Overview
Navigating Latin American regulatory authorities for medical devices involves understanding the specific requirements set by agencies such as ANVISA in Brazil and COFEPRIS in Mexico, as well as the unique regulations in Colombia. The article emphasizes the importance of thorough preparation, local expertise, and continuous monitoring of regulatory changes to facilitate successful market entry and compliance across these diverse jurisdictions.
Introduction
Navigating the regulatory landscape for medical devices in Latin America is a formidable challenge characterized by a diverse array of requirements that vary significantly across countries. With key regulatory authorities such as:
- ANVISA in Brazil
- COFEPRIS in Mexico
- INVIMA in Colombia
manufacturers must adeptly maneuver through complex registration processes and compliance obligations. This article delves into the intricacies of medical device regulation within these regions, offering insights into the specific requirements set forth by each authority and the essential strategies for successful market entry. By highlighting the importance of collaboration with local experts and staying informed on regulatory changes, it aims to equip stakeholders with the knowledge necessary to thrive in this multifaceted environment, ultimately enhancing access to safe and effective medical technologies.
Navigating the Regulatory Landscape for Medical Devices in Latin America
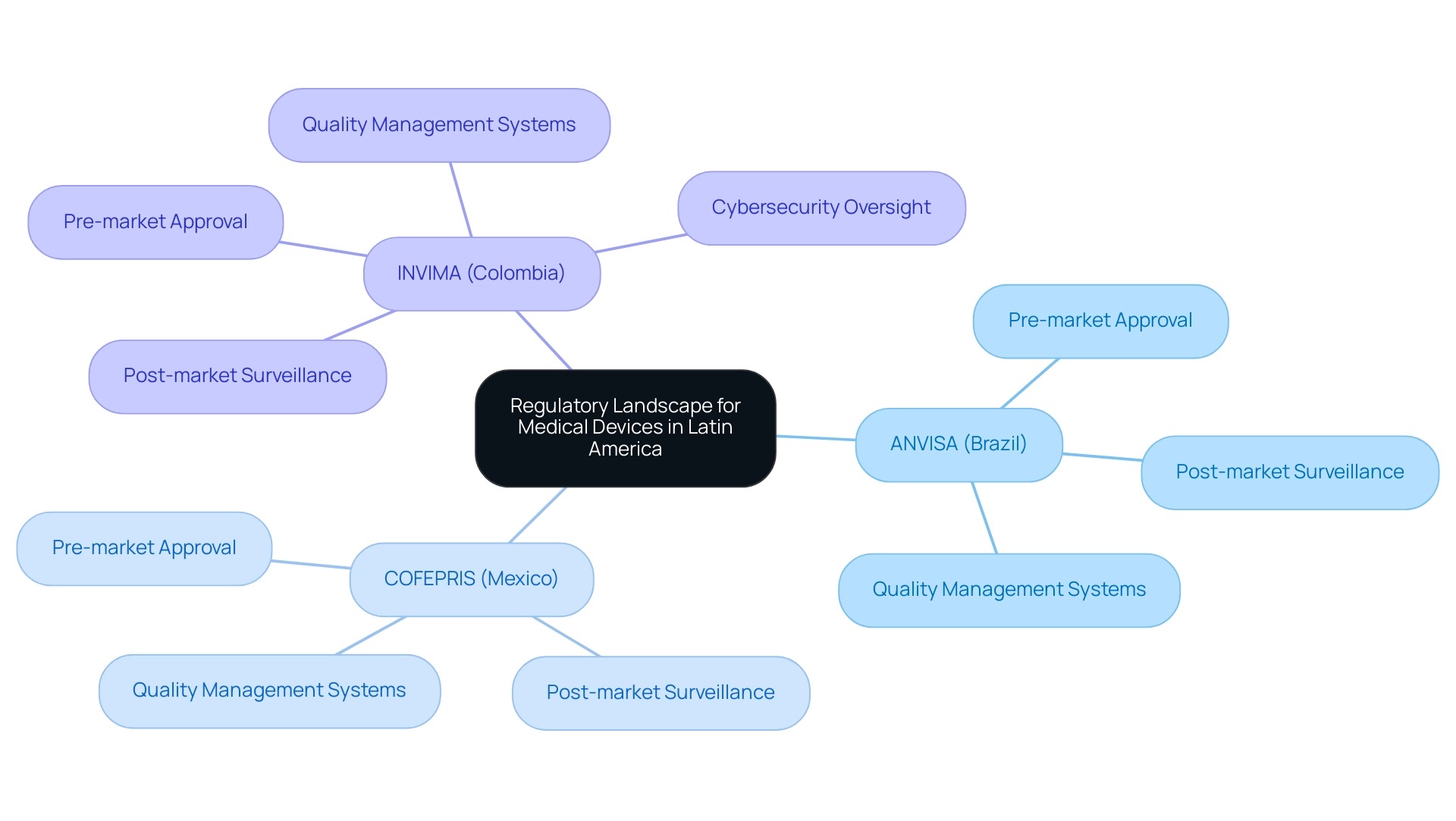
The compliance environment for health equipment in Latin America is detailed in A Guide to Latin American Regulatory Authorities for Medical Devices, which shows a complicated set of requirements that differ from one nation to the next. The main regulatory bodies supervising these products consist of:
- ANVISA in Brazil
- COFEPRIS in Mexico
- Another authority in Colombia
Founded in 1992 under the Ministry of Health and Social Protection, the Colombia National Food and Drug Surveillance Institute is responsible for inspecting and supervising the marketing and manufacturing of health products, evaluating compliance with health standards, and overseeing regulations related to health technologies through its Directorate for Health Technologies and other Technologies.
This directorate oversees and regulates healthcare instruments, proposing technical standards and guaranteeing quality assurance through a strong framework that includes pre-market approval processes and post-market surveillance activities. The health authority is acknowledged as a Level 4 regional reference by the Pan American Health Organization/World Health Organization, highlighting its expertise in health regulation functions crucial for ensuring the safety, efficacy, and quality of healthcare products. A Guide to Latin American Regulatory Authorities for Medical Devices outlines that each of these agencies has established specific requirements for health product registration, including:
- Pre-market approval
- Post-market surveillance
- Adherence to quality management systems
For instance, manufacturers must navigate ANVISA's rigorous documentation processes, engage with COFEPRIS's evaluation protocols, and adhere to regional standards. Given the growing emphasis on cybersecurity, particularly concerning AI-based medical devices, INVIMA has begun to implement oversight approaches that address these challenges, ensuring that manufacturers are equipped to meet the evolving standards. Katherine Ruiz, an expert in Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasizes the necessity for manufacturers to conduct comprehensive research on the unique requirements of each jurisdiction and utilize the knowledge of local compliance professionals.
Denise McDermott, Senior Compliance Specialist at Compliance & Risks, emphasizes this necessity, stating, 'Manufacturers need to continuously monitor and assess requirements to ensure compliance.' This highlights the specific regulatory challenges faced by manufacturers in Latin America, which is detailed in A Guide to Latin American Regulatory Authorities for Medical Devices, underscoring the importance of informed navigation through this multifaceted landscape. With the right guidance, companies can effectively facilitate smoother market entry across these diverse regions.
Understanding Country-Specific Regulations: A Focus on Brazil
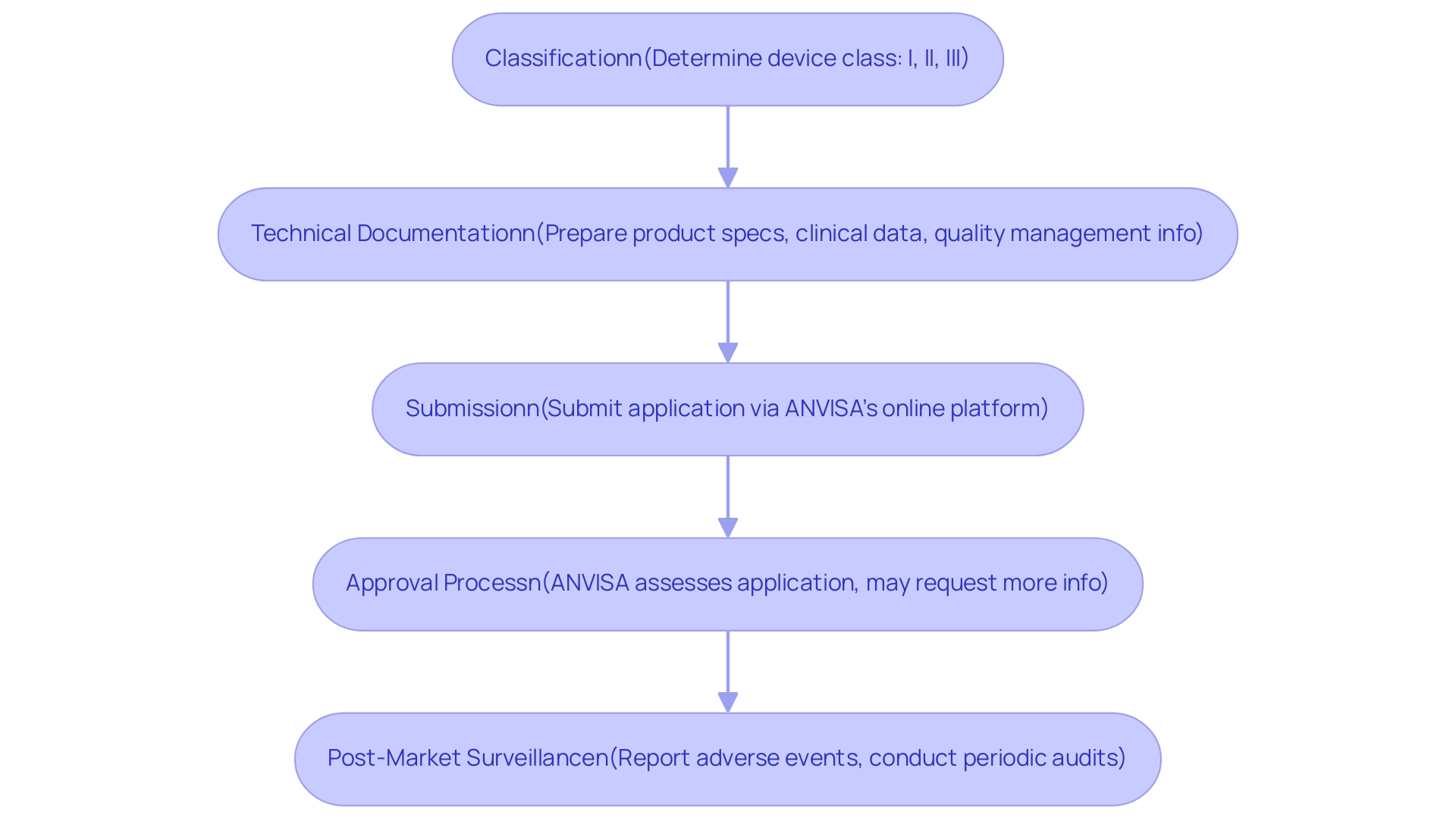
In Brazil, the National Health Surveillance Agency (ANVISA) serves as A Guide to Latin American Regulatory Authorities for Medical Devices, being the main authority responsible for the regulation of healthcare equipment. The registration process is intricate and involves several essential steps:
-
Classification: The first step is to determine the classification of your medical apparatus, which can be categorized as Class I, II, or III, based on a comprehensive risk assessment.
Class I and II devices receive a registration number (Notificacao), while Class III and IV devices undergo a complete approval and testing procedure (Registro). Each class carries distinct requirements that must be met for registration.
-
Technical Documentation: It is crucial to prepare thorough technical documentation, which should include detailed product specifications, relevant clinical data, and information regarding the quality management system.
-
Submission: The registration application must be submitted through ANVISA’s online platform, ensuring that all necessary documents are included to avoid delays.
-
Approval Process: ANVISA will carry out an assessment of the application; this may entail additional requests for further information or clarification to ensure adherence to compliance standards.
-
Post-Market Surveillance: Following approval, it is imperative to adhere to post-market surveillance requirements. This includes reporting any adverse events and undertaking periodic audits to ensure ongoing compliance with regulations.
Navigating Brazil's governance framework, detailed in A Guide to Latin American Regulatory Authorities for Medical Devices, necessitates meticulous attention to detail and strict adherence to specific timelines. Staying informed about regulatory updates and changes is essential for successful registration of the equipment.
As Margret Seidenfaden from the Johner Institute emphasizes, 'The Johner Institute team can assist you with the authorization of your healthcare products in Brazil.' Moreover, with the expertise of bioaccess®, which specializes in accelerated clinical study services in Latin America, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), manufacturers can effectively manage their trials through to success.
The case study titled 'Requirements for the Approval of Medical Devices in Brazil' emphasizes that manufacturers must meet specific requirements before submitting approval documents to the Brazilian authority, underscoring the importance of thorough preparation. For those seeking additional insights, a free webinar titled 'INMETRO and its Role in the Brazilian Medical Equipment Registration Process' will provide further guidance on certification processes relevant to the registration of health products.
Engaging with Local Regulatory Experts
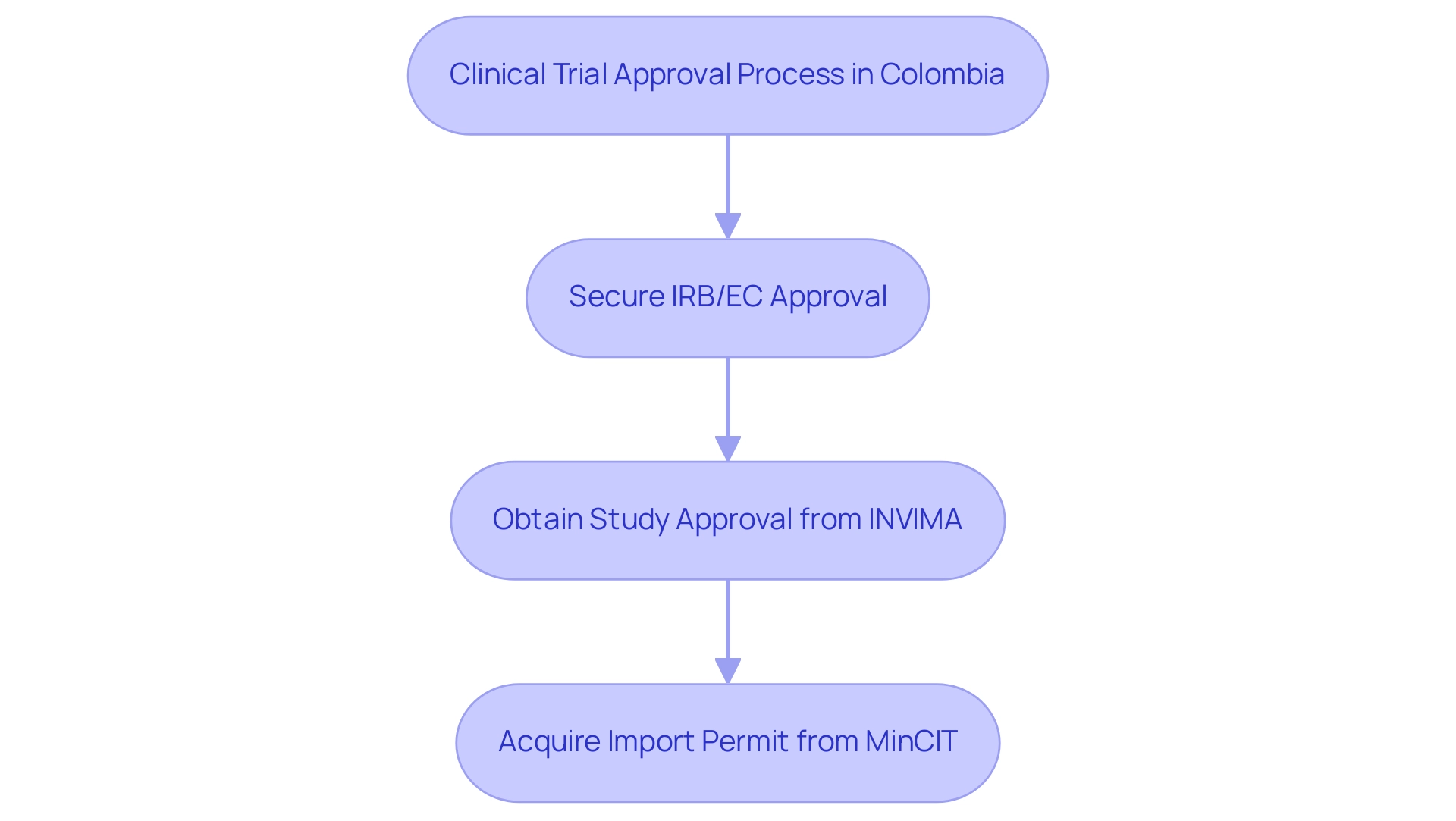
For a comprehensive grasp of the registration environment for healthcare instruments in Latin America, especially in Colombia, it is essential to work with local regulatory specialists, as emphasized in A Guide to Latin American Regulatory Authorities for Medical Devices. The process for obtaining clinical trial approval involves several key steps:
- Securing approval from your site's Institutional Review Board (IRB) or ethics committee (EC), which is responsible for ensuring the ethical conduct of the trial.
- Obtaining study approval from the Colombian National Food and Drug Surveillance Institute, which oversees the safety and efficacy of medical products.
- Acquiring an import permit from the Ministry of Industry and Commerce (MinCIT) to ship investigational devices.
Regulatory experts like Ana Criado, Director of Regulatory Affairs at Mahu Pharma, and Katherine Ruiz, an authority in Regulatory Affairs for Medical Devices, can provide invaluable guidance as outlined in A Guide to Latin American Regulatory Authorities for Medical Devices.
Their expertise in navigating the complexities of Colombian regulations, including the oversight functions of INVIMA, which is classified as a Level 4 health authority by PAHO/WHO, is essential for A Guide to Latin American Regulatory Authorities for Medical Devices. Such professionals can help you understand best practices, documentation requirements, and common pitfalls encountered during the registration process. To identify trustworthy consultants, consider participating in networking opportunities at industry conferences, joining relevant professional associations, or utilizing online platforms that facilitate connections with compliance specialists.
By forming a solid partnership with local specialists, as detailed in A Guide to Latin American Regulatory Authorities for Medical Devices, you can not only simplify your registration process but also ensure compliance with the constantly changing legal landscape. This method improves the chances of successful market entry and aligns with the World Health Organization's focus on local production and technology transfer, ultimately increasing access to essential health products in low- and middle-income countries. Furthermore, referencing the FDA Premarket Approval Database Analysis highlights how oversight processes can impact market entry, underscoring the importance of engaging local experts in navigating these complexities.
Preparing for Regulatory Audits and Inspections
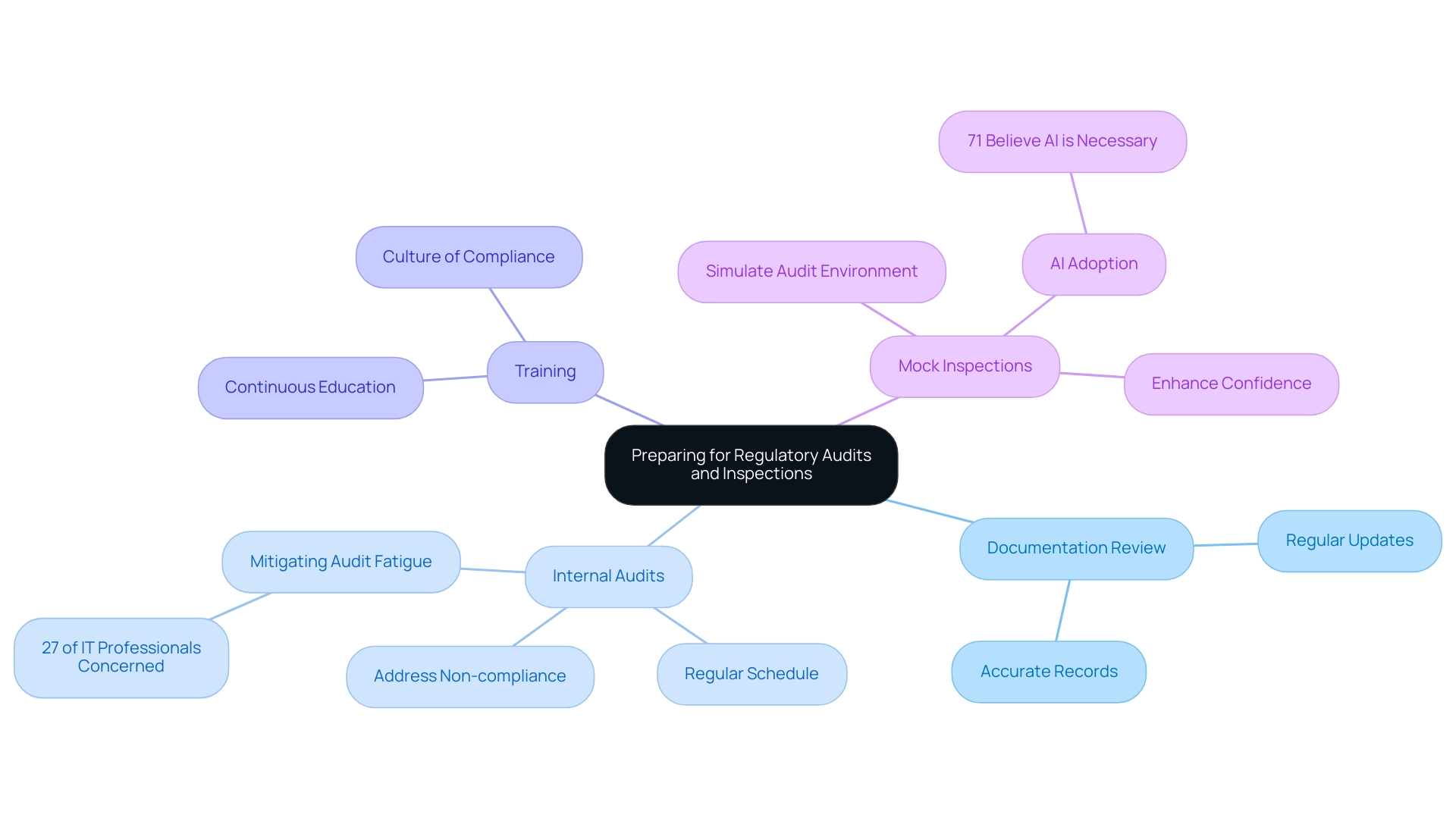
To adequately prepare for compliance audits and inspections, particularly concerning Colombian regulations managed by the authority, companies in the healthcare equipment sector should adopt a multi-faceted approach that incorporates the following strategies:
- Documentation Review: It is crucial to regularly review and update compliance-related documentation, such as quality management systems, technical files, and clinical evaluations. This ensures that all records are accurate and reflect current practices, thereby reducing audit discrepancies.
- Internal Audits: Conducting regular internal audits is essential for identifying areas of non-compliance before they become significant issues. By proactively addressing these areas, companies can better align their processes with the guidelines established by the authority responsible for monitoring and controlling medical devices, as detailed in A Guide to Latin American Regulatory Authorities for Medical Devices, to ensure their safety and efficacy. Recent insights indicate that 27% of security and IT professionals find mitigating internal audit fatigue a top compliance challenge, highlighting the importance of maintaining a structured audit schedule. Furthermore, 67% of global executives believe that ESG regulation is too complex, which underscores the necessity for robust internal processes to navigate these challenges effectively.
- Training: Continuous education is essential for keeping your team updated on compliance standards, including those required by authorities, and best practices. This fosters a culture of compliance and ensures that all personnel are prepared for audit processes.
- Mock Inspections: Implementing mock inspections can be an effective way to simulate the audit environment. This practice allows teams to rehearse their responses to potential audit questions and ensures that all necessary documentation is easily accessible. Companies that engage in mock inspections often report enhanced confidence and performance during actual audits. Additionally, the adoption of new technologies, including AI, is recognized as essential for competitiveness, with 71% of industry respondents affirming its necessity. Businesses utilizing AI can optimize their compliance procedures, further boosting audit preparedness.
By actively adopting these strategies, organizations can greatly enhance their preparedness for audits, reducing the risk of penalties while ensuring ongoing market access under INVIMA's supervision. Furthermore, with 40% of supply chain professionals identifying regulatory and compliance pressures as primary concerns, it is evident that a strong compliance framework is vital for long-term success in the industry. As highlighted by global executives, there is a clear need for more guidance from regulators like INVIMA, classified as a Level 4 health authority by PAHO/WHO, as discussed in A Guide to Latin American Regulatory Authorities for Medical Devices, to navigate these complexities effectively.
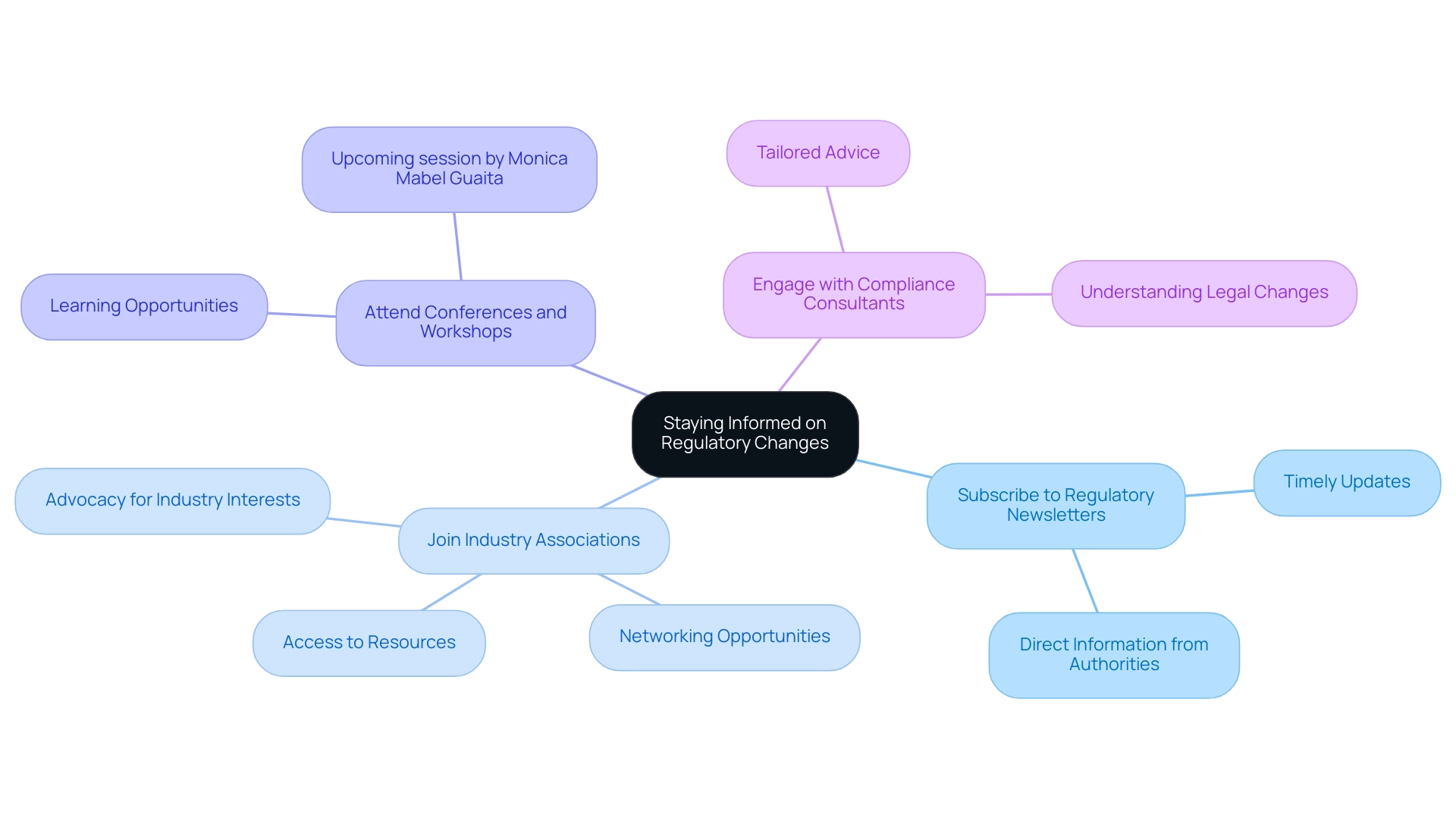
Staying Informed on Regulatory Changes
To effectively navigate the evolving landscape of medical device regulations in Latin America, companies should consult A Guide to Latin American Regulatory Authorities for Medical Devices and adopt proactive strategies to stay informed. With a population of approximately 657 million as of 2024, the implications of compliance are significant, especially given the multifaceted challenges faced by Medtech companies, such as compliance hurdles, language barriers, fragmentation of resources, limited financial resources, and prolonged subject recruitment timelines. Efficient communication with local authorities like INVIMA, Colombia's National Food and Drug Surveillance Institute, which is recognized as a Level 4 health authority by PAHO/WHO, is crucial for following A Guide to Latin American Regulatory Authorities for Medical Devices.
Here are key approaches to consider:
- Subscribe to Regulatory Newsletters: Regulatory authorities frequently distribute newsletters detailing updates on changes in regulations and guidelines. Subscribing to these publications ensures that companies receive timely information directly from the source.
- Join Industry Associations: Membership in industry associations provides access to a wealth of resources, networking opportunities, and insights into emerging compliance trends. These organizations often advocate for industry interests and keep members informed about significant changes, facilitating collaboration, such as that between Greenlight Guru and bioaccess™ to accelerate Medtech innovations in the region.
- Attend Conferences and Workshops: Participation in industry conferences and workshops focused on compliance offers invaluable learning opportunities. Events like the upcoming session featuring Monica Mabel Guaita, CEO of MMGC SRL, will delve into 'Main Regulatory Issues for LATAM,' making them ideal for gaining insights from experts in the field.
- Engage with Compliance Consultants: Building and maintaining relationships with compliance consultants can provide companies with tailored advice and timely updates on navigating legal changes. Their expertise is essential for understanding the complexities of compliance in various jurisdictions, particularly in the context of medical device oversight by INVIMA.
Davis Rolfe, a senior financial analyst, highlights the significance of remaining aware of compliance changes, stating that "proactive engagement with updates is essential for maintaining adherence and competitive advantage." Applying these strategies, as detailed in A Guide to Latin American Regulatory Authorities for Medical Devices, will assist companies in staying compliant with the dynamic legal environment and maintaining a competitive advantage.
For instance, the concept of centralized regional management of Regulatory Affairs allows manufacturers to efficiently handle Regulatory issues across multiple Latin American countries, optimizing documentation use and improving market access while avoiding unnecessary costs and delays. By staying informed and agile, organizations can better adapt to the latest medical device regulations and effectively meet market demands. The urgency for a solution-driven approach to bridge the gap between innovation and execution cannot be overstated, as addressing these challenges is vital for the success of Medtech companies in the regions.
Conclusion
Navigating the regulatory landscape for medical devices in Latin America is undeniably complex, with each country presenting unique challenges and requirements. Key regulatory authorities, including:
- ANVISA in Brazil
- COFEPRIS in Mexico
- INVIMA in Colombia
play critical roles in ensuring the safety, efficacy, and quality of medical devices. Understanding the intricacies of registration processes, classification requirements, and post-market surveillance obligations is essential for manufacturers aiming to successfully enter these diverse markets.
Collaboration with local regulatory experts is paramount. Their insights can significantly streamline the registration process, helping manufacturers avoid common pitfalls and ensuring compliance with evolving regulations. Staying informed about regulatory changes through newsletters, industry associations, and conferences is equally important. These proactive strategies not only enhance compliance but also foster a competitive edge in a rapidly changing environment.
Ultimately, the success of medical device manufacturers in Latin America hinges on their ability to navigate this multifaceted regulatory landscape effectively. By leveraging local expertise, staying updated on regulatory developments, and adopting robust compliance practices, companies can enhance their market access while contributing to the availability of safe and effective medical technologies in the region.
Frequently Asked Questions
What are the main regulatory authorities for medical devices in Latin America?
The main regulatory authorities for medical devices in Latin America are ANVISA in Brazil, COFEPRIS in Mexico, and the Colombia National Food and Drug Surveillance Institute in Colombia.
What is the role of the Colombia National Food and Drug Surveillance Institute?
Founded in 1992, it is responsible for inspecting and supervising the marketing and manufacturing of health products, evaluating compliance with health standards, and overseeing regulations related to health technologies.
What are the key requirements for health product registration in Latin America?
Key requirements include pre-market approval, post-market surveillance, and adherence to quality management systems.
How does the registration process work in Brazil under ANVISA?
The registration process involves several steps: classification of the medical device, preparation of technical documentation, submission of the application through ANVISA’s online platform, an approval process that may require additional information, and adherence to post-market surveillance requirements.
What classifications exist for medical devices in Brazil?
Medical devices in Brazil are classified as Class I, II, III, or IV, with Class I and II receiving a registration number (Notificacao) and Class III and IV undergoing a complete approval and testing procedure (Registro).
What is required in the technical documentation for medical device registration?
The technical documentation must include detailed product specifications, relevant clinical data, and information regarding the quality management system.
Why is post-market surveillance important?
Post-market surveillance is crucial for reporting any adverse events and conducting periodic audits to ensure ongoing compliance with regulations after the product has been approved.
What should manufacturers do to ensure compliance with regulatory requirements in Latin America?
Manufacturers should conduct comprehensive research on the unique requirements of each jurisdiction and continuously monitor and assess compliance requirements.
How can manufacturers facilitate smoother market entry in Latin America?
By utilizing the knowledge of local compliance professionals and staying informed about regulatory updates and changes, manufacturers can navigate the complex compliance environment more effectively.

