Introduction
In the ever-evolving landscape of medical device regulation, the De Novo classification pathway emerges as a crucial mechanism for introducing innovative technologies that lack a predicate device. This regulatory route, established by the FDA, not only facilitates the entry of low to moderate-risk medical devices but also emphasizes the importance of safety and effectiveness in their design and implementation.
As evidenced by the recent success of devices like Tioga Cardiovascular's Luna™ TMVR System, which showcased significant advancements in patient care, the De Novo process streamlines the review and approval timeline, allowing manufacturers to bring groundbreaking solutions to market efficiently.
Understanding the intricacies of this pathway is essential for stakeholders aiming to navigate the complexities of regulatory compliance while fostering innovation in the medical device sector.
Understanding De Novo Requests: An Overview
The new application acts as a crucial regulatory route set up by the FDA, specifically intended for categorizing low to moderate-risk medical instruments that are innovative and do not have any predicate apparatus. This classification is essential for systems, such as Tioga Cardiovascular's groundbreaking Luna™ TMVR System, which recently completed its first human trials with promising outcomes, demonstrating significant improvements in patient recovery times and reduced complications. The new process facilitates a streamlined review, ensuring that innovative products can navigate regulatory challenges efficiently while adhering to stringent safety and effectiveness standards.
Manufacturers can expect to receive a letter communicating the FDA's decision within 60 calendar days of their request, providing a clear timeframe for planning. As of September 30, 2024, a total of 128 Breakthrough Devices have received marketing authorization, underscoring the relevance and success of the new pathway in today's regulatory landscape. For products to qualify for Breakthrough Device designation, they must undergo premarket approval applications, 510(k) notifications, or new classification requests, and must provide effective treatment for serious conditions while meeting specific criteria such as demonstrating substantial clinical improvement over existing therapies.
Furthermore, collaboration with regulatory experts like Ana Criado, who holds extensive experience in Regulatory Affairs and biomedical engineering, enhances the successful navigation of these processes. For manufacturers focused on introducing new medical technologies, understanding the de novo request categorization is crucial. It provides a strategic alternative to the traditional 510(k) pathway, especially when no predicate exists, thereby promoting innovation in the medical landscape.
Step-by-Step Process for Submitting a De Novo Request
- Determine Equipment Classification: Start by evaluating if your apparatus meets the criteria for new classification. This pathway is specifically designed for items that are novel and do not have an existing predicate. If your apparatus meets this criterion, you may proceed with the de novo request.
- Prepare a Comprehensive Proposal: Compile a detailed proposal package that includes essential documentation such as a thorough product description, intended use, and proposed labeling. Ensuring completeness and clarity in your entry is critical for a successful evaluation.
- Conduct Risk Analysis: Execute a comprehensive risk analysis to identify any potential hazards associated with your device. This analysis should not only outline the risks but also detail the strategies for effective risk mitigation, demonstrating your commitment to patient safety.
- Submit the Request: Utilize the FDA’s electronic portal to submit your new application. It is imperative to ensure that all components of the delivery are complete and accurate, as any discrepancies may lead to delays or denials.
- Respond to FDA Queries: Be proactive in your approach by preparing to respond promptly to any inquiries or requests for additional information from the FDA during their review process. A prompt response is crucial, as delays in addressing these queries can lead to denial of your designation request. As noted in recent discussions, the average review time for de novo requests in 2023 was 387 days, indicating the importance of efficiency in communication. As one FDA official stated, 'Timely responses to our information requests are essential to keep the review process moving smoothly.'
- Receive FDA Decision: Finally, await the FDA’s ruling regarding your proposal. This decision will determine whether your equipment is classified as a Class I or Class II item, ultimately impacting its market entry strategy. For example, the INSIGHTEC EXABLATE received marketing authorization with submission number P150038 on 07/11/2016, demonstrating a successful outcome from a thoroughly prepared new submission.
Additionally, leveraging insights from regulatory experts like Ana Criado, who has extensive experience in Regulatory Affairs and clinical trial management in Colombia, including services such as feasibility studies, site selection, compliance reviews, and project management, can provide valuable guidance throughout this process. Moreover, insights from Katherine Ruiz, an expert in Regulatory Affairs for medical instruments and in vitro diagnostics in Colombia, can enhance your understanding of the Regulatory landscape.
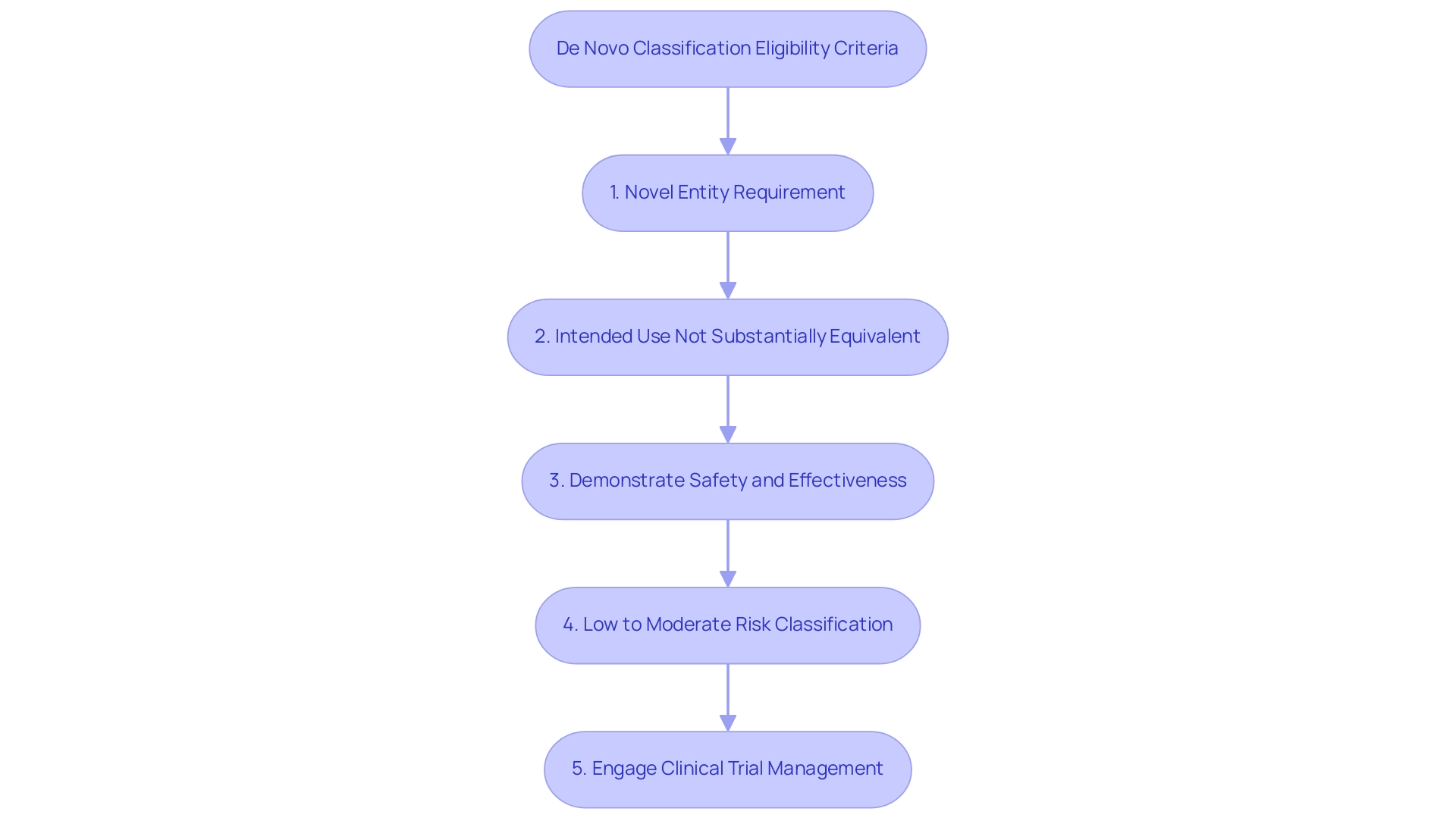
Eligibility Criteria for De Novo Classification
To qualify for De Novo classification, an instrument must adhere to specific eligibility criteria established by the FDA. Firstly, the apparatus must be a novel entity, meaning it does not have a legally marketed predicate apparatus that it can reference. Secondly, the intended use of the equipment should not be substantially equivalent to any existing products on the market.
It is essential for manufacturers to demonstrate that their product is both safe and effective for its intended purpose, a requirement critical in the evaluation process. Furthermore, the device should be classified as posing a low to moderate risk to patients. Understanding these criteria is essential for ensuring that your application aligns with FDA expectations and increases the likelihood of successful approval.
Engaging in comprehensive clinical trial management services, including trial setup, compliance reviews, and project management, can significantly enhance the submission process. As of September 30, 2024, a total of 128 Breakthrough Products have received marketing authorization, illustrating the ongoing evolution in the landscape of novel medical innovations. Additionally, the annualized costs over 10 years for new applications range from $0.1 million to $0.15 million, with a primary estimate of $0.08 million.
This emphasizes the financial factors involved in the process, highlighting the cost-efficiency of involving specialists in Regulatory Affairs, such as Katherine Ruiz, who focuses on medical products and in vitro diagnostics in Colombia. A pertinent case study emphasizes that a commenter proposed that a de novo request should permit the submission of groups of related items; however, the FDA disagreed, stating that grouping multiple items in a single de novo request is generally unsuitable and should be discussed with the FDA before submission. Interacting with these requirements, along with efficient reporting and monitoring practices, can greatly influence the success rates of new classifications, which are becoming increasingly important considering the percentage of products meeting eligibility criteria.
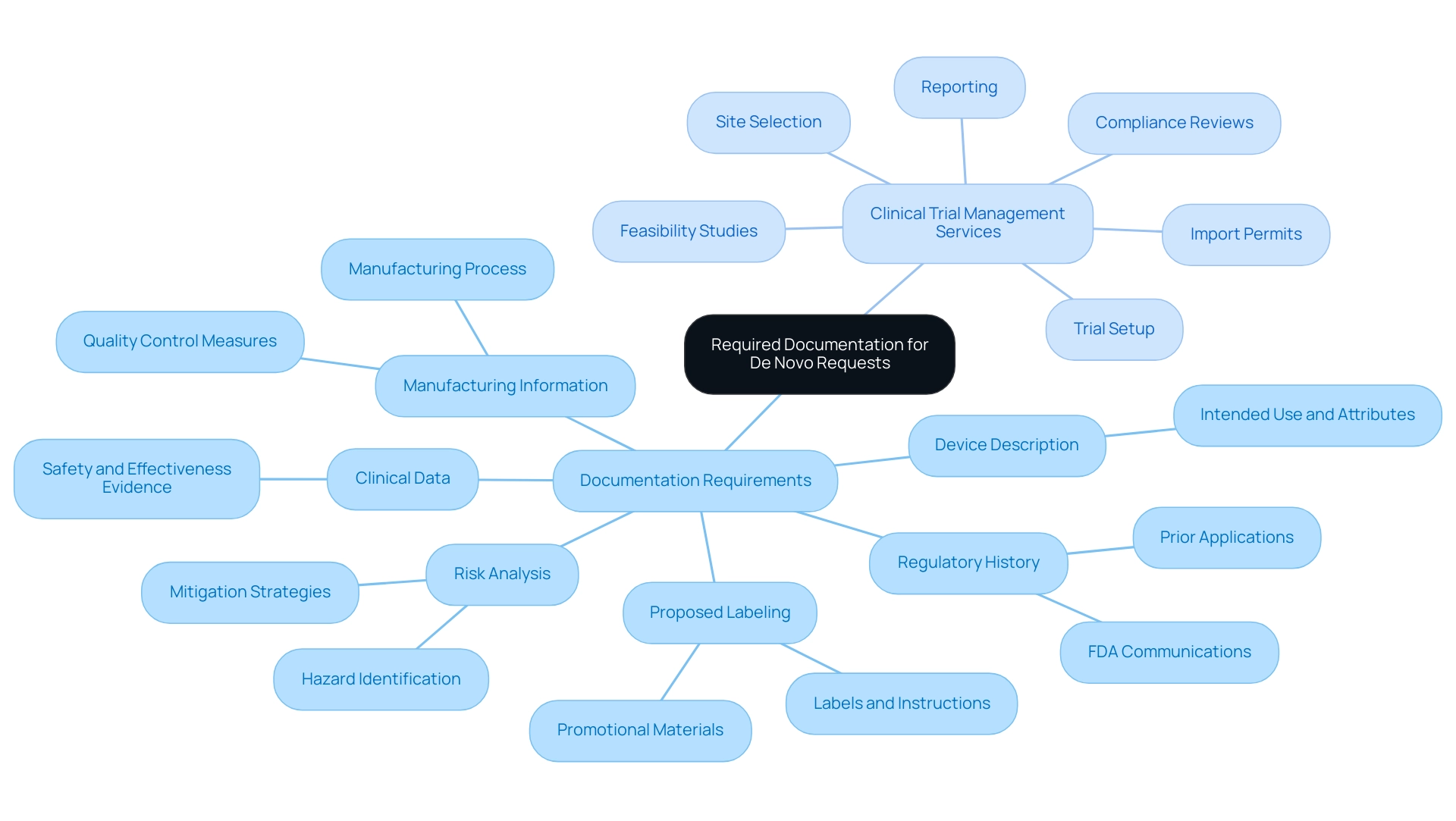
Required Documentation for De Novo Requests
To achieve a successful new request, the following documentation is imperative:
- Device Description: A comprehensive account of the device, detailing its intended use and technological attributes.
- Proposed Labeling: Drafts that encompass proposed labels, instructions for use, and any promotional materials.
- Risk Analysis: A thorough risk analysis that identifies potential hazards and outlines effective mitigation strategies. As emphasized by Adam Newman, Head of Marketing, 'By embracing this pathway and leveraging the resources and expertise available, companies can navigate regulatory challenges with confidence and drive positive impacts on global health.'
- Clinical Data: If relevant, include clinical data that substantiates the safety and effectiveness of the device.
- Manufacturing Information: Information detailing the manufacturing process along with quality control measures in place.
- Regulatory History: Documentation of any prior applications or communications with the FDA concerning the device.
In addition to compiling this crucial documentation, it is vital to engage in comprehensive clinical trial management services, which include:
- Feasibility studies
- Site selection
- Trial setup
- Import permits
- Compliance reviews
- Reporting
These services simplify the process of a de novo request application. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes the need for meticulous project management and reporting throughout the trial to ensure all aspects meet regulatory standards.
Companies must also respond to any Additional Information (AI) requests from the FDA within 180 calendar days, or their application may be deleted. Furthermore, as highlighted in a recent case study, including protocols and reports for all studies is essential for maintaining consistency and credibility in documentation, a requirement that the FDA has now reinforced. For reference, the relevant regulatory updates can be found in the document spanning pages 54826 to 54851 in the Federal Register.
Recent Updates in the De Novo Submission Process
The new application process has experienced several important updates that are crucial for manufacturers to comprehend. Initially, the FDA has required electronic filing for all new requests through its portal, a change intended to simplify the review process. This shift not only enhances efficiency but also reduces the likelihood of delays, making it critical for manufacturers to adapt quickly.
Significantly, the FDA intends to reach a conclusion on new requests within 150 review days, highlighting the importance of prompt entries. Additionally, the FDA has published updated guidance documents that provide comprehensive best practices for de novo requests, detailing the specific types of data required to support those applications. These documents are vital for ensuring compliance and optimizing filing strategies.
Furthermore, the introduction of new pathways for expedited review is particularly noteworthy, as they are designed to facilitate quicker market access for devices addressing unmet medical needs. However, manufacturers should also be aware of the challenges the FDA faces in hiring qualified technical staff, which could impact review timelines. The consequences of not responding to deficiencies within the specified timeframe are significant; if a complete response is not received within 180 days, the request is considered withdrawn, necessitating a new submission for marketing authorization.
By staying informed about these updates, manufacturers can better navigate the evolving landscape of de novo requests and strategically position their products for success.
Conclusion
The De Novo classification pathway represents a vital avenue for the introduction of innovative medical devices that do not have existing predicates. By streamlining the regulatory process, this pathway not only accelerates the approval timeline but also ensures that devices meet rigorous safety and effectiveness standards. The success stories of devices like Tioga Cardiovascular's Luna™ TMVR System underscore the potential of De Novo submissions to enhance patient care and advance medical technology.
Understanding the step-by-step process for submitting a De Novo request is crucial for manufacturers aiming to navigate the complexities of regulatory compliance. From determining device classification to preparing comprehensive documentation and responding to FDA queries, each step plays a significant role in ensuring a successful submission. Engaging with regulatory experts can further strengthen this process, providing valuable insights that enhance the likelihood of approval.
Moreover, the evolving landscape of De Novo submissions, characterized by recent updates and the introduction of expedited review pathways, highlights the FDA's commitment to fostering innovation in the medical device sector. Manufacturers must remain informed about these changes and adhere to the eligibility criteria to optimize their submission strategies effectively.
In summary, leveraging the De Novo pathway not only fosters innovation but also contributes to improved patient outcomes. As the regulatory environment continues to evolve, manufacturers that effectively utilize this classification route will be well-positioned to bring groundbreaking medical devices to market, ultimately benefiting healthcare systems and patients alike.
Frequently Asked Questions
What is the purpose of the new application established by the FDA?
The new application serves as a regulatory route for categorizing low to moderate-risk medical devices that are innovative and lack predicate devices, facilitating a streamlined review process while ensuring safety and effectiveness.
What are some examples of devices that can benefit from this new application?
An example is Tioga Cardiovascular's Luna™ TMVR System, which recently completed its first human trials, showing significant improvements in patient recovery times and reduced complications.
How long does it typically take for manufacturers to receive a decision from the FDA?
Manufacturers can expect to receive a letter communicating the FDA's decision within 60 calendar days of their request.
How many Breakthrough Devices have received marketing authorization as of September 30, 2024?
A total of 128 Breakthrough Devices have received marketing authorization, highlighting the success of the new pathway in the regulatory landscape.
What criteria must products meet to qualify for Breakthrough Device designation?
Products must undergo premarket approval applications, 510(k) notifications, or new classification requests, provide effective treatment for serious conditions, and demonstrate substantial clinical improvement over existing therapies.
Why is collaboration with regulatory experts important for manufacturers?
Collaborating with regulatory experts enhances the successful navigation of the regulatory processes, providing valuable guidance and insights that can facilitate the introduction of new medical technologies.
What is the first step manufacturers should take when seeking a new classification for their device?
Manufacturers should evaluate if their device meets the criteria for new classification, specifically if it is novel and lacks an existing predicate.
What documentation is required for the comprehensive proposal when submitting a new application?
The proposal package should include a thorough product description, intended use, proposed labeling, and a detailed risk analysis to demonstrate commitment to patient safety.
How should manufacturers respond to FDA inquiries during the review process?
Manufacturers should be proactive and prepare to respond promptly to any inquiries or requests for additional information from the FDA, as timely responses are crucial for maintaining the review process.
What determines whether a device is classified as Class I or Class II?
The FDA's decision regarding the proposal will determine the classification of the device, which impacts its market entry strategy.

