional Information Hold' (A Hold) to companies. This emphasizes the significance of clear communication in navigating the evaluation process. The FDA's objective is to reach a conclusion regarding the de novo application in 150 evaluation days under MDUFA IV, highlighting the efficiency of this process.
- Decision: Following the technical assessment, the FDA issues a classification decision for the device. If the request is approved, the applicant receives a grant of marketing authorization, paving the way for the product's entry into the market. Effective navigation of the assessment stages can result in the creation of new regulations and product codes, as shown in the case study on the de novo application process, thus simplifying future submissions. Understanding these stages empowers applicants to prepare effectively for each phase and respond adeptly to FDA inquiries, thereby enhancing the likelihood of a successful review outcome. bioaccess® offers a customized approach to navigate your company towards an acquisition, leveraging their extensive experience and expertise in clinical trial management.
Challenges and Considerations in Preparing a De Novo Application
Navigating the preparation of a de novo application can be fraught with challenges that require careful consideration and strategic planning. Key challenges include:
-
Data Gaps: A critical hurdle is ensuring that ample clinical data supports safety and effectiveness claims.
Experimental tests have identified 56 designs that successfully employed well-folded monomeric structures, underscoring the importance of robust preclinical studies in gathering comprehensive data. This statistic illustrates the necessity of diverse methodologies to effectively fill in potential data gaps. For instance, the case study on 'Record Linkage and Tokenization' highlights how fragmentation of patient data across different healthcare networks can lead to biases in clinical effectiveness analyses.
Tokenization allows for linking patient data across various sources while maintaining privacy, thereby improving the comprehensiveness of data sets.
-
Regulatory Compliance: The regulatory landscape remains complex, necessitating a thorough understanding of FDA guidelines.
Ongoing updates and adjustments in Regulatory requirements indicate that consulting with Regulatory experts, such as Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, is crucial to reduce risks and improve compliance. As mentioned by Nick Tippmann, an experienced marketing professional, 'Navigating these intricacies is fundamental for success in the current medical equipment environment.'
-
Time Constraints: Balancing research timelines with submission deadlines can be particularly challenging. Developing a meticulous project plan that outlines key milestones and deadlines can help manage timelines effectively and ensure a streamlined submission process. Our comprehensive clinical trial management services include trial setup, project management, reporting, and assistance with import permits and nationalization of investigational devices to support this effort.
-
Communication with FDA: Establishing and maintaining open lines of communication with the FDA throughout the assessment process is paramount. Being proactive in addressing inquiries or requests for additional information can significantly influence the assessment timeline and overall success of the submission.
-
Document Review: Review and feedback on study documents to comply with country requirements are critical to ensure all submissions meet regulatory standards. By acknowledging these challenges and employing targeted strategies to address them, including feasibility studies, site selection, and thorough document reviews, applicants can substantially improve their chances of a successful de novo application, paving the way for innovative medical devices to reach the market.
Conclusion
The De Novo application process serves as a vital pathway for bringing innovative medical devices to market, particularly those without a predicate device. Understanding its critical components—from thorough device descriptions and risk assessments to robust clinical data—is essential for manufacturers aiming to comply with FDA regulations and enhance patient care. By navigating the submission and review procedures effectively, applicants can significantly improve their chances of success.
Challenges such as data gaps, regulatory compliance, and time constraints require strategic planning and a proactive approach to communication with the FDA. The insights shared throughout this article underscore the importance of meticulous preparation and the role of expert guidance in overcoming these hurdles. Real-world examples, including successful case studies, highlight the practical implications of the De Novo process and its significance in advancing medical technology.
Ultimately, rigorous adherence to the established guidelines not only streamlines the application journey but also fosters the development of groundbreaking medical devices that can transform patient outcomes. As the landscape of medical device regulation continues to evolve, staying informed and prepared will be paramount for manufacturers seeking to innovate and improve healthcare solutions.
Frequently Asked Questions
What is the de novo application process?
The de novo application is a regulatory pathway established by the FDA for low to moderate-risk instruments that lack a predicate. It allows manufacturers to seek a new classification for their product based on demonstrated safety and effectiveness.
Why is understanding the de novo application procedure important?
Understanding the de novo application procedure is vital for ensuring compliance with regulatory standards and fostering advancements in medical technology.
What are the initial steps involved in the de novo application process?
The process begins with a comprehensive assessment of the item's intended use and associated risks, which informs the preparation steps for the application.
How many Breakthrough Designations has the FDA granted as of September 30, 2024?
As of September 30, 2024, the FDA has granted a total of 1,041 Breakthrough Designations.
What significance does the approval of rare disease treatments have in relation to the de novo application?
The approval of 37 rare disease treatments by the FDA in 2023 highlights the agency's commitment to facilitating access to innovative therapies, which is relevant to the de novo application process.
What role does INVIMA play in the regulation of medical devices?
INVIMA, Colombia's National Food and Drug Surveillance Institute, oversees medical device regulation, ensuring compliance and safety as classified by PAHO/WHO as a Level 4 health authority.
What essential components should be included in a well-prepared de novo application?
Key components include Equipment Description, Risk Assessment, Clinical Data, Trial Setup and Approval, Reporting Information, Labeling Information, and Manufacturing Information.
How does a manufacturer demonstrate the safety of their equipment in the de novo application?
Manufacturers must conduct a thorough risk assessment, identifying potential risks and outlining strategies to mitigate them, which is critical for demonstrating the device's safety.
What is the importance of clinical data in the de novo application?
Robust clinical data is necessary to substantiate claims of safety and effectiveness, and it should be gathered and presented in alignment with the latest FDA expectations.
What happens if a de novo application request is considered withdrawn?
A De Novo request may be considered withdrawn if the requester submits a written notice of withdrawal, fails to respond to information requests, or does not allow FDA inspection of facilities.
How long does the FDA take to review a de novo application?
The FDA will review the de novo application within 120 days after receipt or after additional information that results in acceptance.
What support services does bioaccess® provide for navigating the regulatory landscape?
Bioaccess® offers services such as feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting to assist with regulatory compliance.




