Overview
Navigating ANVISA regulations for Medtech companies involves a systematic approach that includes understanding medical device classification, ensuring compliance with Brazilian standards, and engaging effectively with stakeholders. The article outlines critical steps such as pre-submission preparation, timely responses to ANVISA inquiries, and maintaining post-market surveillance, emphasizing that thorough documentation and proactive communication are essential for successful product approval in Brazil's regulatory landscape.
Introduction
Navigating the complex regulatory landscape for medical devices in Brazil requires a deep understanding of ANVISA's stringent requirements and processes. As the agency responsible for ensuring the safety and efficacy of medical products, ANVISA's classification system ranges from low-risk Class I devices to high-risk Class IV devices, each with its own set of approval criteria.
This article delves into the essential components of engaging with ANVISA, including:
- The initial classification and registration of medical devices
- The critical documentation required for compliance
- Post-market surveillance practices necessary for ongoing adherence to regulations
By exploring best practices and leveraging expert insights, companies can streamline their approach to regulatory challenges and enhance their chances of successful market entry in one of the world's largest healthcare markets.
Understanding ANVISA: The Regulatory Landscape for Medical Devices in Brazil
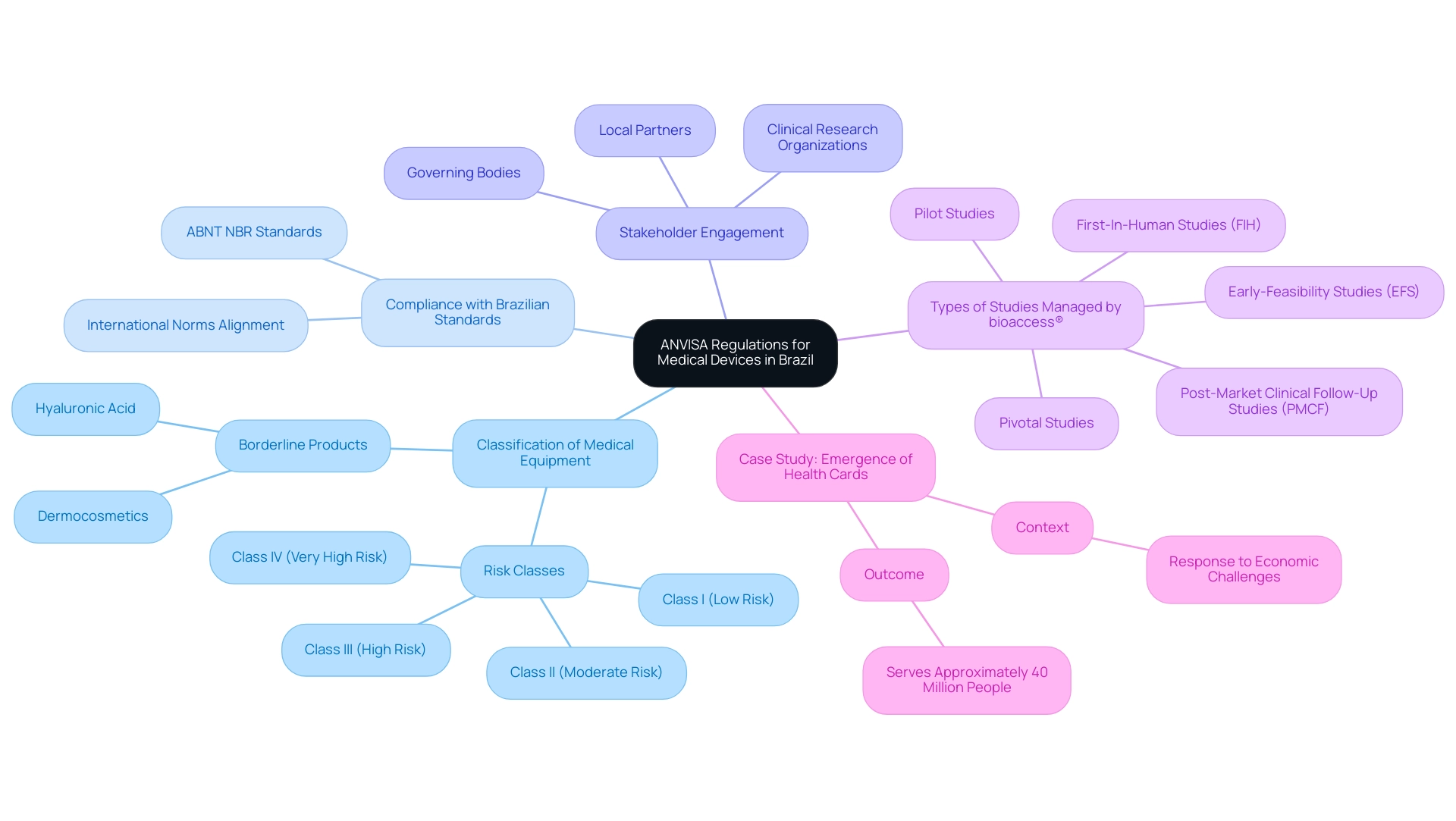
Navigating ANVISA (Brazil) Regulations for Medtech Companies is critical as ANVISA plays a role in regulating medical equipment in Brazil, ensuring that all products meet established safety and efficacy standards. The regulatory framework includes a classification system that categorizes items from Class I (low risk) to Class IV (high risk), with each category imposing distinct requirements for authorization. Medical equipment companies should focus on several key aspects to navigate this landscape effectively, particularly by leveraging comprehensive clinical trial management services offered by bioaccess®:
- Classification of Medical Equipment: Comprehending the categorization of your apparatus according to regulatory standards is crucial, as this directly affects the approval process you will need to follow. Borderline products such as dermocosmetics and hyaluronic acid frequently face considerable classification difficulties, which may require official registration to clarify their compliance status. Navigating ANVISA (Brazil) Regulations for Medtech Companies involves ensuring that any medical device is registered with the regulatory authority before it can be promoted in Brazil. This process requires the submission of comprehensive documentation, including technical specifications and clinical data to demonstrate safety and efficacy. With bioaccess®'s expertise, companies can ensure that their registration process is streamlined and compliant, particularly when navigating ANVISA (Brazil) Regulations for Medtech Companies.
- Compliance with Brazilian Standards: Adhering to national standards, such as those set by the ABNT NBR (Brazilian National Standards Organization), is vital. These standards align closely with international norms, ensuring that products are compliant and competitive in the global marketplace. Bioaccess® offers compliance reviews to assist in meeting these standards.
- Stakeholder Engagement: Identifying and engaging with key stakeholders—such as governing bodies, clinical research organizations, and local partners—is crucial for streamlining the approval process. Bioaccess® provides project management services that facilitate this engagement throughout the clinical study process.
- Types of Studies Managed by bioaccess®: Bioaccess® specializes in various critical studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). These specialized services are tailored for navigating ANVISA (Brazil) regulations for Medtech companies, effectively ensuring that clients can fulfill compliance requirements efficiently.
With the health agency's 2024-2025 agenda currently featuring 172 topics, including 89 in progress, grasping these foundational elements is more important than ever. A pertinent case study is the emergence of health cards, which developed in response to economic challenges and now serves approximately 40 million people, emphasizing how compliance complexities can lead to innovative solutions in healthcare.
Katherine Ruiz, an expert in compliance matters for medical instruments and in vitro diagnostics in Colombia, stresses the importance of staying informed about changes in regulations:
For more information, please reach out to Healthcare, Life Sciences, and Biotech Sectors Commercial Specialist, Jefferson Oliveira at Jefferson.Oliveira@trade.gov.
This insight emphasizes the necessity for ongoing involvement with compliance changes to ensure successful navigation of the intricate oversight environment.
Step-by-Step Guide to Navigating the Medical Device Approval Process with ANVISA
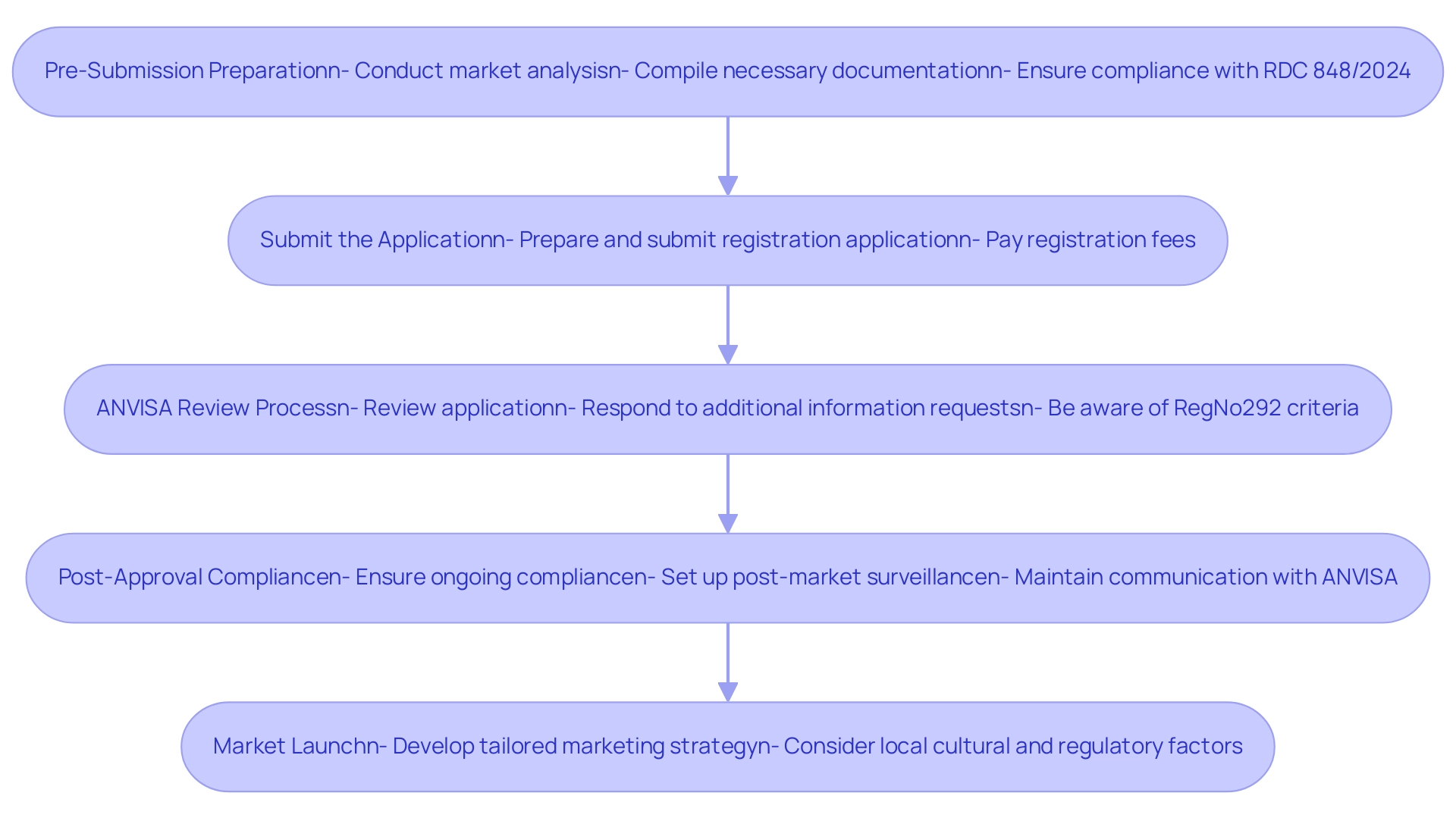
Successfully navigating the medical device approval process with the regulatory agency requires a systematic approach consisting of several critical steps:
-
Pre-Submission Preparation:
- Conduct a comprehensive market analysis to understand both the competitive landscape and specific regulatory requirements, leveraging the expertise of specialized teams like bioaccess® who have over 20 years in Medtech. This foundational step is crucial for aligning your product with market needs.
- Compile all necessary documentation, including technical files, clinical data, and certifications of your quality management system, ensuring that each element meets ANVISA standards. Note that with the new RDC 848/2024 regulation becoming mandatory in 180 days, timely preparation is essential for compliance.
-
Submit the Application:
- Prepare and submit your registration application through ANVISA’s online platform. It is vital to ensure that all required documents are included, as omissions can lead to delays.
- Pay the applicable registration fees. Grasping the fee structure can assist in budgeting and financial planning for the endorsement process.
-
ANVISA Review Process:
- ANVISA will initiate a review of your submitted application, which may involve requests for additional information or clarifications. Being prompt and thorough in your responses is essential for maintaining the momentum of the endorsement process. Additionally, be aware that RegNo292 establishes criteria for defining Equivalent Foreign Regulatory Authorities, which may influence the review process.
- The review timeline can vary significantly based on the classification and complexity of the device, so it is important to be prepared for potential adjustments to your timelines.
-
Post-Approval Compliance:
- Upon receiving approval, it is imperative to ensure ongoing compliance with ANVISA regulations. This involves setting up a strong post-market surveillance system and being careful in reporting any adverse events related to your product. Furthermore, understanding the classification of human biological materials according to risk categories, including specific labeling and transport requirements, is crucial for compliance.
- Maintain continuous communication with ANVISA and stay updated on any regulatory changes that may impact your device's compliance status. Bioaccess® offers specialized knowledge and flexibility in navigating ANVISA (Brazil) Regulations for Medtech Companies, ensuring that you remain compliant throughout the lifecycle of your device.
-
Market Launch:
- Once you have received approval, develop a marketing strategy that is tailored to the Brazilian healthcare market. This approach must consider local cultural subtleties and regulatory factors to connect effectively with your intended audience.
By diligently adhering to these guidelines and taking into account the most recent regulatory changes, medical technology firms can simplify navigating ANVISA (Brazil) regulations for medtech companies during the certification process. Bioaccess® offers extensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, and Post-Market Clinical Follow-Up Studies, ensuring the successful integration of their products into the Brazilian healthcare system.
Preparing Documentation for ANVISA Submission
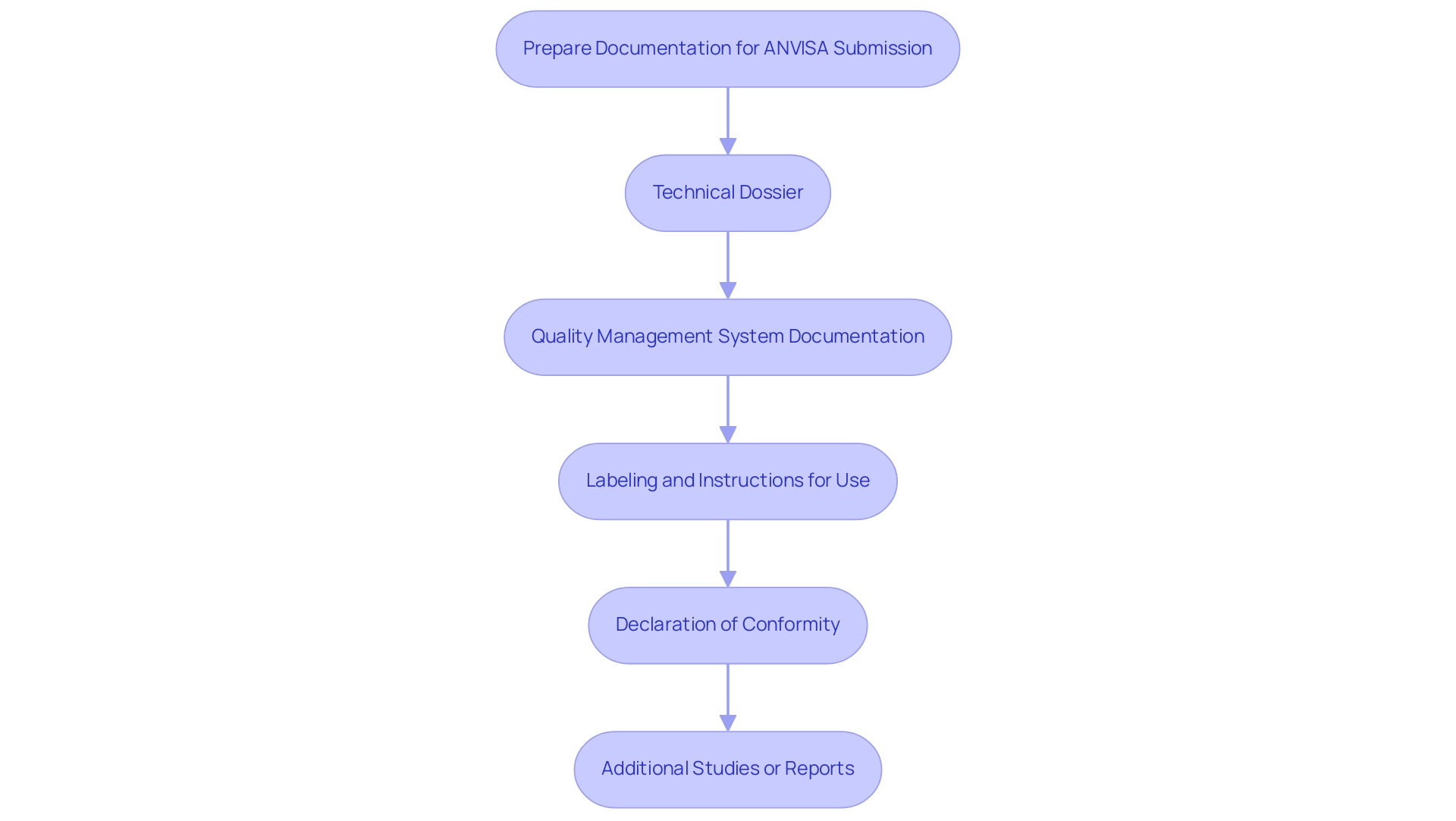
The preparation of documentation for regulatory submission encompasses several essential components, each critical to ensuring compliance and facilitating the approval process:
-
Technical Dossier: A comprehensive technical dossier is fundamental and should include:
- A detailed description of the device and its intended use.
- Thorough documentation of design and manufacturing processes.
- Risk management documentation to assess potential hazards associated with the device.
- Clinical evaluation reports that demonstrate the device’s safety and efficacy through robust data.
-
Quality Management System Documentation: Evidence of adherence to ISO 13485 or other relevant quality management standards is necessary. This should encompass:
- Quality manuals that outline the quality management system.
- Specific procedures and protocols in place.
- Records of audits and evaluations to confirm compliance.
-
Labeling and Instructions for Use: It is crucial that all labeling meets ANVISA’s stringent requirements, which include:
- Clear and concise instructions for use that are easily understandable.
- Comprehensive safety information and warnings to inform users adequately.
- Adherence to Brazilian language requirements, ensuring accessibility for all users.
-
Declaration of Conformity: A declaration of conformity is essential, affirming that the equipment complies with all applicable regulations and standards governing medical instruments in Brazil.
-
Additional Studies or Reports: When applicable, include any clinical trial data or supplementary studies that substantiate the device's safety and efficacy. This can strengthen your submission and provide the agency with the necessary evidence for review.
By carefully compiling these documents, companies can greatly improve their chances of a smooth approval process with the agency, which is essential when navigating ANVISA (Brazil) Regulations for Medtech Companies, thereby aiding market entry and adherence to standards. Furthermore, it's noteworthy that the Brazilian Health Regulatory Agency has representatives in the CIOMS Working Group on Real-World Data and Real-World Evidence in Regulatory Decision Making, which underscores the importance of integrating real-world evidence into submissions. In addition, the National Research Ethics Commission emphasizes the need for assessing each research participant’s needs and capabilities, which can be critical in clinical evaluations.
For practical insights, companies can refer to the case study of BioPharma Services, which has successfully managed submissions from the health authority, providing expertise in drug development projects and assisting pharmaceutical companies in meeting the agency's standards for bioequivalence studies. Notably, experts like Juan Cuya, MD, and Katherine Ruiz can offer invaluable guidance on Regulatory Affairs and compliance, further enhancing the submission process.
Engaging with ANVISA: Best Practices for Effective Communication
Effective engagement with ANVISA necessitates adherence to several best practices that can significantly enhance the approval process for medical devices, especially considering that Brazil ranks as the world's eighth largest market for prescription medicines:
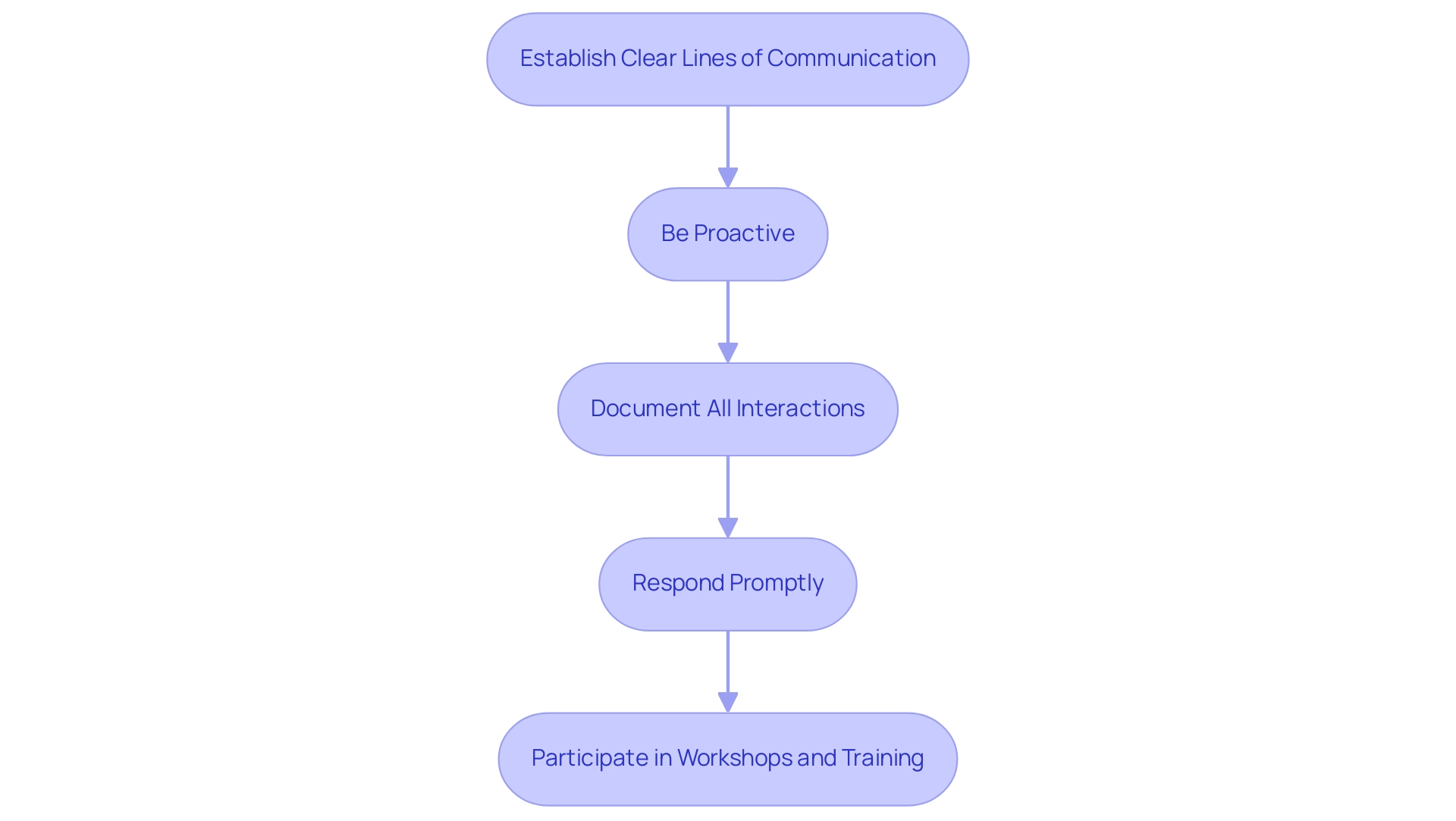
- Establish Clear Lines of Communication: It is crucial to identify the relevant contacts within the regulatory agency specific to your device category. Establishing a connection with these individuals guarantees that you have direct access to the necessary information and support throughout the endorsement process.
- Be Proactive: Initiate contact with the regulatory agency early in your project timeline to clarify requirements and pose any questions. Early engagement helps prevent misunderstandings that could lead to delays in the approval process. As noted by Pankaj P. Nerkar, "Medicinal products registration is a lengthy process in Brazil," making proactive communication even more essential.
- Document All Interactions: Maintain comprehensive records of all communications with the regulatory agency, including emails, phone calls, and meetings. This documentation serves as a valuable reference for future inquiries and can support your case during the review.
- Respond Promptly: If the regulatory agency issues a deficiency letter requesting further information or clarification, respond swiftly. Timely responses demonstrate your commitment to compliance and can expedite the review process, helping to mitigate potential delays.
- Participate in Workshops and Training: Engage in ANVISA-hosted workshops or training sessions to stay informed about compliance updates and best practices. These opportunities not only enhance your understanding but also demonstrate your dedication to adhering to their guidelines.
As an example of successful engagement, bioaccess® has effectively maneuvered the compliance environment in Latin America, especially in carrying out Early-Feasibility Studies (EFS), First-In-Human Studies (FISH), and Post-Market Clinical Follow-Up Studies (PMCF) for submissions. With more than 20 years of experience in Medtech, their knowledge highlights the significance of these best practices in attaining successful results. Furthermore, bioaccess® offers a customized approach that adapts to the specific needs of each study, enhancing their competitive advantage.
By integrating these strategies, medical technology firms can foster positive connections with ANVISA, which is crucial for navigating ANVISA (Brazil) Regulations for Medtech Companies and ensuring the successful endorsement of their medical products.
Post-Market Surveillance and Compliance with ANVISA Regulations
Post-market surveillance is a crucial element of regulatory compliance with INVIMA, acting as a safeguard for both patient safety and product efficacy. Companies must engage in several critical practices:
- Monitor Equipment Performance: Continuously collect and analyze data regarding the equipment's performance, including any adverse events reported by users. This ongoing assessment is vital for ensuring the equipment meets safety standards throughout its lifecycle.
- Report Adverse Events: Establish a robust system for reporting adverse events to INVIMA within the stipulated timelines. Adhering to these requirements is imperative for maintaining compliance and safeguarding patient health.
- Conduct Periodic Reviews: Regular review of product performance and compliance with INVIMA regulations is necessary to identify potential issues early. This proactive approach can mitigate risks and enhance quality control processes.
- Implement Corrective Actions: Should any issues arise, it is crucial to promptly implement corrective actions and communicate these to INVIMA as required. The Clinical Hazards List (CHL), which relies on post-market surveillance data, underscores the importance of maintaining up-to-date safety knowledge. Without sufficient post-market data, the completeness and reliability of the CHL cannot be assured, as highlighted in its ongoing updates based on surveillance data.
- Stay Updated on Regulatory Changes: Vigilance in tracking any changes in INVIMA regulations is paramount, as these may affect the product or its market usage. As noted by experts in compliance affairs including Katherine Ruiz, "transparency should be an essential feature of authorities, yet this requirement remains fragile and fragmented," emphasizing the need for robust compliance practices.
By prioritizing post-market monitoring and compliance with standards, medtech companies not only enhance the ongoing safety and effectiveness of their products but also safeguard their market position amid changing oversight environments. Notably, INVIMA's classification as a Level 4 health authority by PAHO/WHO underscores its competence in ensuring the safety, efficacy, and quality of medical products in Colombia. Additionally, yearly data show an increasing number of adverse events reported to INVIMA, reinforcing the need for diligent monitoring and reporting practices to ensure patient safety and compliance.
The Directorate for Medical Devices and other Technologies plays a crucial role in this process, overseeing the compliance of medical devices with established standards and regulations, thereby enhancing the overall regulatory framework in Colombia.
Conclusion
Navigating the regulatory landscape for medical devices in Brazil, particularly through ANVISA, is a multifaceted endeavor that demands thorough preparation and strategic engagement. The classification of medical devices, with its varying risk levels from Class I to Class IV, serves as the cornerstone of this process, influencing the subsequent registration and compliance requirements. Companies must meticulously prepare comprehensive documentation, including:
- Technical dossiers
- Quality management system records
to meet ANVISA's stringent standards.
Effective communication with ANVISA is equally critical. Establishing clear lines of communication, being proactive in inquiries, and responding promptly to requests can significantly streamline the approval process. Engaging in workshops and maintaining thorough documentation of interactions further enhance the likelihood of a successful outcome.
Post-market surveillance remains a vital aspect of regulatory compliance, ensuring that devices continue to meet safety and efficacy standards throughout their lifecycle. Continuous monitoring, timely reporting of adverse events, and readiness to implement corrective actions are essential practices for maintaining compliance and safeguarding patient health.
In conclusion, understanding and adhering to ANVISA's regulatory framework is essential for medical device companies aiming to enter the Brazilian market. By leveraging expert insights, engaging in best practices, and committing to ongoing compliance, companies can effectively navigate the complexities of this landscape and enhance their chances of success in one of the world's largest healthcare markets.
Frequently Asked Questions
What is the role of ANVISA in regulating medical equipment in Brazil?
ANVISA is responsible for regulating medical equipment in Brazil, ensuring that all products meet established safety and efficacy standards.
How are medical devices classified by ANVISA?
Medical devices are classified into four categories: Class I (low risk) to Class IV (high risk), with each class imposing distinct requirements for authorization.
What are the key aspects that medical equipment companies should focus on when navigating ANVISA regulations?
Companies should focus on classification of medical equipment, compliance with Brazilian standards, stakeholder engagement, and managing various types of studies.
Why is understanding the classification of medical equipment important?
Understanding the classification is crucial because it directly affects the approval process and the specific requirements that need to be met for registration.
What documentation is required for registering a medical device with ANVISA?
Comprehensive documentation is required, including technical specifications and clinical data to demonstrate the safety and efficacy of the medical device.
What standards must companies comply with to ensure their products are competitive in the global marketplace?
Companies must adhere to national standards set by the ABNT NBR (Brazilian National Standards Organization), which align closely with international norms.
How can bioaccess® assist companies in navigating ANVISA regulations?
Bioaccess® offers comprehensive clinical trial management services, compliance reviews, project management services, and expertise in various critical studies to streamline the regulatory process.
What types of studies does bioaccess® manage?
Bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What steps are involved in the medical device approval process with ANVISA?
The steps include pre-submission preparation, submitting the application, the ANVISA review process, post-approval compliance, and market launch.
What should companies do after receiving approval from ANVISA?
Companies must ensure ongoing compliance with ANVISA regulations, establish a post-market surveillance system, report adverse events, and maintain communication with ANVISA.
How can companies prepare for the upcoming RDC 848/2024 regulation?
Companies should conduct a comprehensive market analysis and compile necessary documentation to ensure timely compliance with the new regulation, which becomes mandatory in 180 days.




