Introduction
In the intricate world of medical device regulation, understanding the concept of predicate devices is paramount for manufacturers seeking to navigate the approval landscape effectively. Predicate devices serve as benchmarks, enabling new devices to demonstrate their safety and efficacy through the FDA's 510(k) approval process.
As the medical device market continues to evolve, particularly with advancements in technology and shifts in consumer demand, the relevance of predicate devices becomes increasingly significant. This article delves into the nuances of predicate devices, exploring their role in regulatory pathways, the challenges faced in their identification, and the critical considerations manufacturers must account for when selecting appropriate predicate devices.
With insights from industry experts, it aims to provide a comprehensive overview that equips stakeholders with the knowledge needed to ensure compliance and facilitate successful market entry.
Defining Predicate Devices: An Overview
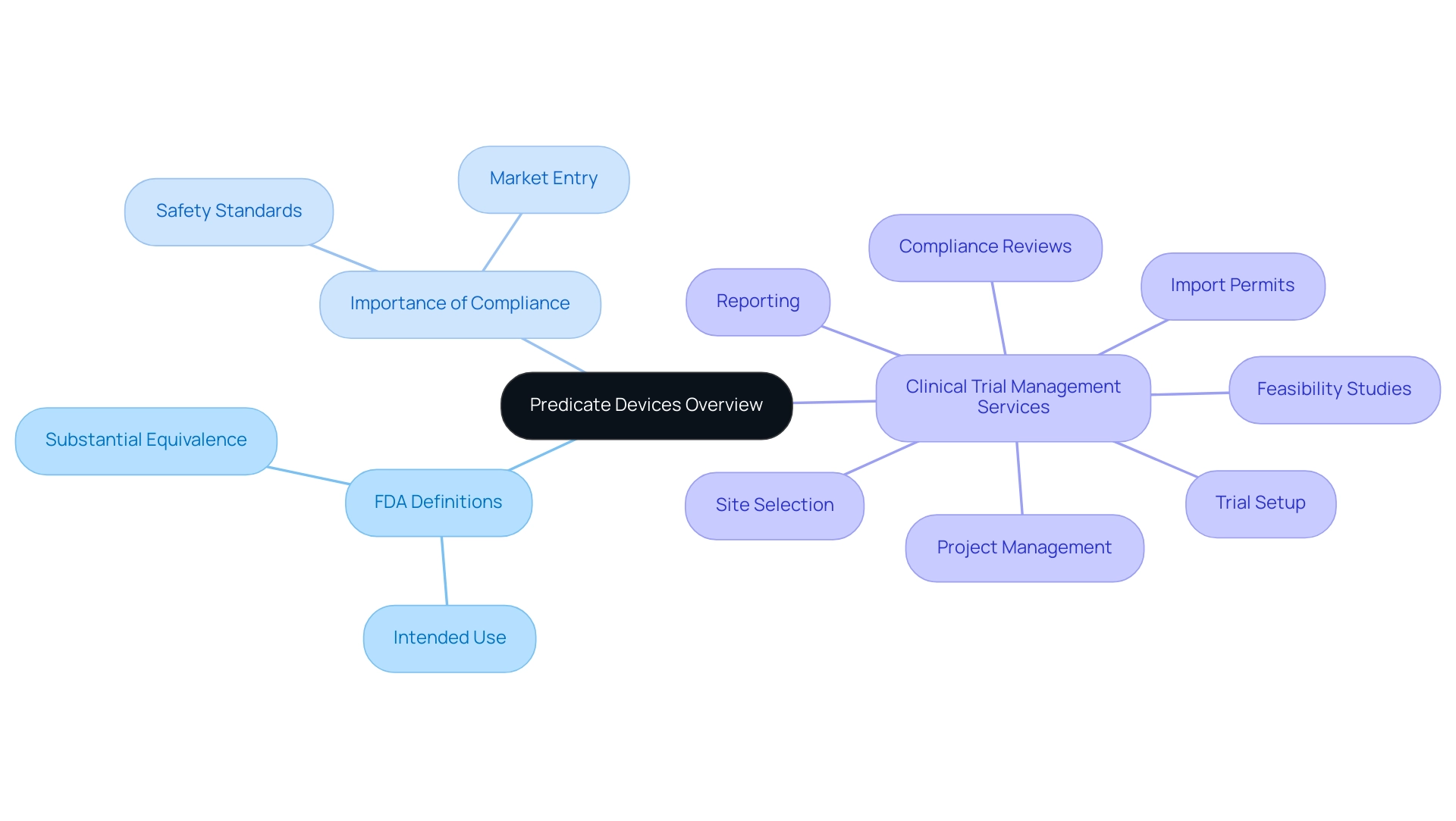
A classification item is a medical instrument that has been legally sold, acting as an important standard for showing the safety and efficacy of a new instrument. The U.S. Food and Drug Administration (FDA) defines a new item as substantially equivalent to another item when considering what is a predicate device, provided it shares the same intended use and technological characteristics. This comprehension is crucial for producers as they traverse the intricate approval environment to obtain authorizations for new medical instruments.
The FDA's stringent assessment system lays the groundwork for innovation while guaranteeing that new products adhere to established safety and effectiveness standards. As the medical equipment market progresses, especially with an expected 1.6% value-added shift in 2024, it is essential to remain knowledgeable about what is a predicate device and its compliance implications for successful product development and market entry. Comprehensive clinical trial management services, including:
- feasibility studies
- site selection
- compliance reviews
- trial setup
- import permits
- project management
- reporting
are critical in supporting this process.
Katherine Ruiz, a specialist in compliance matters for medical instruments and in vitro diagnostics in Colombia, embodies the knowledge necessary to maneuver through these complexities. Furthermore, the trend of at-home diagnostics, which gained traction during the pandemic, underscores the importance of such tools in adjusting to consumer needs and enhancing healthcare access.
The Role of Predicate Devices in the 510(k) Approval Process
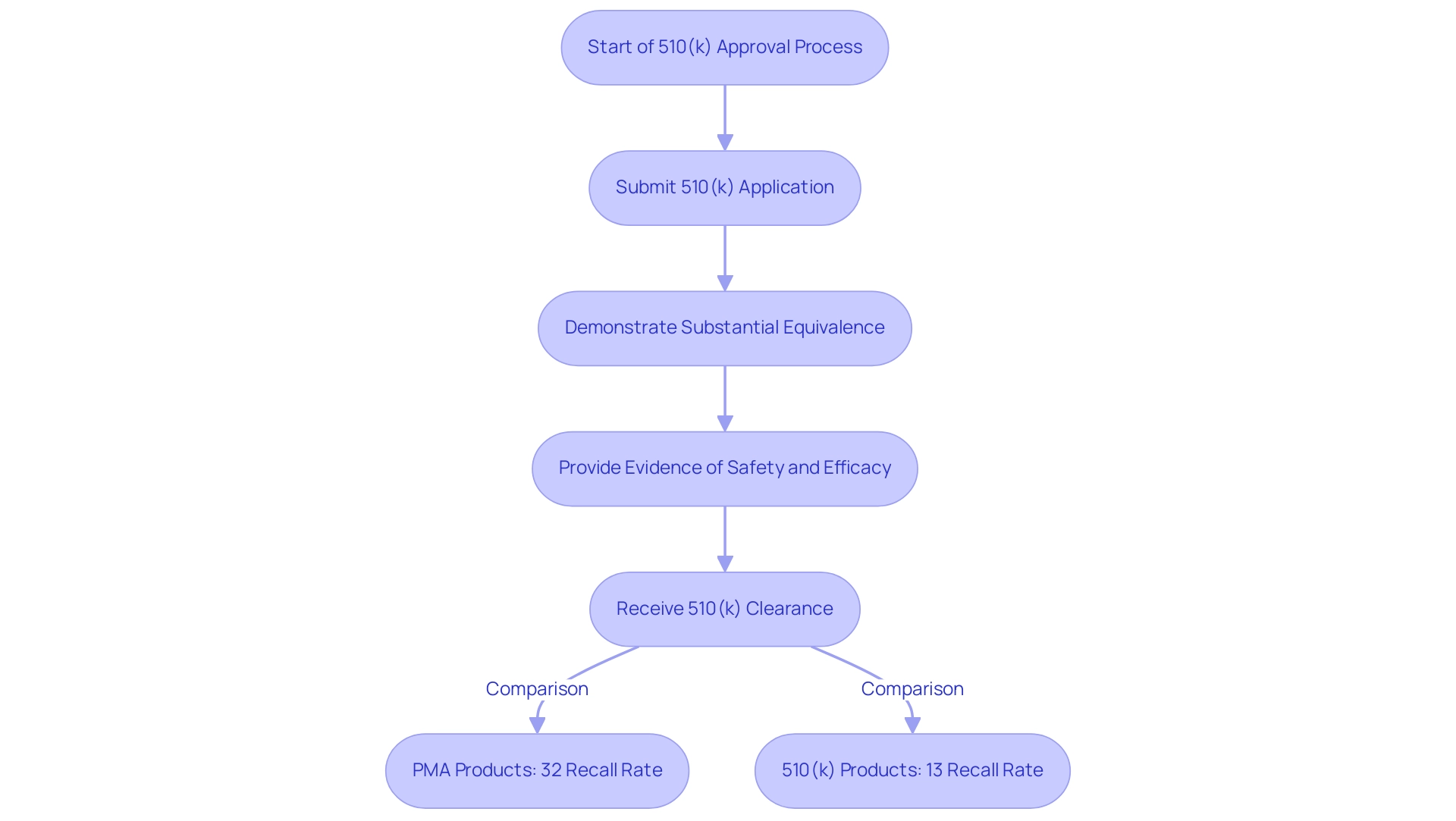
The 510(k) approval procedure acts as an essential regulatory route for manufacturers looking to prove that their new medical products are significantly comparable to existing reference items already available. This pathway is essential as it offers a more expedited route to market than the more rigorous Premarket Approval (PMA) procedure, allowing manufacturers to bring innovations to healthcare settings more swiftly. To achieve 510(k) clearance, it is imperative for manufacturers to present robust evidence demonstrating that their product meets safety and efficacy standards comparable to what is a predicate device.
Significantly, the risk of recall for products with PMA clearance is 32% in contrast to 13% for 510(k) items, emphasizing the comparative safety and effectiveness of the 510(k) method. This structure not only facilitates efficient market entry for new products but also prioritizes patient safety and effectiveness. Recent advancements, including completed guidelines aimed at improving transparency, consistency, and predictability in the 510(k) evaluation system, are responding to the changing requirements of the medical equipment landscape.
Comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial set-up, import permits, and reporting on study status and adverse events—are crucial for ensuring that manufacturers navigate these compliance pathways effectively. Professionals such as Katherine Ruiz, specializing in compliance matters for medical instruments and in vitro diagnostics in Colombia, play a crucial role in this undertaking, especially given INVIMA's supervision as a Level 4 health authority by PAHO/WHO. Moreover, real-world implications, such as the recent ransomware attack affecting Artivion's shipping processes, underscore the challenges manufacturers face in complying with regulatory requirements while ensuring operational integrity.
Selecting the Right Predicate Device: Key Considerations
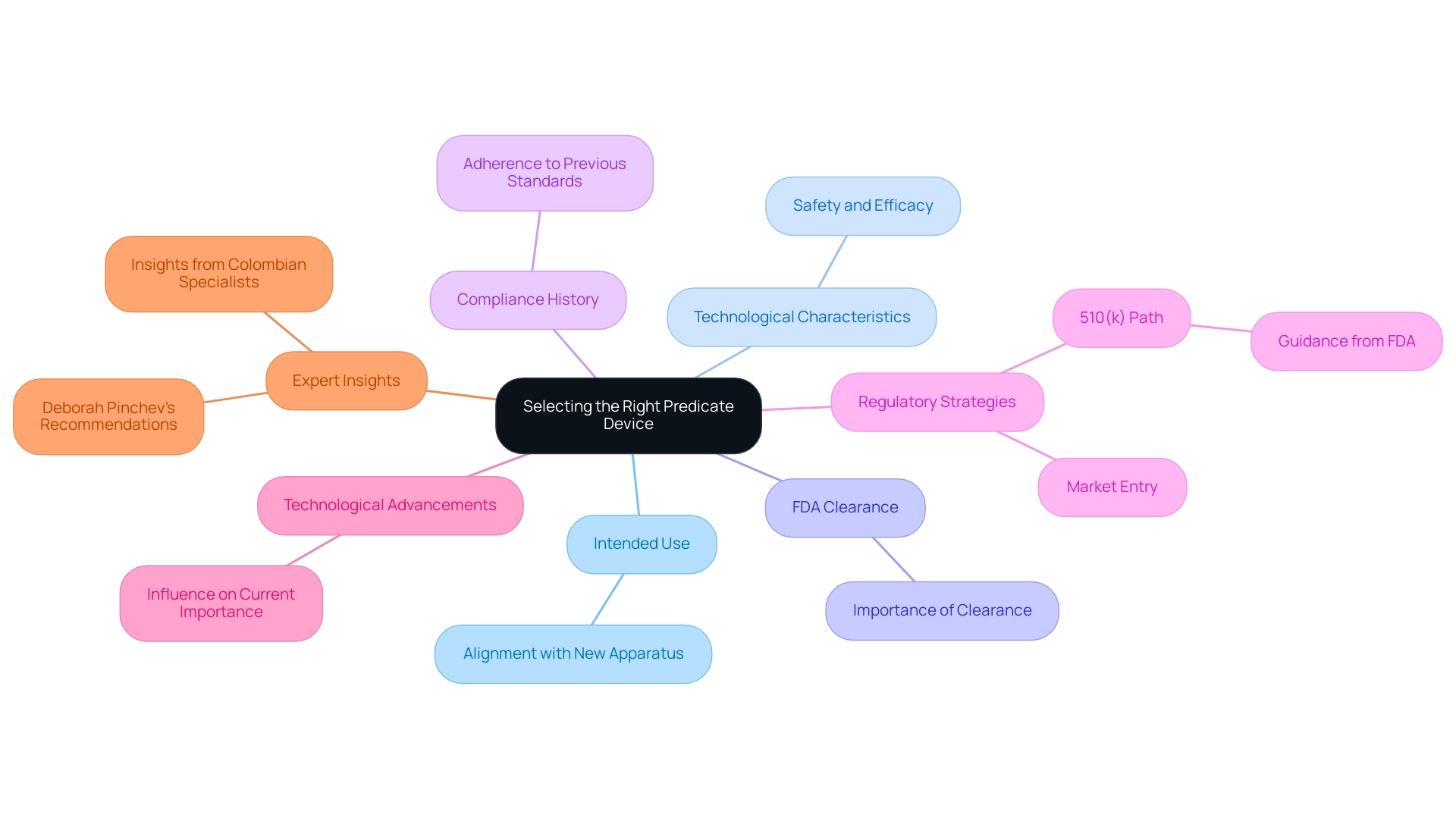
When choosing a reference product, manufacturers must meticulously evaluate several essential factors. Foremost among these are the intended use of the apparatus and its technological characteristics, which must align closely with those of the new apparatus. It is essential to confirm that the reference item has received FDA clearance, ensuring it has been evaluated for safety and efficacy.
Furthermore, grasping the compliance history of the original device is essential, as it demonstrates its adherence to previous standards. As Deborah Pinchev, QA/RA Manager at StarFish Medical, advises, 'If you are still developing your regulatory strategy and are considering the 510(k) path, I highly recommend reading this guidance and following the FDA’s advice when choosing your reference device.' Manufacturers should also consider any advancements in technology or shifts in clinical practices that may influence the current importance of the item.
Such considerations are essential as they can greatly impact the assessment of substantial equivalence, which is crucial for understanding what is a predicate device in the FDA's approval system for new medical products. Additionally, to encourage openness, the FDA recommends that sponsors incorporate a narrative in the 510(k) summary that clarifies the basis for selection. This narrative aids in elucidating what is a predicate device by providing safety and effectiveness information associated with the predicate product, making it publicly accessible.
Lastly, all written comments must include the docket number: FDA-2023-D-3134. In Colombia, specialists like Ana Criado, Director of Compliance and CEO of Mahu Pharma, along with Katherine Ruiz, who focuses on Affairs for Medical Instruments and In Vitro Diagnostics, offer invaluable insights into navigating these compliance challenges, particularly in the context of accelerated medical instrument clinical studies provided by bioaccess®. Bioaccess® offers tailored services that include Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring that manufacturers are well-equipped to meet both local and international regulatory requirements.
Their knowledge in handling the intricacies of classification products is crucial for effective compliance and market entry.
Understanding Substantial Equivalence in Predicate Device Selection
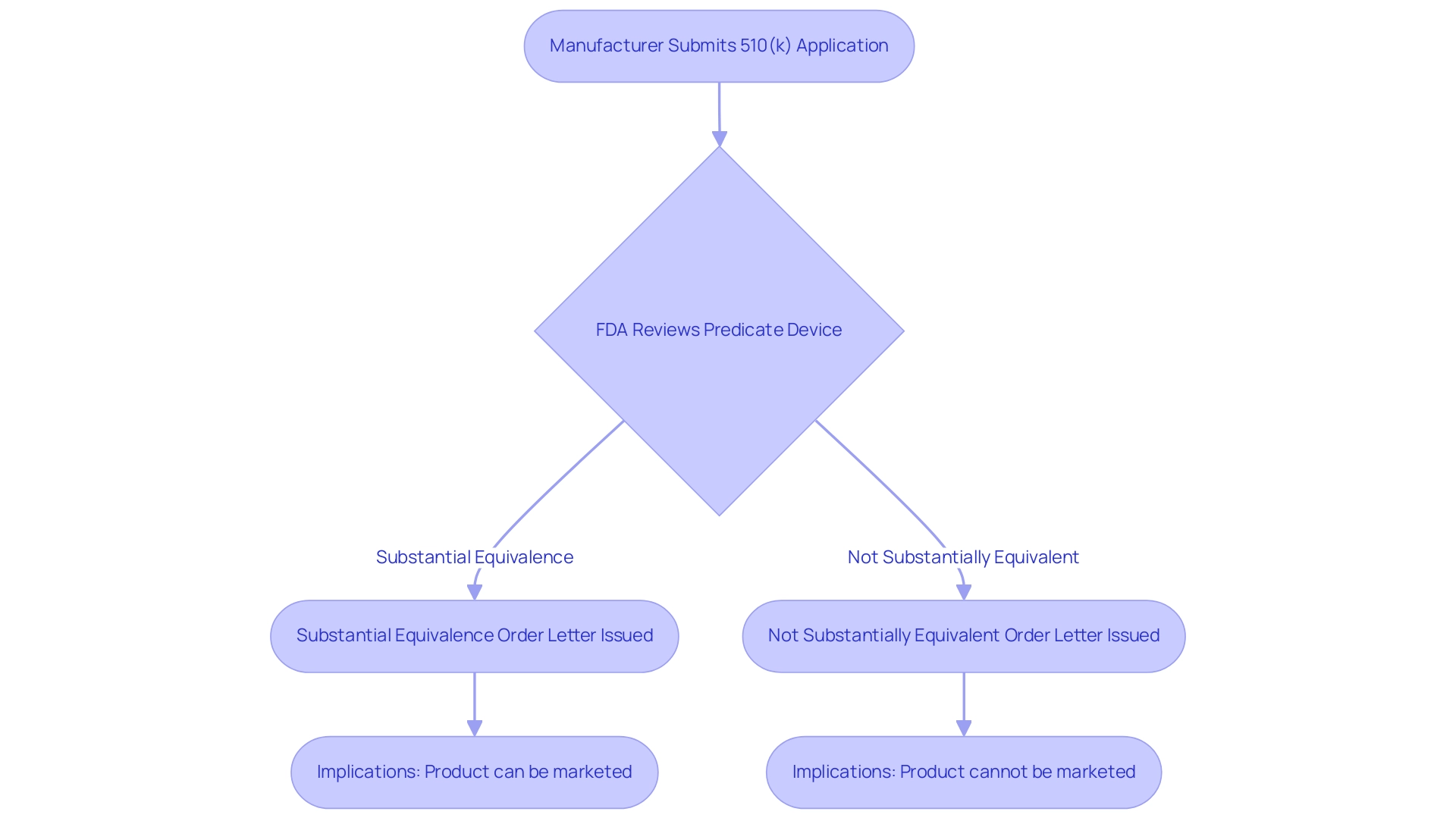
Substantial equivalence is a critical assessment conducted by the FDA to determine what is a predicate device and whether a new medical instrument can be regarded as safe and effective as that device. To establish substantial equivalence, manufacturers must demonstrate that their new product shares the same intended use and possesses similar technological characteristics. In cases where differences exist, it is imperative for the manufacturer to provide robust evidence indicating that these differences do not introduce new questions regarding safety and effectiveness.
This determination is crucial for the 510(k) submission procedure, as it directly impacts the regulatory route for new products. The review process culminates in either a substantial equivalence order letter or a not substantially equivalent order letter, which clearly delineates the outcome of the assessment. Notably, as of 2024, four products under the Center for Biologics Evaluation and Research (CBER) have successfully obtained marketing authorization based on substantial equivalence determinations.
For example, the BONE HEALTH TECHNOLOGIES, INC. OSTEOBOOST BELT, with marketing submission number DEN230015, received authorization on January 12, 2024, showcasing the successful navigation of the substantial equivalence pathway. These instances underline the significant role that substantial equivalence plays in determining approval rates for products, as well as the broader implications for the medical market, particularly in light of ongoing updates to FDA guidelines affecting substantial equivalence determinations.
With specialists like Ana Criado, who serves as the Director of Affairs and has extensive experience in consultancy for leading global companies, including her pivotal role at Colombia's agency INVIMA, and her founding of Mahu Pharma, the complexities surrounding these rules can be expertly navigated.
Ana's extensive background in biomedical engineering, health economics, and compliance affairs ensures adherence and facilitates market entry for innovative medical solutions.
Challenges in Identifying and Using Predicate Devices
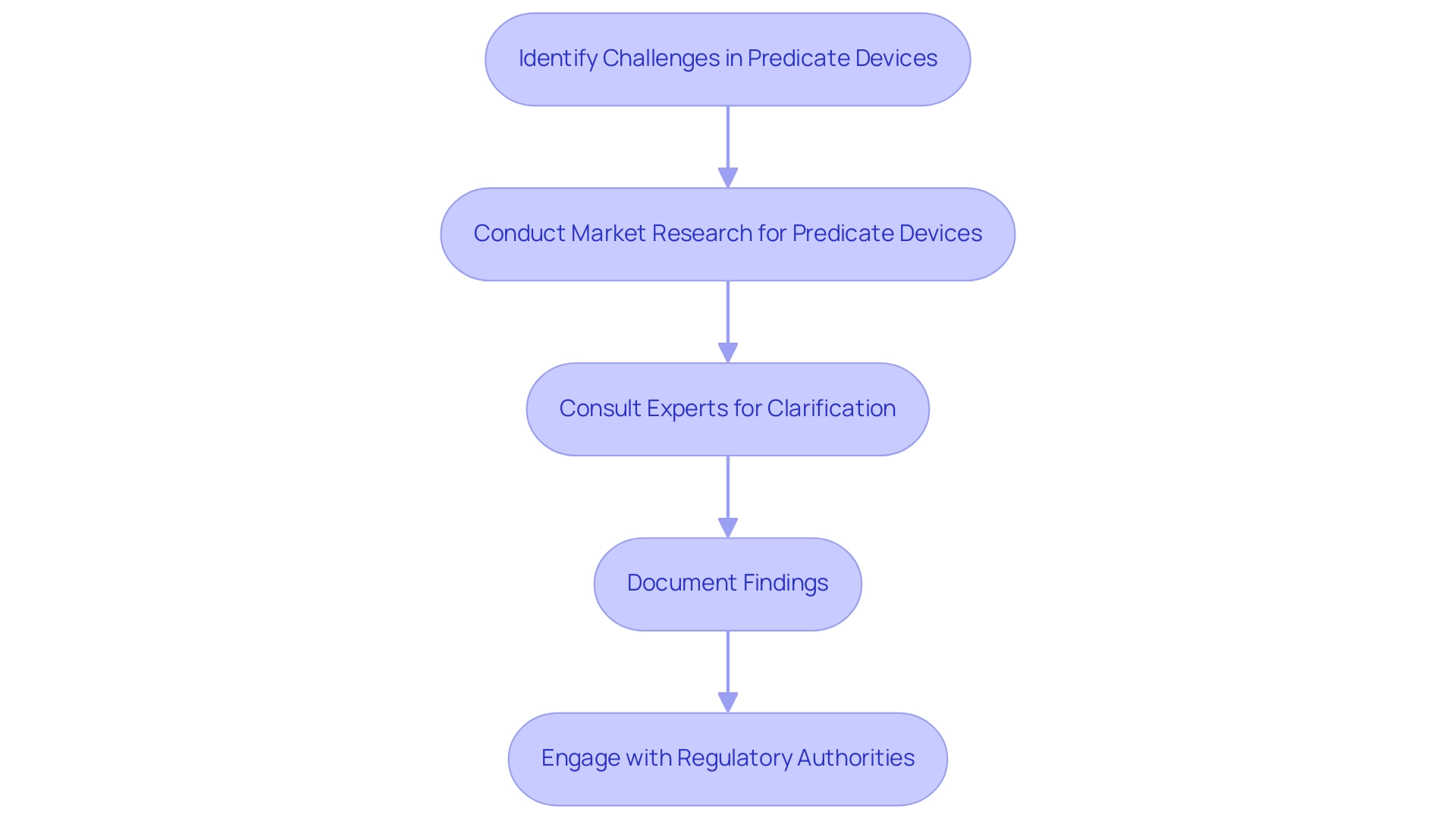
The procedure of recognizing and employing reference products presents considerable difficulties for producers, especially in the changing regulatory environment of 2024. A primary concern is the shortage of reference instruments that closely resemble the characteristics of new products, complicating the demonstration of substantial equivalence and raising questions about what is a predicate device—a critical requirement for 510(k) submissions. Furthermore, the rapid pace of technological advancement can render previously accepted products outdated, leading to questions about what is a predicate device, as approximately 30% of these products used in submissions are deemed obsolete, increasing the likelihood of additional scrutiny from the FDA.
Significantly, specialists such as Ana Criado, Director of Compliance Affairs and CEO of Mahu Pharma, highlight the importance of understanding these nuances. To effectively navigate these complexities, manufacturers must engage in diligent research and maintain proactive communication with Regulatory experts, including specialists like Katherine Ruiz, who focus on Regulatory Affairs for medical products and in vitro diagnostics in Colombia. Bob Forlenza, Managing Partner at Spring Lake Equity Partners, stresses, 'Comprehending the subtleties of classification tools is vital in guaranteeing a seamless approval procedure.'
For instance, the case study titled 'When Are 510(k) Submissions Required?' underscores the significance of recognizing compliant items to ensure a smoother approval process, illustrating key scenarios where a 510(k) submission is necessary. Producers who have effectively tackled these challenges often highlight the importance of comprehensive documentation and early interaction with oversight institutions.
Specific strategies recommended by these experts include:
- Conducting comprehensive market research to identify suitable predicate devices
- Leveraging expert consultations to clarify what is a predicate device and regulatory expectations
Conclusion
The exploration of predicate devices highlights their essential role in the medical device approval process, particularly through the FDA's 510(k) pathway. By serving as benchmarks for safety and efficacy, predicate devices enable manufacturers to demonstrate substantial equivalence, facilitating a more streamlined route to market. Understanding the intricacies involved in the identification and selection of appropriate predicate devices is crucial, as it directly impacts the success of new product submissions and compliance with regulatory standards.
As the medical device landscape evolves, manufacturers face increasing challenges, including the rapid pace of technological advancements and the potential obsolescence of existing predicate devices. The importance of conducting thorough research and maintaining open lines of communication with regulatory experts cannot be overstated. Engaging with knowledgeable professionals in regulatory affairs, such as those highlighted in the article, is vital for navigating the complexities of predicate device selection and ensuring adherence to evolving FDA guidelines.
In conclusion, a robust understanding of predicate devices is not only beneficial but essential for manufacturers aiming to achieve successful market entry. By prioritizing diligent research, strategic planning, and expert consultation, stakeholders can effectively address the challenges presented in today's dynamic regulatory environment. Emphasizing the importance of predicate devices will ultimately contribute to the development of innovative medical technologies that meet the needs of healthcare providers and patients alike.
Frequently Asked Questions
What is a classification item in the context of medical instruments?
A classification item is a medical instrument that has been legally sold and serves as a standard for demonstrating the safety and efficacy of new instruments.
How does the FDA determine if a new medical item is substantially equivalent to a predicate device?
The FDA defines a new item as substantially equivalent to another when it shares the same intended use and technological characteristics as the predicate device.
Why is understanding predicate devices important for manufacturers?
Understanding predicate devices is crucial for manufacturers as it helps them navigate the complex approval environment necessary for obtaining authorizations for new medical instruments.
What is the 510(k) approval procedure?
The 510(k) approval procedure is a regulatory pathway for manufacturers to demonstrate that their new medical products are significantly comparable to existing reference items already available, allowing for a more expedited route to market compared to the Premarket Approval (PMA) procedure.
What are the safety and efficacy standards required for 510(k) clearance?
To achieve 510(k) clearance, manufacturers must provide robust evidence that their product meets safety and efficacy standards comparable to those of a predicate device.
What is the difference in recall rates between PMA and 510(k) products?
The risk of recall for products with PMA clearance is 32%, while the risk for 510(k) items is 13%, indicating a comparative safety advantage for the 510(k) method.
What role do clinical trial management services play in the approval process?
Clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, and reporting, are critical for helping manufacturers navigate compliance pathways effectively.
Who is Katherine Ruiz and what is her significance in this context?
Katherine Ruiz is a specialist in compliance matters for medical instruments and in vitro diagnostics in Colombia, providing essential knowledge for navigating the complexities of regulatory compliance.
How has the trend of at-home diagnostics impacted the medical equipment market?
The trend of at-home diagnostics, which gained traction during the pandemic, highlights the importance of such tools in adapting to consumer needs and enhancing healthcare access.
What recent advancements have been made in the 510(k) evaluation system?
Recent advancements include completed guidelines aimed at improving transparency, consistency, and predictability in the 510(k) evaluation system to better meet the changing requirements of the medical equipment landscape.




