Introduction
Navigating the complex landscape of medical device approval is imperative for manufacturers seeking to bring innovative products to market. The FDA's rigorous approval process, encompassing various pathways such as the 510(k) and Premarket Approval (PMA), demands a thorough understanding of regulatory requirements and strategic planning.
As the industry evolves, recent trends indicate an increase in approval timelines, necessitating proactive engagement with regulatory bodies to mitigate delays.
This article delves into the critical stages of the FDA approval process, highlighting the importance of:
- Early communication
- Meticulous submission practices
- Ongoing compliance post-approval
By exploring these essential elements, stakeholders can better position themselves for success in the competitive medical technology arena.
Overview of the FDA Medical Device Approval Process
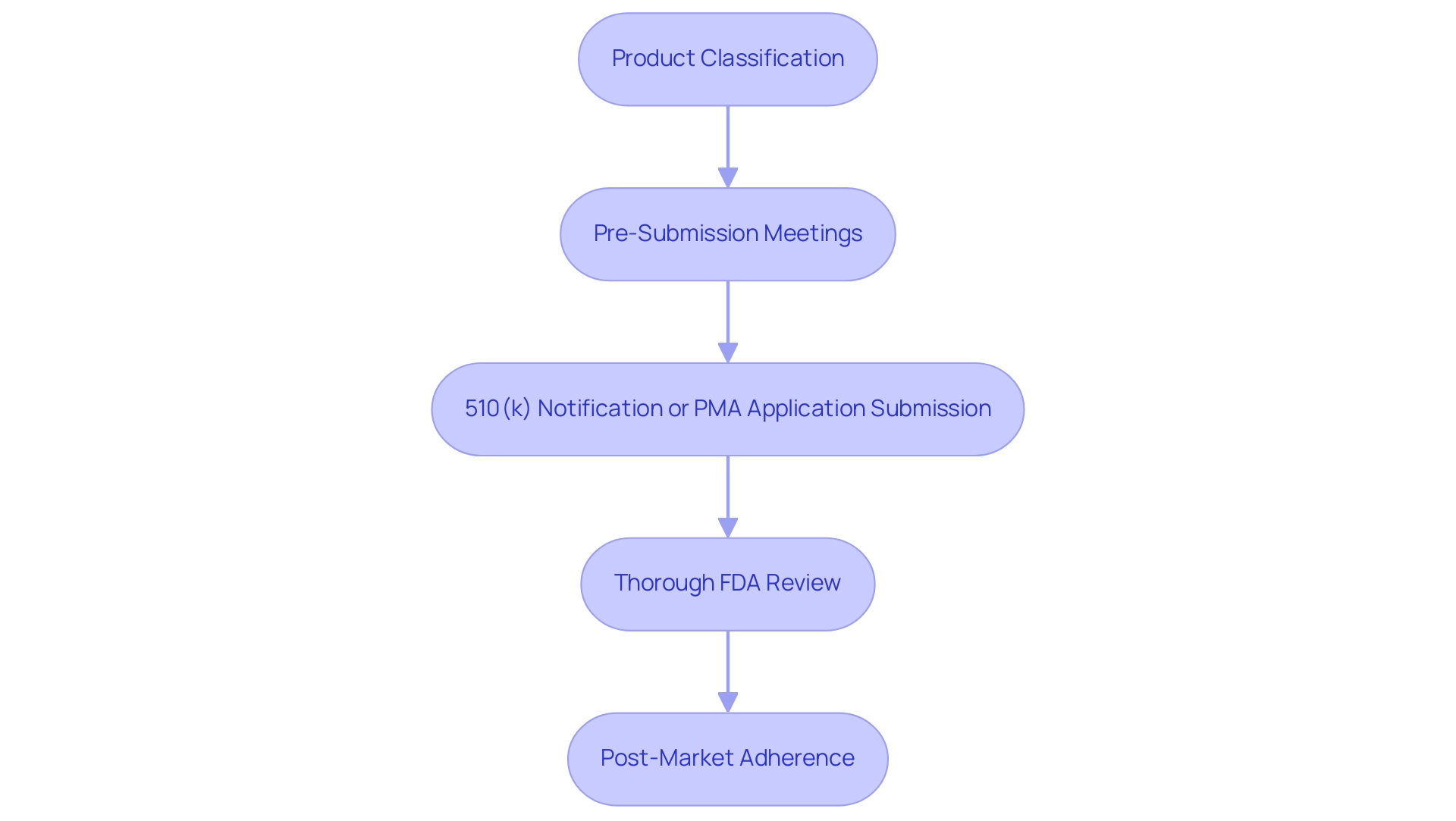
The procedure for FDA medical equipment certification follows a timeline that includes several essential phases, such as pre-market submission, thorough evaluation, and post-market adherence in the FDA medical device approval process. Initially, manufacturers must ascertain their product's classification, as this determination significantly influences the selection of the appropriate approval pathway. The procedure often begins with pre-submission meetings with FDA officials, providing clarity and guidance on requirements.
Following this, manufacturers submit either a 510(k) notification or a Premarket Approval (PMA) application as part of the FDA medical device approval process timeline, contingent upon the device's assessed risk level. Once submitted, the FDA medical device approval process timeline initiates a thorough review, which often includes the examination of clinical data to assess safety and efficacy before granting authorization. Recent statistics indicate that the average timeline for De Novo authorizations has expanded slightly, reflecting the increasing complexities within the regulatory framework—rising from 415 days in 2023 to 420 days in 2024.
This trend emphasizes the significance of comprehending the subtleties of the FDA endorsement system, as pointed out by analyst Iseult McMahon, who remarked,
We believe that this is a directional positive for the wider medical technology sector as it offers both enhanced certainty to firms (and investors) and decreases cash expenditure during the interim period while a company is anticipating a decision.
Moreover, the minor extensions in wait times for De Novo and Panel Track authorizations signify persistent challenges in the regulatory framework for these categories. For stakeholders in the clinical research field, mastering this overview is essential to successfully navigate the FDA medical device approval process timeline and ensure compliance at every stage.
Additionally, with bioaccess®'s expertise in managing a variety of clinical studies including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), the process can be streamlined. Bioaccess® also provides essential services such as compliance reviews, trial setup, project management, and monitoring to ensure adherence to Regulatory standards. Katherine Ruiz, a recognized authority in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, provides invaluable insights into navigating INVIMA's regulatory functions, ensuring compliance with the highest standards of oversight.
INVIMA plays an essential role in the Colombian regulatory environment, supervising the authorization and monitoring of medical equipment. The recent FDA clearance of the UNIPURE SF6 Ophthalmic Gas UNIFEYE Gas Delivery System and UNIPURE SF6 Ophthalmic Gas UNIPEXY Gas Delivery System on 08/26/2024 further exemplifies the changing landscape of medical equipment authorizations.
Key Pathways to FDA Approval: 510(k) vs. PMA
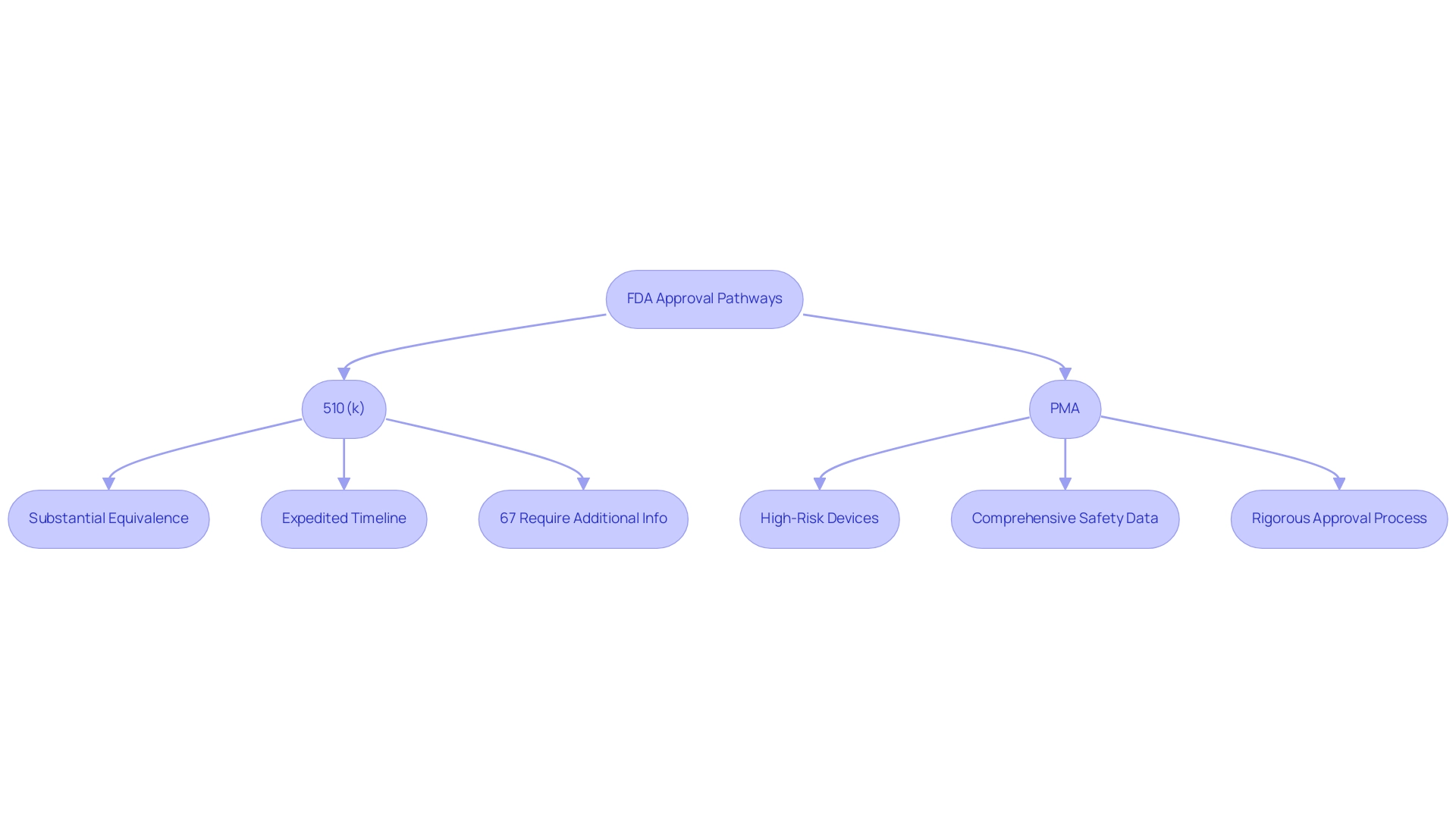
The 510(k) pathway facilitates the approval of products that demonstrate substantial equivalence to existing marketed products, typically resulting in a more expedited approval timeline. In stark contrast, the Premarket Approval (PMA) pathway is reserved for high-risk medical instruments classified as Class III. These instruments, which may support or sustain human life or pose significant risks of illness or injury, necessitate a comprehensive submission of safety and effectiveness data, often derived from extensive clinical trials.
The PMA procedure is notably rigorous, requiring manufacturers to provide robust evidence of a device's safety and efficacy, which is critical given the potential risks associated with these high-risk devices. Currently, data indicates that 67% of 510(k) submissions require an additional information request, highlighting the complexities embedded in this procedure. Manufacturers must diligently prepare detailed documentation tailored to the stringent requirements of their selected pathway.
Understanding the distinct processes of the FDA medical device approval process timeline for 510(k) and PMA influences not only planning timelines but also resource allocation strategies. Our comprehensive clinical trial management services at bioaccess® include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures that you are well-prepared for success. We specialize in:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
As industry expert Katrina Rogers states, 'Readily available data from the FDA tells us we can expect a reasonably high (though not 100%) success rate for our PMA and 510(k) medical product submissions.' This insight highlights the significance of understanding these regulatory pathways for clinical research directors maneuvering through the complex terrain of medical product authorizations in Latin America. Bioaccess® brings a customized approach to managing these clinical trials, ensuring compliance with both FDA and Anvisa regulations while leveraging our extensive expertise.
The Importance of Early Engagement with the FDA
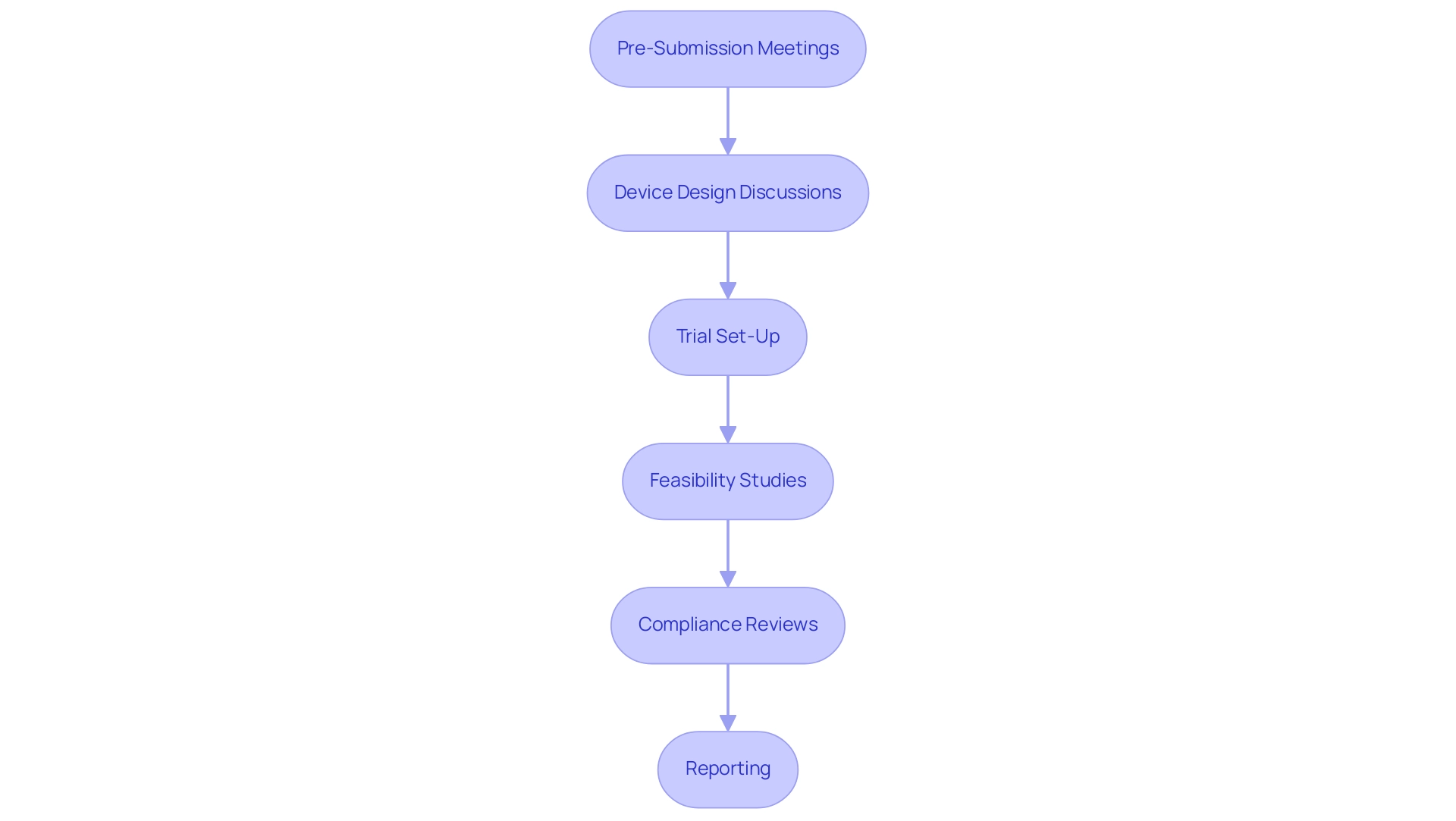
Engaging early with the FDA through pre-submission meetings is a strategic move for manufacturers aiming to simplify the authorization pathway for medical equipment. These meetings promote conversations about the device's design, intended use, and the specific information needed for endorsement, closely aligning with the detailed procedures outlined in our clinical trial management services, such as:
- Feasibility studies
- Trial set-up
- Compliance reviews
- Reporting
By addressing potential concerns at this stage, manufacturers can significantly mitigate the risk of delays during the evaluation phase.
Furthermore, obtaining timely feedback from the FDA regarding study designs and data requirements fosters a more efficient submission pathway. This proactive approach not only enhances clarity regarding regulatory expectations but also plays a critical role in shortening the FDA medical device approval process timeline. Research indicates that structured communication within the review teams and with sponsors could further enhance this approach; notably, questions with ≥ 80% agreement among stakeholders can lead to a more cohesive review effort.
As Peter Neumann states, 'Structured communications within the review team and with sponsors could enhance the review system.' Additionally, our expertise in navigating FDA and Anvisa regulations, as demonstrated in recent case studies, illustrates the real-world implications of regulatory changes and emphasizes the importance of proactive engagement with the FDA to effectively navigate potential challenges. Notably, recent statistics suggest that companies engaging in pre-submission meetings often experience higher success rates in securing approvals, underscoring the value of such strategic engagements in the competitive landscape of medical product development.
To learn more about how we can assist you, BOOK A MEETING.
Navigating the Submission and Review Process
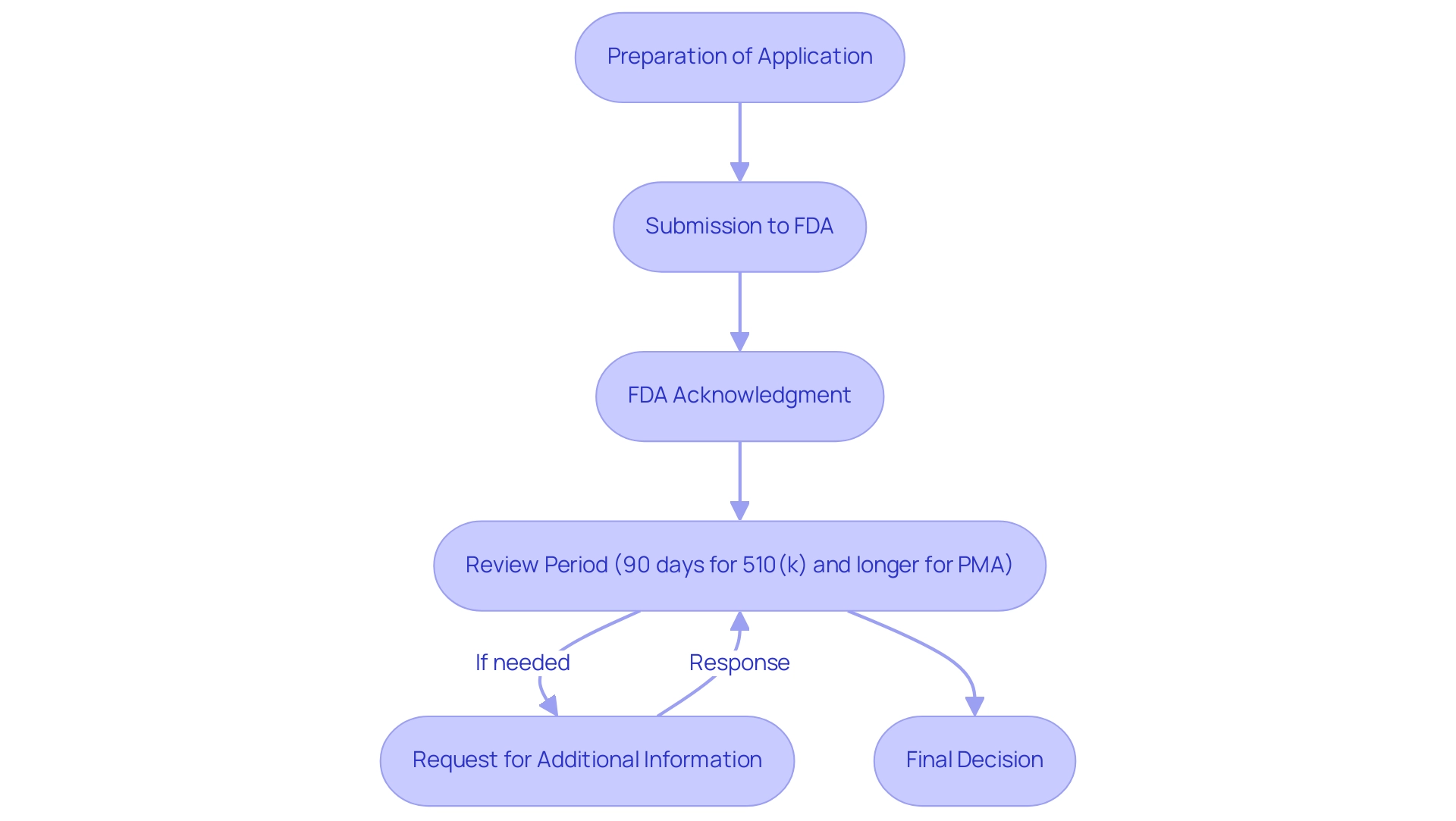
The submission procedure for medical products to the FDA begins with the meticulous preparation of a comprehensive application, encompassing all necessary documentation and clinical data. Upon submission, the FDA acknowledges receipt and begins the review. For 510(k) submissions, the review typically spans around 90 days, while the FDA medical device approval process timeline for Pre-Market Approval (PMA) applications can extend significantly longer, often reaching several months or more, particularly in complex cases.
In 2024, recent data shows that around 90 percent of leaders in the medical equipment sector are emphasizing US regulatory clearance, reflecting a strategic focus on the FDA's review process. A notable development includes the FDA's endorsement of a novel device for the non-invasive treatment of spinal cord injuries, showcasing the agency's active role in advancing medical technology. It is common for the FDA to request additional information during the review period, underscoring the importance of manufacturers responding swiftly to mitigate delays.
Furthermore, the FDA is anticipated to enhance transparency by publishing quarterly lists of authorizations in the Federal Register and making PMA file data publicly accessible. Understanding the nuances of the FDA medical device approval process timeline and the expectations throughout the review process is vital for effective project management. This is particularly relevant in Latin America, where bioaccess® offers comprehensive clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial set-up
- Start-up approvals
- Import permits
- Project management
- Reporting on study status and adverse events
—ensuring a streamlined approach tailored to local regulations.
Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, emphasizes the critical nature of compliance during this phase. Additionally, a case study on optimizing pricing and reimbursement strategies underscores how informed decision-making based on market intelligence can enhance financial performance and market positioning, highlighting the significance of strategic planning during the submission process and its impact on overall outcomes.
Post-Approval Compliance and Monitoring Requirements
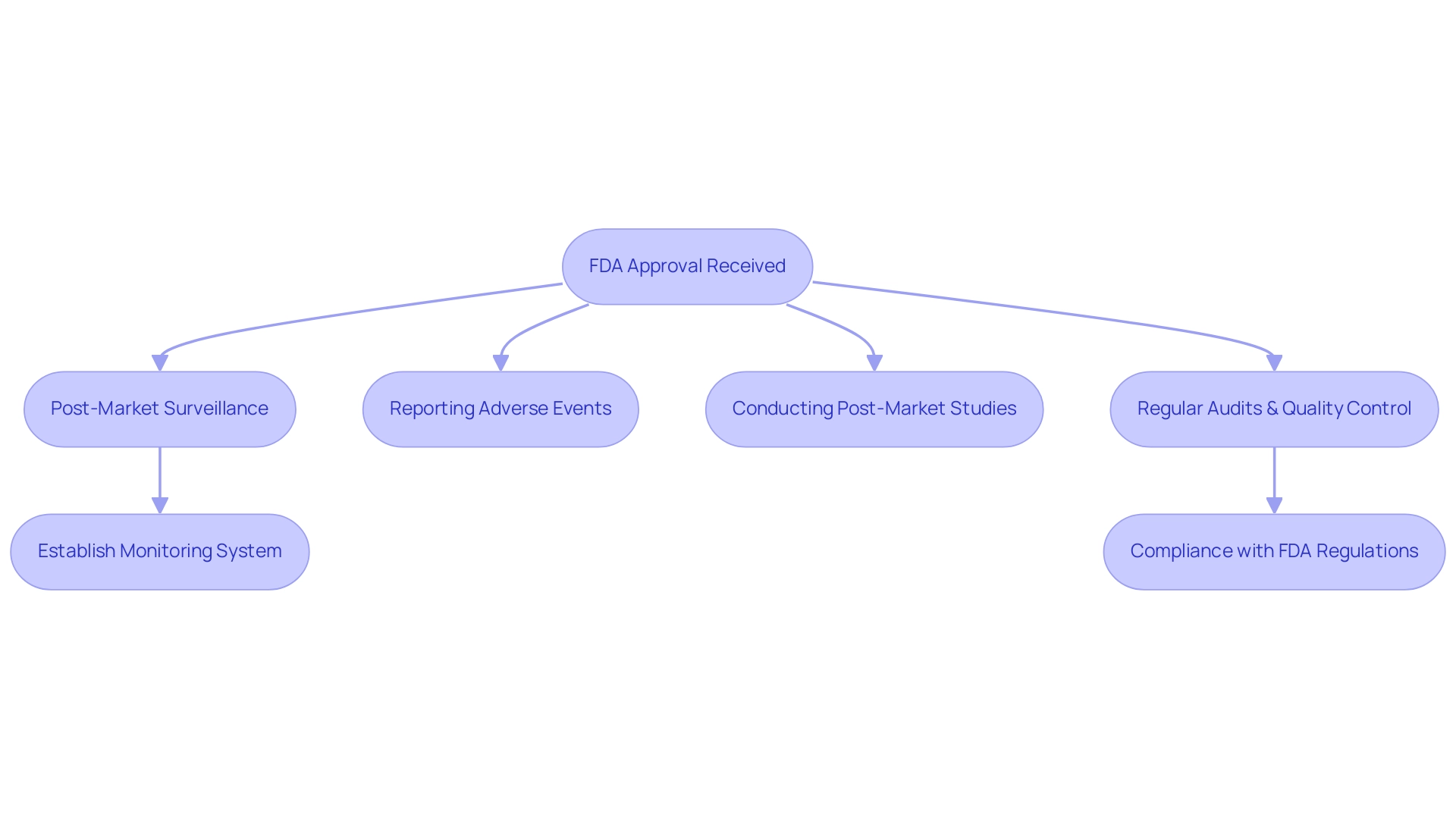
Upon receiving FDA approval, manufacturers are tasked with stringent post-market surveillance obligations. These requirements encompass not only the reporting of adverse events but also the execution of post-market studies and adherence to all relevant FDA regulations. Regular audits and comprehensive quality control measures are essential for maintaining ongoing compliance.
Establishing a robust monitoring system to track device performance and safety is paramount; neglecting these responsibilities can result in significant penalties and jeopardize patient safety. Notably, errors preventing the loading of collections can complicate the monitoring procedure, highlighting the challenges manufacturers face in maintaining compliance. As detailed in the case study 'Pathways to Medical Device Authorization,' understanding the FDA medical device approval process timeline and various clearance procedures, such as the Pre-market Authorization (PMA) and the Pre-market Notification (PMN) mechanisms, is essential for managing post-market responsibilities.
Furthermore, the FDA's response time of 45 days for applications for designation as a Humanitarian Use Device (HUD) underscores the importance of timely compliance efforts. With comprehensive clinical trial management services provided by bioaccess®, including feasibility studies, site selection, compliance reviews, project management, and reporting, as well as expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), clinical researchers can navigate these challenges effectively. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, provides invaluable insights into the unique regulatory issues related to the device approval process.
With over 20 years of experience in Medtech, bioaccess® has the expertise and customized approach needed to navigate your company towards an acquisition. Therefore, understanding and fulfilling these obligations is crucial for ensuring a sustained and successful presence in the market.
Conclusion
Navigating the FDA medical device approval process is a multifaceted endeavor that requires a thorough understanding of various regulatory pathways, including the 510(k) and Premarket Approval (PMA) systems. Early engagement with FDA officials through pre-submission meetings can significantly streamline the approval process, allowing manufacturers to address potential concerns and align their submissions with regulatory expectations. By meticulously preparing applications that meet the stringent requirements of these pathways, stakeholders can optimize their chances of timely approval.
As the approval timelines evolve, particularly for De Novo and PMA submissions, it becomes increasingly important for manufacturers to remain proactive and responsive throughout the review process. The data indicates a notable shift toward longer review periods, highlighting the necessity for strategic planning and resource allocation. Emphasizing ongoing compliance post-approval is equally critical, as manufacturers must adhere to rigorous surveillance and reporting obligations to ensure patient safety and maintain their market position.
In conclusion, success in the competitive medical technology landscape hinges on a comprehensive understanding of the FDA approval process, early and effective communication with regulatory bodies, and a commitment to compliance at every stage. By embracing these principles, manufacturers can enhance their likelihood of navigating the complexities of device approval and ultimately contribute to advancing medical innovation.
Frequently Asked Questions
What are the main phases of the FDA medical device approval process?
The main phases include pre-market submission, thorough evaluation, and post-market adherence.
How do manufacturers determine the appropriate approval pathway for their medical device?
Manufacturers must ascertain their product's classification, as this significantly influences the selection of the appropriate approval pathway.
What is the initial step manufacturers take before submitting their medical device for FDA approval?
Manufacturers typically begin with pre-submission meetings with FDA officials to gain clarity and guidance on requirements.
What types of submissions can manufacturers make as part of the FDA approval process?
Manufacturers can submit either a 510(k) notification or a Premarket Approval (PMA) application, depending on the assessed risk level of the device.
What does the FDA review process involve after a submission is made?
The review process often includes a thorough examination of clinical data to assess the safety and efficacy of the device before granting authorization.
How has the average timeline for De Novo authorizations changed recently?
The average timeline for De Novo authorizations has increased from 415 days in 2023 to 420 days in 2024, reflecting the increasing complexities within the regulatory framework.
What is the significance of understanding the FDA endorsement system?
Understanding the FDA endorsement system offers enhanced certainty to firms and investors and decreases cash expenditure during the interim period while awaiting a decision.
What challenges do De Novo and Panel Track authorizations currently face?
There are persistent challenges in the regulatory framework for these categories, leading to minor extensions in wait times for authorizations.
How does bioaccess® assist in the FDA medical device approval process?
Bioaccess® streamlines the process by managing various clinical studies, providing services like compliance reviews, trial setup, project management, and monitoring to ensure adherence to regulatory standards.
What role does INVIMA play in the Colombian regulatory environment?
INVIMA supervises the authorization and monitoring of medical equipment in Colombia.
What are the differences between the 510(k) and PMA pathways?
The 510(k) pathway facilitates approval for products showing substantial equivalence to existing products, typically resulting in a faster timeline, while the PMA pathway is for high-risk devices requiring comprehensive safety and effectiveness data.
What percentage of 510(k) submissions require additional information requests?
Currently, 67% of 510(k) submissions require an additional information request.
What services does bioaccess® offer for clinical trial management?
Bioaccess® offers feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting.
What types of studies does bioaccess® specialize in?
Bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).




