Overview
The article centers on how clinical researchers can adeptly navigate trial challenges in Mexico, highlighting the critical importance of understanding regulatory requirements, cultural considerations, and logistical factors. It offers a comprehensive step-by-step guide that encompasses strategies for:
- Patient recruitment
- Compliance with ethical standards
- Establishment of partnerships with local institutions
These elements collectively illustrate how they contribute to the success of clinical trials in the region, underscoring the necessity of collaboration and the pivotal role of local expertise.
Introduction
Mexico is rapidly emerging as a key player in the global clinical trial landscape, attracting researchers and sponsors with its unique blend of regulatory advantages, cost-effective solutions, and a diverse patient population. As the number of clinical trials continues to surge, particularly in the Medtech sector, understanding the intricacies of conducting research in this dynamic environment becomes paramount.
Navigating the regulatory landscape established by COFEPRIS and implementing effective patient recruitment strategies are crucial. The success of clinical trials hinges on a comprehensive approach that addresses cultural sensitivities and logistical challenges.
This article delves into the essential components of conducting successful clinical trials in Mexico, offering insights into best practices, regulatory requirements, and the importance of building strong partnerships with local institutions.
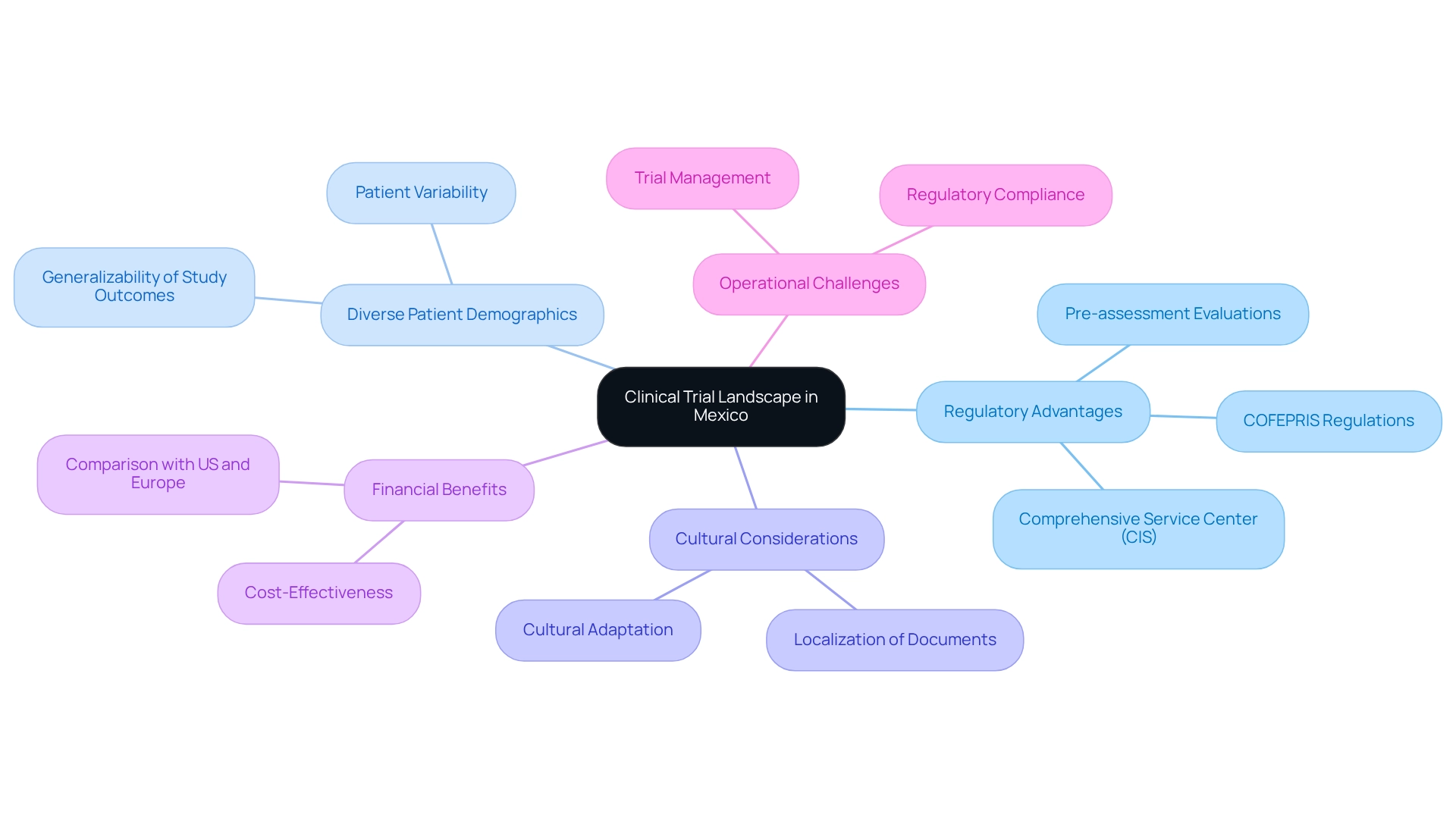
Understanding the Clinical Trial Landscape in Mexico
Mexico has established itself as a pivotal player in the global medical study arena, characterized by a blend of regulatory advantages, a diverse patient demographic, and cost-effective research solutions. By 2025, the nation has witnessed a remarkable increase in the number of medical studies, particularly within the Medtech sector, fueled by a robust healthcare infrastructure and an expanding network of research organizations (CROs). Recent statistics reveal that the number of medical studies in Mexico has surged by over 30% in the past year, underscoring the country's appeal to global sponsors.
Navigating the regulatory landscape is essential for success. Researchers must familiarize themselves with the regulations imposed by COFEPRIS, Mexico's health product regulatory authority, which has streamlined its processes through the establishment of the Comprehensive Service Center (CIS). This initiative plays a vital role in enhancing the efficiency of regulatory submissions, allowing COFEPRIS a maximum of 60 business days to issue decisions on sanitary registration processes.
Moreover, candidates can benefit from pre-assessment evaluations conducted by approved third parties, facilitating smoother application submissions.
The diverse demographics of Mexico present a unique opportunity for researchers to enhance the generalizability of study outcomes. By leveraging this diversity, medical studies can more accurately reflect the varied responses to healthcare interventions across different populations, particularly in the Medtech domain where understanding patient variability is crucial. However, it is imperative to address cultural differences that may influence patient recruitment and retention.
A case analysis highlighting the importance of localization in research emphasizes the necessity for native Spanish speakers to translate regulatory documents accurately, ensuring cultural relevance and improving patient comprehension and adherence.
Furthermore, the financial advantages of conducting research in Mexico are noteworthy. Compared to markets such as the United States and Europe, the cost-effectiveness of research in this region can be up to 50% lower, making it an attractive option for Medtech companies seeking to expedite their trials. bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting, ensuring that your studies are conducted efficiently and effectively.
As the research study landscape in Mexico continues to evolve, navigating trial challenges while staying attuned to current trends and regulatory benefits will be crucial for investigators aiming to adeptly manage the complexities of study execution.
Navigating Regulatory Requirements for Clinical Trials
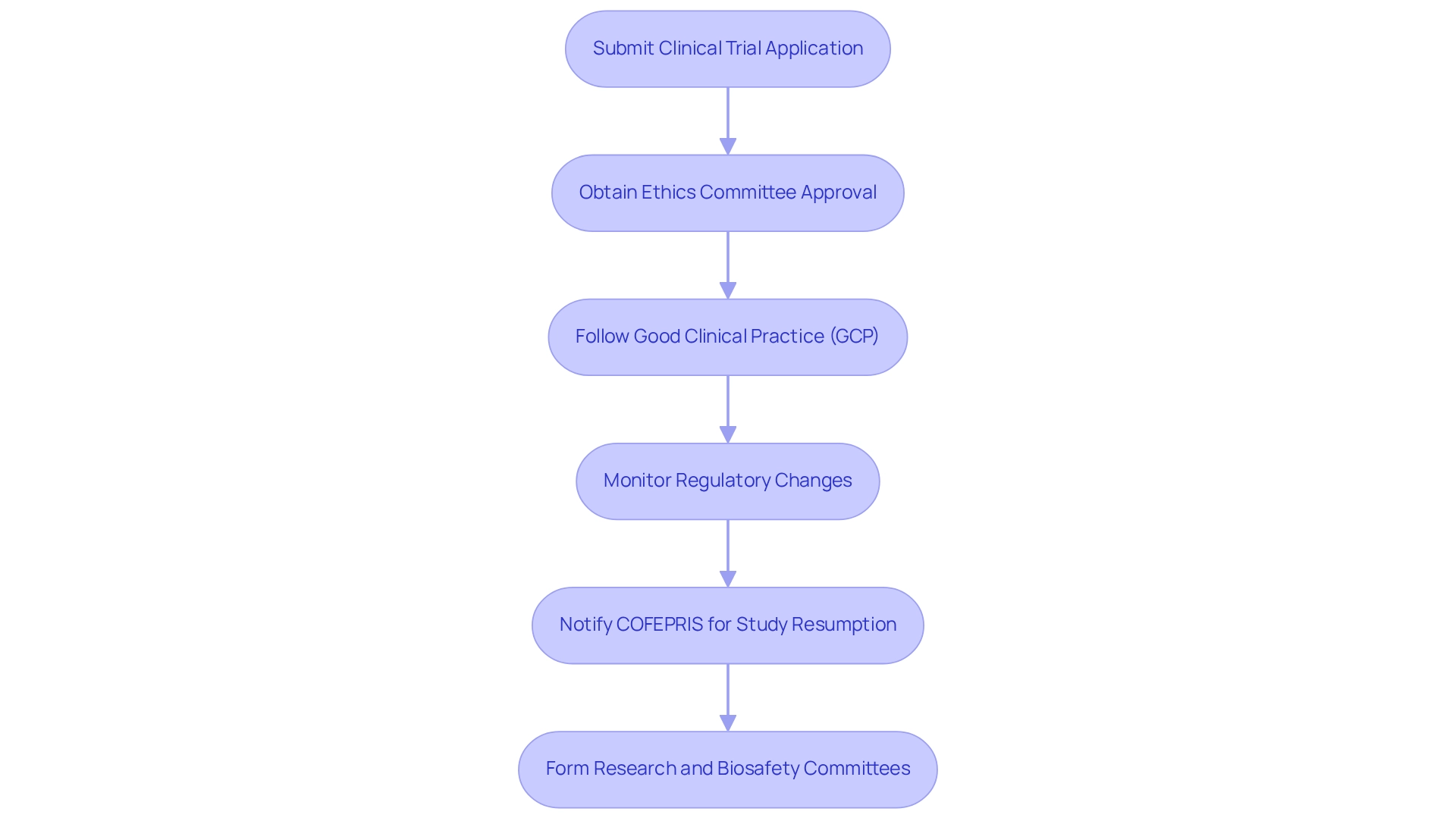
Navigating the regulatory landscape in Mexico necessitates a comprehensive understanding of the requirements established by COFEPRIS. Researchers must ensure that all clinical studies comply with the General Health Law and the Health Research Secondary Regulations. The essential steps to follow are as follows:
- Submit a Clinical Trial Application: Prepare and submit a detailed application to COFEPRIS, which should include study protocols, informed consent forms, and qualifications of the investigators involved.
- Obtain Ethics Committee Approval: It is crucial to secure approval from an independent ethics committee prior to commencing any study, as this is a mandatory requirement.
- Follow Good Clinical Practice (GCP): All study activities must comply with GCP guidelines to uphold the integrity and reliability of the research outcomes.
- Monitor Regulatory Changes: Regularly stay informed about any updates in regulations that could affect ongoing or future studies.
Additionally, collaborating with local regulatory experts can streamline the application process and enhance compliance. Researchers should leverage resources from organizations such as ACRP for comprehensive guidance on regulatory compliance. It is important to note that if a study is resumed, COFEPRIS must be notified within a maximum of 15 working days. Furthermore, every health organization performing research is obligated to form a Research Committee and a Biosafety Committee, with essential approvals required for each research site.
In December 2024, a new equivalence agreement was published, allowing for the importation of certain drugs and medical devices without COFEPRIS marketing authorization, provided they have commercialization authorization from recognized regulatory agencies. This agreement aims to facilitate participation in consolidated public procurement, ensuring that imported products meet regulatory standards while streamlining the import process.
As Bernardo Martinez-Negrete notes, "Galicia is ranked as the top firm in Mexico by renowned international publications including Chambers and Partners, among others," highlighting the importance of working with established firms to navigate these complexities.
By following these steps and utilizing available resources, researchers in the medical field can ensure successful applications and compliance with ethical standards while navigating trial challenges in Mexico. 'bioaccess®'s expertise and tailored approach can greatly assist Medtech firms in this process, facilitating the advancement of medical devices more quickly through extensive research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
Effective Strategies for Patient Recruitment in Mexico
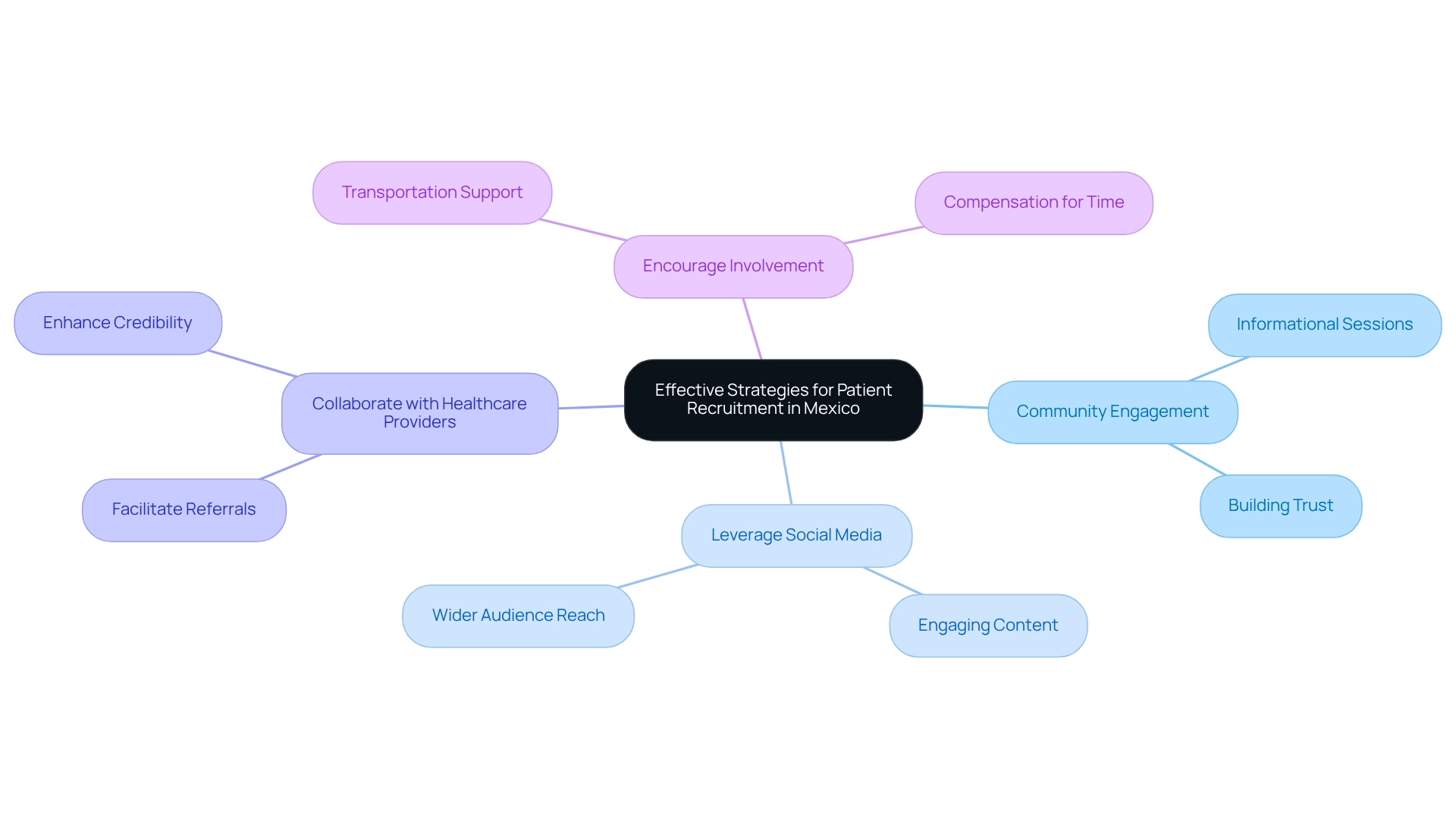
Navigating trial challenges in Mexico is crucial for enlisting participants in medical studies, influenced by cultural perspectives and varying levels of understanding regarding medical opportunities. To enhance recruitment efforts, consider implementing the following effective strategies:
- Community Engagement: Establish strong relationships with local communities to build trust and enhance awareness about research studies. Organizing informational sessions can inform potential participants about the advantages and procedures involved in medical research. This method reflects the effective partnership between bioaccess™ and Caribbean Health Group, which seeks to establish Barranquilla as a prominent location for medical research in Latin America, backed by Colombia's Minister of Health.
- Leverage Social Media: Utilize popular social media platforms to reach a wider audience. Engaging content can effectively spread information about ongoing studies and encourage participation among various demographics.
- Collaborate with Healthcare Providers: Form partnerships with local healthcare providers to identify potential participants. These providers can facilitate referrals and lend credibility to the recruitment process, making patients more likely to consider participation. This strategy is crucial, particularly as bioaccess® collaborates with organizations such as GlobalCare Clinical Trials to improve ambulatory services in Colombia, attaining over 50% reduction in recruitment time and 95% retention rates.
- Encourage Involvement: Providing rewards, such as transportation support or payment for time invested in the study, can greatly enhance participation rates. This approach acknowledges the commitment required from participants and can enhance their willingness to engage.
Best Practices:
- Tailor recruitment messages to resonate with local cultural values, ensuring that they are relatable and respectful.
- Ensure recruitment materials are available in both Spanish and English, broadening accessibility and understanding among potential participants.
The pediatric population in Latin America, which comprises approximately 30% of individuals under 14 years old, underscores the importance of effective recruitment strategies targeting this demographic. As the region's demographics evolve, particularly with a growing elderly patient population, the potential for new drug markets expands. Effective community involvement in medical trials has been demonstrated to enhance recruitment results, as indicated by examples where biotechnology firms outsourcing early-phase trials to Mexico have achieved considerable cost reductions and expedited development schedules.
Sergio A. Sanchez, a previous research consultant, highlights that "sponsors must carefully evaluate the partner’s performance in choosing research locations and investigators, enrolling participants, and adhering to project timelines." By utilizing these strategies and insights, medical researchers can improve their effectiveness in navigating trial challenges in Mexico, while bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies further facilitates the advancement of medical devices in the Medtech sector. Additionally, the economic impact of Medtech studies on local economies, including job creation and healthcare improvement, underscores the importance of these initiatives.
Addressing Socioeconomic Factors in Clinical Trial Participation
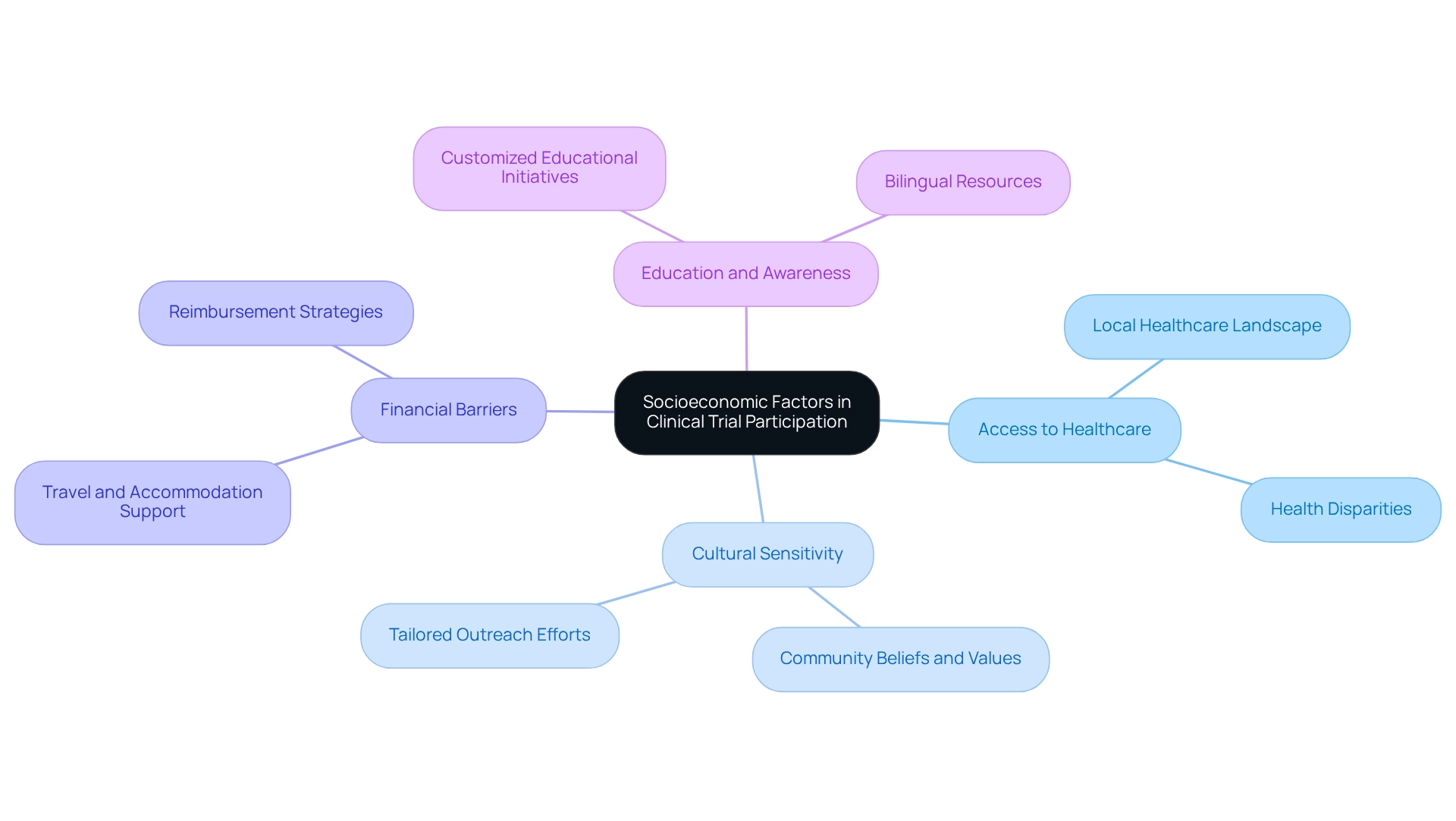
Navigating trial challenges in Mexico is essential, given the significant impact of socioeconomic factors on participation in medical studies. This necessitates a thorough strategy aimed at improving enrollment and retention. Researchers should focus on several key aspects:
- Access to Healthcare: Understanding the local healthcare landscape is crucial. Acknowledging how disparities in healthcare access can influence potential participants' willingness to engage in studies is vital. For instance, individuals in disadvantaged regions may face restricted access to healthcare services, discouraging participation in clinical studies. Notably, research indicates that first-time stroke cases among individuals aged 45 and older highlight considerable health disparities that can complicate the navigation of trial challenges in Mexico.
- Cultural Sensitivity: Cultural attitudes towards medical research significantly affect participation rates. Researchers must be attuned to the beliefs and values of different communities, ensuring that research designs and outreach efforts are culturally appropriate and resonate with potential participants. As noted by Lewis B Morgenstern, minority groups are more likely to participate in studies when outreach efforts are effectively tailored to their communities, despite initial limited engagement.
- Financial Barriers: Financial constraints often pose considerable challenges for study participants. To mitigate these challenges, it is crucial to provide support for travel and accommodation costs. Implementing reimbursement strategies or offering stipends can alleviate the financial burden and encourage participation.
- Education and Awareness: Enhancing understanding of the advantages of joining research studies is essential. Customized educational initiatives that address the specific issues and interests of different socioeconomic groups can foster greater comprehension and curiosity about research. The case analysis titled 'Strategies for Enhancing Minority Participation in Clinical Research' underscores the importance of community-based strategies for navigating trial challenges in Mexico to improve participation rates among underserved populations.
In this context, bioaccess® offers extensive management services for research studies, focusing on efficiently navigating trial challenges in Mexico. Their expertise in Early-Feasibility Assessments, First-In-Human Experiments, feasibility assessments, site selection, compliance reviews, setup, import permits, project management, and reporting ensures that experiments are not only compliant but also accessible to diverse populations. Furthermore, the impact of Medtech research studies on local economies—such as job creation, economic growth, and healthcare enhancement—highlights the significance of international cooperation in promoting medical research in Latin America.
Recommendations:
- Conduct focus groups to gather insights on community perceptions of clinical trials, which can inform more effective outreach strategies.
- Collaborate with local NGOs to enhance access to underserved populations, ensuring that recruitment efforts are inclusive and representative.
- Create bilingual educational resources to improve communication and comprehension among various participant groups, thereby boosting involvement in research studies. Additionally, understanding that Latino patients may place a greater emphasis on the potential benefits of experimental treatments compared to white patients can inform how outreach is structured.
Logistical Considerations for Conducting Trials in Mexico
Carrying out medical studies in Mexico necessitates careful attention to various logistical factors that can greatly influence the success of the research.
Site Selection: Choosing the appropriate research locations is essential. Researchers should evaluate sites based on patient demographics, accessibility, and the availability of necessary medical resources. The choice of site can influence patient recruitment and retention, making it essential to consider local healthcare infrastructure and community engagement. Understanding local dialects, influenced by education, socioeconomic status, and ethnic background, can also enhance communication and trust with participants. Partnerships, like the one between bioaccess™ and Caribbean Health Group, showcase the potential for transforming areas such as Barranquilla into prominent locations for medical research in Latin America, backed by local health authorities.
Supply Chain Management: A robust supply chain strategy is crucial for guaranteeing the prompt delivery of testing materials and medications. This includes establishing relationships with local suppliers and understanding the regulatory landscape that may affect logistics. Effective supply chain management can mitigate delays that frequently arise from bureaucratic obstacles, especially in public healthcare environments, where numerous research specialists encounter challenges navigating trial complexities in Mexico.
Data Management: Implementing effective data management systems is vital for securely handling patient information while complying with local regulations. This involves employing electronic data capture systems that enable real-time data entry and monitoring, ensuring data integrity and accessibility throughout the study. bioaccess® is dedicated to ensuring information security and client trust, underscoring the significance of data protection in medical device research trials.
Transportation and Accommodation: Planning for participant transportation and accommodation is critical, especially for those traveling from remote areas. Providing logistical support can enhance participant experience and retention rates. Researchers should explore collaborations with nearby hotels and transportation services to simplify these arrangements.
Best Practices:
- Establish clear communication channels with all stakeholders involved in the experiment to ensure alignment and address any issues promptly.
- Leverage local expertise while navigating trial challenges in Mexico effectively, as local professionals can provide insights into regional nuances that may affect execution.
- Consider the recent experiences of companies like AEGEA Medical, which successfully completed enrollment in the PACE MX research project, highlighting the importance of strategic site selection and logistical planning in achieving critical milestones in research. This case analysis demonstrates how careful planning can result in successful outcomes. Furthermore, as highlighted by Luis Armando Bravo Castillo, founder of Probiotics, economical solutions in the Medtech sector can greatly influence project feasibility. His insights highlight the significance of balancing quality with expense in medical research. Ultimately, the statistic that a study reached information saturation after conducting interviews with 14 participants stresses the necessity of careful site selection and participant involvement in research studies.
Building Partnerships with Local Institutions
Establishing strong collaborations with local organizations is crucial for navigating trial challenges in Mexico and ensuring the success of clinical studies. Here are key strategies to consider:
-
Identify Key Stakeholders: Engage with local hospitals, universities, and research organizations that can provide critical resources and support. Understanding the landscape of potential partners is essential when navigating trial challenges in Mexico for effective collaboration.
-
Establish Collaborative Agreements: Develop formal agreements that clearly outline the roles and responsibilities of each partner. This clarity fosters accountability and ensures that all parties are aligned in their objectives.
-
Leverage Local Knowledge: Utilize the expertise of local partners when navigating trial challenges in Mexico and effectively handle the cultural and regulatory landscapes. Their insights can help mitigate risks and enhance the overall efficiency of the testing process. For example, bioaccess provides extensive management services for research projects, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, which can greatly simplify the process.
-
Foster Long-Term Relationships: Invest in establishing lasting connections with partners to enable future collaborations and boost the credibility of your studies. Navigating trial challenges in Mexico can lead to long-term partnerships that result in more streamlined processes and improved outcomes, contributing to job creation and economic growth in the region.
Best Practices:
- Attend local conferences and networking events to connect with potential partners and stay informed about the latest developments in the field.
- Share successes and challenges openly to build trust and foster a collaborative environment.
- Reflect on the results from the case study titled "Strategy Management in Collaborative Research Partnerships," which examines the challenges and strategies associated with managing various research partnerships. Engaged leadership and careful monitoring are crucial for successful outcomes.
- As highlighted by Sergio A. Sanchez, former research consultant at Deallus Consulting, "By thoroughly assessing past performance and capabilities, sponsors should be able to choose the appropriate partner for their specific drug candidate with the demonstrated ability for navigating trial challenges in Mexico, including regulatory, logistical, and even cultural aspects of studies."
- Furthermore, note that COFEPRIS mandates sponsors or CROs to adhere to Good Clinical Practice standards for executing research studies, which is vital for navigating trial challenges in Mexico. This encompasses review and feedback on research documents to comply with country requirements, along with monitoring and reporting on research status, inventory, and serious and non-serious adverse events.
Ethical Considerations and Patient Rights in Clinical Trials
Ethical considerations are paramount in conducting medical studies in Mexico, where adherence to established principles is essential for the integrity of research and the protection of participants. Key ethical principles include:
- Informed Consent: It is crucial that all participants provide informed consent, fully understanding the risks and benefits associated with their involvement in the research. Recent statistics indicate that a significant portion of participants in Mexican clinical trials feel adequately informed about their rights and the implications of the research, underscoring the necessity of effective communication in the consent process.
- Confidentiality: Researchers must implement rigorous data protection measures to ensure the privacy of participants. This encompasses secure data handling practices and guaranteeing that personal information is accessible only to authorized personnel. At bioaccess®, we are dedicated to maintaining information security and client trust, as reflected in our grievance and data protection procedures. Participants with inquiries or concerns regarding the processing of their information can contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. We address all concerns in accordance with applicable law, ensuring transparency and compliance. Our privacy policy, generated with CookieYes, further details our commitment to data protection.
- Vulnerable Populations: Special care must be taken with vulnerable populations, such as minors and individuals facing mental health challenges. Ethical guidelines dictate that the risks associated with studies must be justified by the potential benefits, and informed consent should be obtained when appropriate. A significant case analysis highlights the ethical principles governing investigations involving prisoners, emphasizing the importance of minimizing risks and confirming that participation is warranted, particularly for individuals deemed mentally incompetent. This case study illustrates the critical nature of adhering to ethical and legal frameworks in sensitive investigative contexts.
- Compliance with Ethical Guidelines: Adherence to ethical guidelines established by international organizations, such as the Declaration of Helsinki, is vital. These guidelines provide a framework for ethical conduct in studies, ensuring the rights and welfare of participants are prioritized.
Recommendations:
- Conduct regular training sessions for research staff on ethical standards and patient rights to cultivate a culture of compliance and respect. Additionally, ensure that all study participants are covered by local insurance to enhance their protection.
- Establish a clear protocol for addressing ethical concerns that may arise during the process, ensuring that all team members are equipped to handle such issues effectively.
As Max Baumann observed, "Entering 2025, we still perceive biotech encountering essential business model obstacles as therapeutic end-markets grow increasingly saturated." By prioritizing these ethical considerations, researchers can adeptly navigate trial challenges in Mexico while upholding the highest standards of integrity and respect for participants.
Best Practices for Successful Clinical Trials in Mexico
To ensure the success of clinical trials in Mexico, researchers should adopt the following best practices:
-
Thorough Planning: Allocate sufficient time for meticulous planning that encompasses regulatory compliance, patient recruitment strategies, and logistical arrangements. This foundational step is crucial for aligning with local regulations and ensuring smooth trial execution. Additionally, sponsors must ensure that investigational products are stored and handled according to applicable Good Manufacturing Practices (GMPs), which is essential for regulatory compliance. Partnerships, such as the collaboration between bioaccess™ and Caribbean Health Group, illustrate the significance of strategic planning in establishing medical study locations that comply with regulatory standards. This partnership was revealed on March 29, 2019, during a gathering in Miami, FL, highlighting the dedication to improving healthcare studies in Barranquilla.
-
Engagement with Local Communities: Establish strong relationships with local communities to foster trust and enhance recruitment efforts. Understanding cultural nuances and employing native language communication can significantly improve participant engagement and retention. A case study titled 'Importance of Localization in Clinical Trials' highlights that navigating trial challenges in Mexico through native language communication and localization strategies can boost patient recruitment and adherence, ultimately enhancing the success of research. This method is additionally supported by initiatives such as the one endorsed by Colombia's Minister of Health, which seeks to position Barranquilla as a leading location for clinical studies in Latin America.
-
Continuous Monitoring: Establish a robust system for ongoing observation of study progress and participant feedback. This proactive approach enables the timely identification and resolution of issues, ensuring that the process remains on track and adheres to ethical standards. Thorough research study management services, including project oversight, feasibility assessments, compliance evaluations, and reporting, are vital for maintaining supervision and ensuring adherence to protocols.
-
Adaptability: Maintain flexibility in strategies to respond to real-time feedback and changing conditions within the testing environment. The ability to pivot based on participant needs and external factors can greatly enhance the overall success of clinical research. As Max Baumann, Head of Execution at Treehill Partners, states, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
Key Takeaways:
- Uphold ethical considerations and prioritize patient rights throughout the trial process.
- Leverage local expertise and resources for effectively navigating trial challenges in Mexico and optimizing trial outcomes.
- Ensure that communication, such as the Informed Consent Form (ICF), is presented in plain language that participants can understand, generally at a grade 6-8 reading level, to facilitate better comprehension and engagement.
Conclusion
Mexico's emergence as a key hub for clinical trials is propelled by its regulatory advantages, cost-effectiveness, and diverse patient demographics, particularly in the Medtech sector. This article underscores the critical importance of navigating COFEPRIS regulations and the necessity for effective patient recruitment strategies that honor cultural sensitivities. By comprehensively understanding the local healthcare landscape and addressing socioeconomic factors, researchers can significantly improve enrollment and retention rates in clinical trials.
Establishing strong partnerships with local institutions is crucial for the successful execution of trials. Collaborative agreements, the leveraging of local expertise, and the nurturing of long-term relationships not only streamline processes but also enhance the credibility and efficacy of research initiatives. Ethical considerations, especially those related to informed consent and the safeguarding of vulnerable populations, must remain central to all clinical research activities.
In conclusion, to excel in Mexico's clinical trial landscape, a comprehensive approach that integrates meticulous planning, community engagement, and strict adherence to ethical standards is essential. The potential for accelerated development timelines and substantial cost savings, coupled with the opportunity to address diverse patient needs, positions Mexico as a highly attractive destination for clinical research. As the landscape continues to evolve, remaining informed and adaptable will be pivotal in achieving successful outcomes in this dynamic environment.
Frequently Asked Questions
What makes Mexico a significant player in the global medical study arena?
Mexico is recognized for its regulatory advantages, diverse patient demographics, and cost-effective research solutions, leading to a significant increase in medical studies, especially in the Medtech sector.
How much has the number of medical studies in Mexico increased recently?
The number of medical studies in Mexico has surged by over 30% in the past year.
What is COFEPRIS and why is it important for researchers in Mexico?
COFEPRIS is Mexico's health product regulatory authority responsible for overseeing medical studies. Researchers must adhere to its regulations to ensure compliance and successful study execution.
What is the Comprehensive Service Center (CIS)?
The CIS is an initiative by COFEPRIS that streamlines the regulatory submission process, allowing for decisions on sanitary registration processes within a maximum of 60 business days.
How can candidates facilitate smoother application submissions in Mexico?
Candidates can benefit from pre-assessment evaluations conducted by approved third parties, which help streamline the application process.
Why is the diverse demographic of Mexico beneficial for medical studies?
The diverse demographics allow researchers to enhance the generalizability of study outcomes by reflecting varied responses to healthcare interventions across different populations.
What challenges might researchers face due to cultural differences in Mexico?
Cultural differences may influence patient recruitment and retention, necessitating careful consideration in study design and execution.
What is the importance of localization in research documentation?
Accurate translation of regulatory documents by native Spanish speakers ensures cultural relevance, improving patient comprehension and adherence to study protocols.
How does the cost of conducting research in Mexico compare to the United States and Europe?
Conducting research in Mexico can be up to 50% lower in cost compared to the United States and Europe, making it a more attractive option for Medtech companies.
What services does bioaccess® provide for medical studies in Mexico?
bioaccess® offers comprehensive management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project management, and reporting.
What essential steps must researchers follow when navigating the regulatory landscape in Mexico?
Researchers must submit a Clinical Trial Application, obtain Ethics Committee Approval, follow Good Clinical Practice (GCP), and monitor regulatory changes.
What is the significance of the new equivalence agreement published in December 2024?
The agreement allows for the importation of certain drugs and medical devices without COFEPRIS marketing authorization, provided they have commercialization authorization from recognized regulatory agencies, streamlining the import process.
How can researchers ensure successful applications and compliance with ethical standards in Mexico?
By following regulatory steps, collaborating with local experts, and utilizing resources from organizations like ACRP, researchers can navigate trial challenges effectively.




