Overview
Medtech companies can overcome barriers to market entry by developing comprehensive strategies that address regulatory hurdles, funding challenges, market access issues, competition, and technological advancements. The article emphasizes the importance of conducting SWOT analyses, setting clear objectives, engaging stakeholders, and establishing market access strategies, which collectively enhance a company's ability to navigate the complexities of the medtech landscape and achieve successful product introduction.
Introduction
The medtech industry stands at a crossroads, facing a myriad of challenges that can impede market entry for innovative companies. As these firms strive to introduce groundbreaking medical technologies, they encounter a complex landscape marked by:
- Regulatory hurdles
- Funding difficulties
- Intense competition
Understanding the multifaceted barriers to market penetration is crucial for success in this dynamic environment. This article delves into the key obstacles medtech companies face and outlines strategic approaches to navigate these challenges effectively. By examining:
- Regulatory requirements
- Funding strategies
- Market access issues
Firms can develop robust plans that not only facilitate entry but also foster sustainable growth in an ever-evolving sector.
Identifying Key Barriers to Market Entry for Medtech Companies
Medtech companies frequently encounter several significant barriers when attempting to enter the industry, which include the following:
-
Regulatory Hurdles: Navigating the intricate regulatory landscape is a primary challenge for medtech firms. Compliance with stringent regulations established by authorities such as the FDA and EMA is essential. This requires a profound understanding of clinical trial requirements, product approvals, and post-market surveillance. As noted by Mercè Guerra, Manager of Regulatory Affairs,
To achieve your goals in this evolving regulatory environment, you need not only a comprehensive regulatory strategy but also a thorough clinical and access analysis.
The complexity of these regulations can significantly affect the time and resources required for successful participation. Notably, Phase III studies are usually very large studies (N=hundreds to 3000) designed to confirm a product’s safety and effectiveness, further complicating the regulatory process. The FDA's policies for developing guidance documents were formalized by congressional regulation in 2000, adding additional layers of compliance for new products.
-
Funding Challenges: Securing sufficient funding for research and development remains critical for medtech startups. Many companies struggle to attract investors, often due to the perceived risks associated with clinical trials and entering the industry. Developing a strong business plan and demonstrating potential demand can be crucial in attracting the necessary investment. In 2024, funding trends indicate an increasing focus on the viability of innovative products, emphasizing the need for startups to clearly articulate their value propositions. bioaccess® can help address these funding challenges by offering strategic insights and links to potential investors, utilizing their extensive network and experience in the medical technology sector.
-
Market Access Issues: Attaining access to healthcare systems and reimbursement pathways poses another challenge. Engaging with payers and effectively demonstrating the clinical and economic value of products is vital for ensuring adoption by healthcare providers. The ability to navigate these complexities can determine the success of a product in the market.
-
Competition and Industry Saturation: The medical technology sector is defined by fierce rivalry, with many participants competing for share. Understanding the competitive landscape and effectively differentiating products is crucial for success. Companies must continually assess their positioning to remain relevant in a crowded marketplace.
-
Technological Advancements: Rapid advancements in technology can quickly render existing products obsolete. Staying knowledgeable about emerging innovations and adapting to changing industry needs is essential for maintaining relevance and competitiveness. This dynamic environment requires a proactive strategy for product development and commercial approach.
Overall, overcoming barriers to market entry for medtech companies is essential for successful access, particularly in a setting where regulatory challenges and funding issues are becoming more significant. For instance, bioaccess® provides accelerated medical device clinical study services in Latin America, leveraging over 20 years of expertise in managing Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Comprehending the functions of regulatory bodies, like INVIMA in Colombia, and their influence on access to the marketplace can further improve a company’s approach. Furthermore, bioaccess® distinguishes itself from rivals by delivering customized solutions that not only simplify the regulatory process but also offer essential assistance in obtaining funding, ensuring a comprehensive strategy for launching. A recent analysis of FDA Premarket Approval Data emphasizes the significant role of cardiovascular devices, summarizing approval times and orders, which highlights the importance of comprehending these regulatory timelines in the launch strategy.
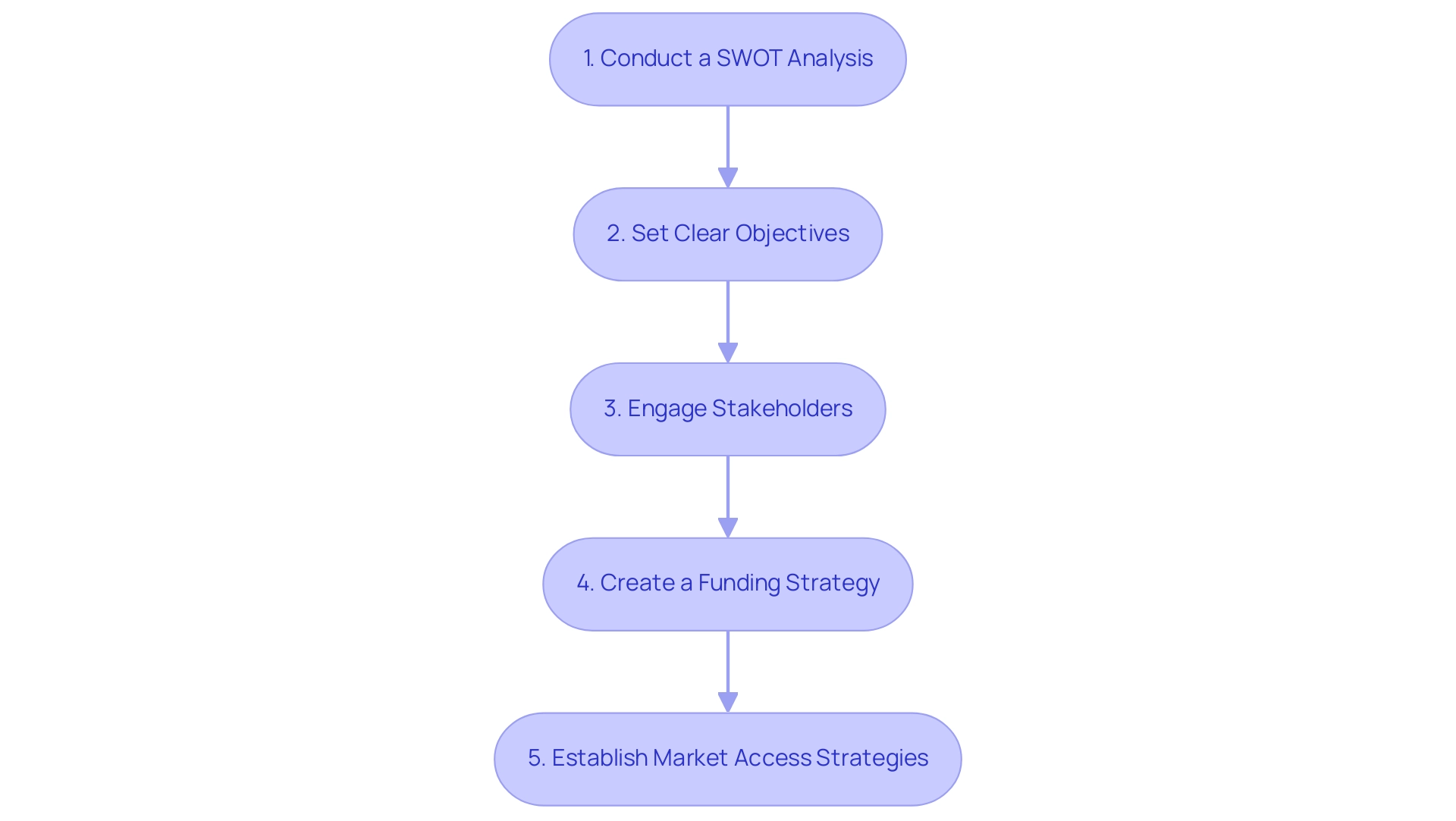
Developing a Strategic Plan to Address Barriers
Creating a strong strategic plan is crucial for medtech firms focused on overcoming barriers to market entry for medtech companies during their introduction. Here are essential steps to consider:
-
Conduct a SWOT Analysis: Initiate by conducting a thorough SWOT analysis to assess the organization's Strengths, Weaknesses, Opportunities, and Threats.
This foundational step not only identifies internal capabilities but also external challenges, which is critical in overcoming barriers to market entry for medtech companies in an industry marked by evolving regulations and competitive risks. As Ahsan Siddiqui from the Quality Management & Patient Safety Department notes,
SWOT analysis also helps to identify the 7 Muda wastes of the organization to reduce the wastes to make the organization reliable, productive, and profitable.
-
Set Clear Objectives: Define SMART objectives—specific, measurable, achievable, relevant, and time-bound—for entry into the sector.
Clear objectives help align the team's efforts in overcoming barriers to market entry for medtech companies, ensuring that all members are working towards common goals and can effectively allocate resources. It is essential to consider external factors, such as the potential impacts on earnings, similar to the regulatory and competitive risks faced by companies like Alphabet.
-
Engage Stakeholders: Actively involve key stakeholders, including regulatory experts, investors, and healthcare professionals, throughout the planning process.
Their combined insights are essential in forming a comprehensive plan that focuses on overcoming barriers to market entry for medtech companies and anticipates various industry dynamics. Develop a regulatory plan aimed at overcoming barriers to market entry for Medtech companies by designing a comprehensive strategy to navigate the complex landscape of medical device regulations. This should include detailed timelines for clinical trials, submissions, and approvals, ensuring compliance with all necessary regulations from the start.
Our comprehensive clinical trial management services encompass feasibility studies, selection of research sites, and principal investigator (PI) identification, along with compliance reviews that are instrumental in aligning with country-specific requirements. Furthermore, the regulatory strategy should incorporate overcoming barriers to market entry for Medtech companies, such as obtaining import permits and nationalizing investigational devices, to facilitate smooth entry.
-
Create a Funding Strategy: Identify potential funding sources, such as venture capital, grants, or strategic partnerships.
Crafting a compelling pitch is crucial to attract the right investors who share the vision for innovative medical solutions. For instance, BD's management has provided conservative guidance for fiscal year 2025, expecting approximately 10% EPS growth, which underscores the importance of a robust funding strategy.
-
Establish Market Access Strategies: Establishing market access strategies is essential for overcoming barriers to market entry for medtech companies, with early engagement with payers and healthcare providers being vital.
Grasping reimbursement pathways and establishing these connections can greatly ease access, particularly in a sector where heart disease continues to be a primary cause of death, with more than 735,000 heart attacks happening each year in the U.S. This focus on early access to the industry is crucial for overcoming barriers to market entry for medtech companies aiming to create a significant influence. Moreover, examining financial metrics such as BAC stock performance (currently at 47.11, up by 0.40 points or 0.86%) can offer a wider financial perspective that impacts strategic planning choices.
By adhering to these steps, healthcare technology firms can position themselves strategically in a competitive environment, which is essential for overcoming barriers to market entry for medtech companies and achieving sustainable growth. Our services also include comprehensive project management and monitoring throughout the clinical trial process, ensuring that every stage is executed efficiently. Effective reporting on study status, inventory, and both serious and non-serious adverse events enhances transparency and trust with stakeholders.
Implementing the Strategic Plan
To effectively execute a tactical plan in the medtech sector, companies must consider several key approaches:
- Assign Responsibilities: It is imperative to clearly define roles and responsibilities within the team. This ensures accountability across all aspects of the plan, which is crucial given that nearly half of all project spending is at risk of being wasted due to a lack of effective team-based communication. As Bob Kantor, Principal, notes, 'Effective communication is the backbone of any successful project.'
- Establish Timelines: Setting realistic timelines for each phase of implementation is essential. Doing so helps ensure that milestones are met, keeping the project on track and facilitating timely entry.
- Monitor Progress: Regularly reviewing progress against the strategic plan is vital. Employing key performance indicators (KPIs) to assess success enables data-informed modifications to plans as needed, promoting a proactive approach to project management. This includes monitoring study status, inventory, and serious and non-serious adverse events.
- Maintain Open Communication: Cultivating a culture of open communication within the team and with stakeholders is crucial. This practice allows for prompt and collaborative resolution of challenges, enhancing team cohesion and project success.
- Adjust to Changes: The capability to modify the plan in reaction to new information, regulatory shifts, or economic dynamics is essential. In Colombia, for instance, understanding the role of INVIMA as a Level 4 health authority in medical device oversight is critical for ensuring compliance and successful trial setup, including the feasibility and selection of research sites and principal investigators (PIs).
- Document Lessons Learned: Keeping detailed records of the implementation process, including successes and challenges faced, is important. This documentation not only informs future approaches to new ventures but also aids in ongoing enhancement of overall processes. Furthermore, understanding the influence of medical technology clinical studies on regional economies—such as job creation and healthcare advancements—can further validate investments in these sectors.
By implementing these approaches, medical technology firms can improve their project management practices and increase their chances of overcoming barriers to market entry for medtech companies during successful product introduction. The RASCI model, as highlighted in the case study, ensures that all vital personnel are engaged and supported throughout the process, ultimately contributing to improved project outcomes.
Evaluating Market Entry Success
To effectively assess entry success, medtech companies should undertake the following strategies:
-
Analyze Key Metrics: Regularly review critical data such as sales figures, industry share, and customer feedback. This quantitative analysis acts as a basis for evaluating product performance and comprehending economic dynamics, particularly in the context of overcoming barriers to market entry for Medtech companies. Notably, the total first-page keywords show a 20% growth year-over-year, highlighting the importance of monitoring these metrics to gauge market presence.
-
Solicit Feedback: Actively gather insights from key stakeholders, including healthcare providers and patients. Their experiences and satisfaction levels provide invaluable information that can inform product enhancements and marketing strategies. Recent trends indicate a growing emphasis on customer feedback statistics in medtech, highlighting the importance of overcoming barriers to market entry for medtech companies to significantly influence product development and positioning.
-
Review Regulatory Compliance: Ensure that all regulatory requirements, particularly those outlined by INVIMA, have been meticulously met. INVIMA, as Colombia's National Food and Drug Surveillance Institute, oversees compliance and quality assurance for health products. An evaluation of compliance not only protects the company’s reputation but also provides insights into how adherence affects overall performance, which is crucial for overcoming barriers to market entry for Medtech companies, particularly considering INVIMA's classification as a Level 4 health authority by PAHO/WHO.
-
Conduct Post-Mortem Analysis: After a defined period, engage in a comprehensive analysis of the market entry process. Identify both successful elements and shortcomings in the context of overcoming barriers to market entry for medtech companies to gain a nuanced understanding of what approaches worked and which did not. This reflective practice is essential for ongoing enhancement, highlighting the necessity to modify approaches based on assessments.
-
Modify Approaches for Future Involvements: Utilize the knowledge acquired from assessments to enhance and adjust methods for upcoming engagements. Incorporating lessons learned is essential for overcoming barriers to market entry for Medtech companies, as it enhances the likelihood of success in future endeavors.
-
Celebrate Successes: Acknowledge and celebrate the achievements within the team. Recognizing milestones is essential for overcoming barriers to market entry for Medtech companies, as it fosters a culture of motivation and reinforces a commitment to continuous improvement. As Podymos aptly puts it,
Book a call with our team to break away from the same old way of doing things and create disruptive sales and marketing channels that set you apart, ensuring your device is impossible to miss.
This mindset is essential for overcoming barriers to market entry for medtech companies, driving innovation, and maintaining competitive advantage in the medical technology landscape.
-
Utilize Case Studies: For instance, analyzing engagement rates in emails, similar to CTR in social media, shows that A/B testing can improve engagement by identifying layouts that drive better interaction. This not only enhances customer feedback but also informs strategies for overcoming barriers to market entry for Medtech companies. Furthermore, comprehensive clinical trial management services, including feasibility studies, trial setup processes, and project management, are vital for optimizing the overall process. The role of the principal investigator (PI) is crucial in overseeing trial execution, ensuring compliance with protocols, and maintaining participant safety. Additionally, the economic impact of medtech clinical studies can lead to job creation and healthcare improvements, fostering international collaboration and growth.
Conclusion
Navigating the complexities of the medtech industry requires a strategic approach to overcome the significant barriers that companies face. This article has highlighted the critical challenges, including:
- Regulatory hurdles
- Funding difficulties
- Market access issues
- Competition
- Rapid technological advancements
By understanding these obstacles, medtech firms can develop and implement effective strategies that facilitate successful market entry and sustainable growth.
Developing a comprehensive strategic plan is essential for addressing these barriers. Key steps include:
- Conducting a SWOT analysis
- Setting clear objectives
- Engaging stakeholders
- Formulating robust regulatory and funding strategies
Furthermore, implementing the plan with well-defined responsibilities, realistic timelines, and continuous monitoring will enable companies to adapt to changing circumstances and maintain alignment with their goals.
Ultimately, the success of medtech companies hinges on their ability to evaluate their market entry strategies effectively. By analyzing key performance metrics, soliciting stakeholder feedback, reviewing regulatory compliance, and celebrating achievements, firms can refine their approaches for future endeavors. Embracing these practices not only enhances the likelihood of success but also positions companies to thrive in a competitive and rapidly evolving landscape. As the medtech sector continues to advance, those that proactively address these challenges will emerge as leaders in innovation and patient care.
Frequently Asked Questions
What are the primary barriers faced by medtech companies when entering the industry?
Medtech companies encounter several significant barriers, including regulatory hurdles, funding challenges, market access issues, competition and industry saturation, and technological advancements.
What do regulatory hurdles entail for medtech firms?
Regulatory hurdles involve navigating complex regulations set by authorities like the FDA and EMA, requiring a deep understanding of clinical trial requirements, product approvals, and post-market surveillance. Compliance is essential and can significantly impact the time and resources needed for market participation.
Why is funding a challenge for medtech startups?
Securing sufficient funding for research and development is critical yet challenging for medtech startups due to perceived risks associated with clinical trials. A strong business plan and clearly articulated value propositions are essential for attracting investors.
What market access issues do medtech companies face?
Medtech companies must engage with payers and effectively demonstrate the clinical and economic value of their products to gain access to healthcare systems and reimbursement pathways, which is vital for product adoption by healthcare providers.
How does competition affect medtech companies?
The medical technology sector is highly competitive, requiring companies to understand the competitive landscape and differentiate their products effectively. Continuous assessment of their market positioning is crucial for remaining relevant.
What impact do technological advancements have on medtech products?
Rapid technological advancements can quickly make existing products obsolete, necessitating that companies stay informed about emerging innovations and adapt to changing industry needs to maintain competitiveness.
How can bioaccess® assist medtech companies in overcoming these barriers?
Bioaccess® provides accelerated medical device clinical study services and offers strategic insights, links to potential investors, and customized solutions to simplify the regulatory process and assist in funding, ensuring a comprehensive strategy for market entry.
What role do regulatory bodies like INVIMA play in market access for medtech companies?
Understanding the functions of regulatory bodies such as INVIMA in Colombia can enhance a company's approach to market access by clarifying the regulatory landscape and its influence on product launch strategies.




