Overview
This article primarily addresses the pressing need to overcome challenges in clinical trial design within Colombia, emphasizing strategies that enhance the efficiency and effectiveness of research studies. It underscores the critical importance of local expertise, regulatory engagement, and robust community relationships. By adopting patient-centric approaches and leveraging advanced technological tools, significant improvements can be made in recruitment, retention, and overall study success in the Colombian healthcare landscape.
Introduction
Colombia has emerged as a significant player in the clinical trials arena, particularly in the realm of medical technology. With a diverse patient population and a regulatory framework that is evolving to meet international standards, the country presents a unique landscape for clinical research.
However, navigating this environment comes with its own set of challenges, from regulatory delays to patient recruitment issues.
As stakeholders seek to leverage Colombia's potential for clinical trials, understanding the intricacies of its healthcare system and the cultural context becomes paramount.
This article delves into the multifaceted aspects of conducting clinical trials in Colombia, highlighting the opportunities and obstacles that Medtech companies face in this dynamic setting.
Understanding the Landscape of Clinical Trials in Colombia
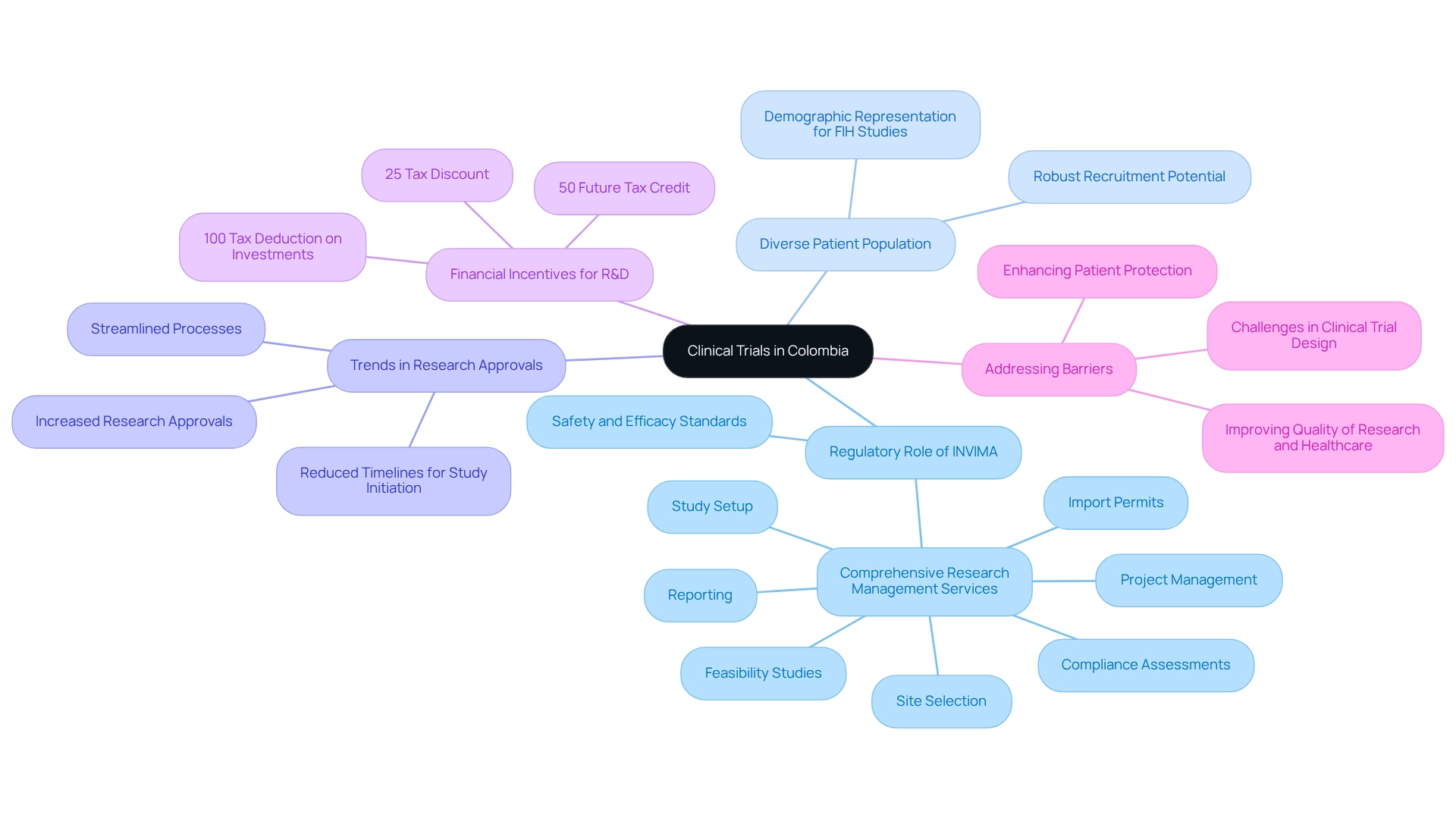
This nation has positioned itself as a crucial participant in the research landscape, particularly within the medical technology field. Designated as an upper-middle-income (UMC) nation by the World Bank, Colombia's economic condition significantly influences its research environment in healthcare. The nation is characterized by a diverse population, which is essential for successful study recruitment, and a regulatory system overseen by the Instituto Nacional de Vigilancia de Alimentos y Medicamentos (INVIMA).
Understanding the challenges in clinical trial design for Colombia—such as the complexities of the local healthcare system, patient demographics, and cultural nuances—is vital for crafting effective trials.
The Colombian government, in collaboration with INVIMA, has made substantial strides in enhancing the regulatory framework, aligning it more closely with international standards. This commitment is reflected in the research support services market, which generated a revenue of USD 79.7 million in 2024 and is projected to reach USD 126.7 million by 2030, indicating a strong growth trajectory. The market is expected to expand at a compound annual growth rate (CAGR) of 8.1% from 2025 to 2030, with Phase III studies being the largest revenue-generating segment in 2024, while Phase I studies are anticipated to exhibit the fastest growth.
Key factors for navigating the research landscape in South America include:
- INVIMA's Regulatory Role: INVIMA plays a critical role in overseeing studies, ensuring they meet safety and efficacy standards while facilitating a smoother approval process. This encompasses comprehensive research management services such as feasibility studies, site selection, compliance assessments, study setup, import permits, project management, and reporting, all of which are provided by bioaccess®.
- Diverse Patient Population: The rich diversity of the nation's population enhances the potential for robust recruitment, allowing for a wide range of demographic representation, which is crucial for first-in-human (FIH) studies.
- Trends in Research Approvals: Recent regulatory reforms have led to an increase in research approvals, streamlining processes and reducing timelines for study initiation, making the nation an attractive destination for Medtech companies.
- Financial Incentives for R&D: The country offers significant financial incentives for R&D, including a 100% tax deduction on investments in science, technology, and innovation projects, a 25% tax discount, and a 50% future tax credit, making it economically advantageous for companies to conduct studies in the region.
- Addressing Barriers: Effectively addressing the challenges in clinical trial design for Colombia is crucial for enhancing patient protection and improving the overall quality of research and healthcare in the region.
By addressing these factors, stakeholders can effectively leverage the local research environment to advance medical technology innovations, ultimately improving patient outcomes and healthcare quality in the area. With bioaccess® as a prominent CRO in Latin America, companies can benefit from expedited research services tailored to their needs.
Identifying Key Challenges in Clinical Trial Design
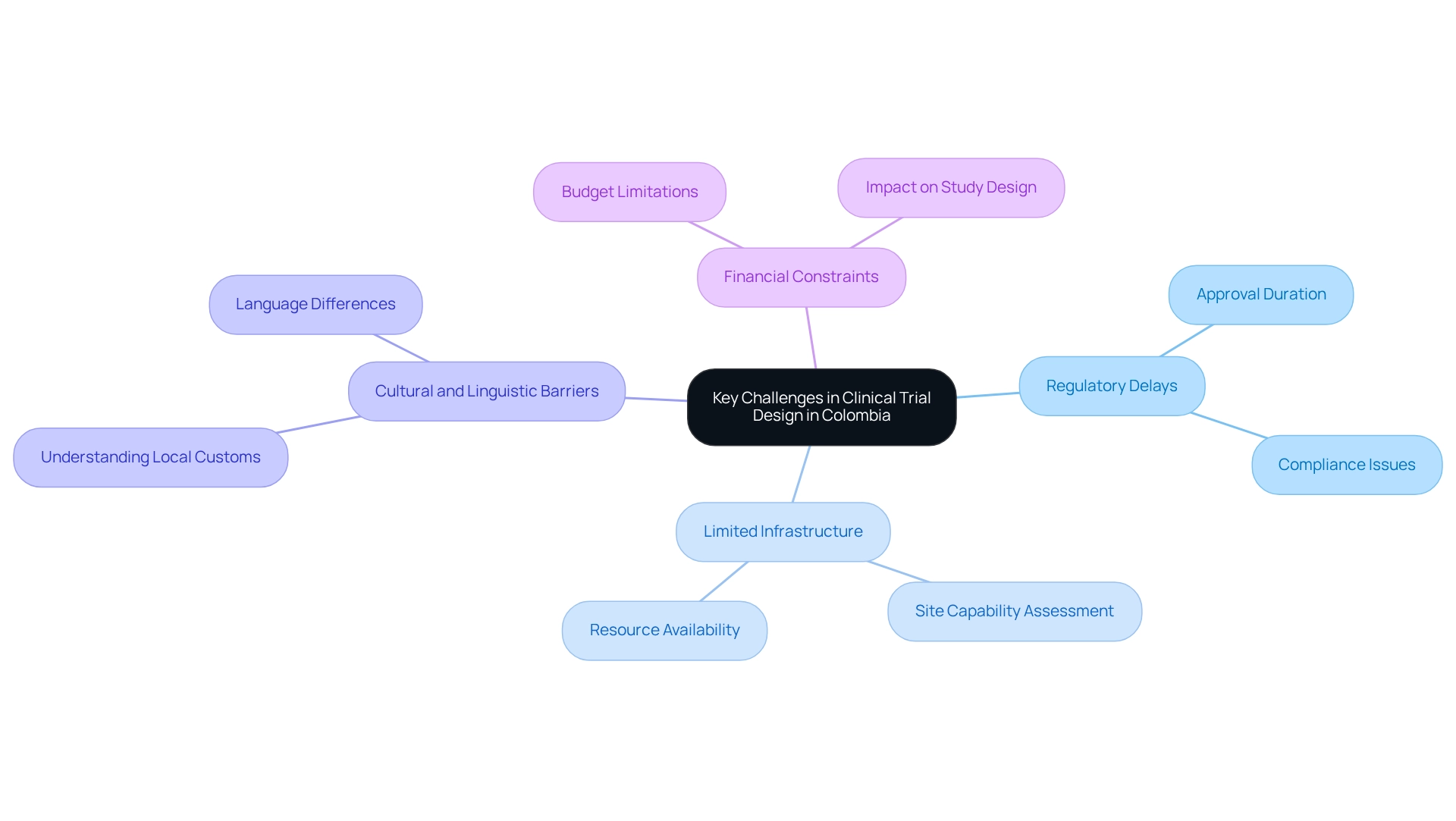
Creating medical studies in Colombia necessitates navigating the challenges inherent in clinical trial design, which can significantly impact the research process. Key obstacles include:
-
Regulatory Delays: The approval process for research studies can be protracted, often extending over several months due to bureaucratic complexities. These delays hinder timely access to critical data and slow down the overall research timeline. For instance, the typical approval duration for medical studies can exceed six months, posing considerable challenges for Medtech firms aiming to expedite their research. bioaccess™ offers extensive assistance in overcoming these regulatory hurdles, ensuring compliance with INVIMA, the Colombian National Food and Drug Surveillance Institute, and streamlining the approval process through services such as compliance evaluations and study setup.
-
Limited Infrastructure: While there has been progress in the healthcare sector, certain areas in Colombia still lack the essential infrastructure to support complex studies. This limitation can compromise the quality and reliability of the data collected, making it crucial for researchers to thoroughly assess the capabilities of potential sites. Collaborations like that of bioaccess™ with Caribbean Health Group aim to enhance the infrastructure and resources available for clinical trials in Barranquilla, positioning it as a premier location for research.
-
Cultural and Linguistic Barriers: A profound understanding of local customs, languages, and participant perceptions is vital for effective recruitment and retention in clinical trials. Cultural nuances can influence individuals' willingness to participate, necessitating a culturally sensitive approach from researchers. Additionally, linguistic differences can result in misunderstandings that may affect compliance and data integrity. bioaccess™ underscores the importance of local knowledge in tackling these challenges, ensuring that studies are designed with the needs of the participant group in mind.
-
Financial Constraints: Budget limitations often restrict the scope of research trials, impacting the ability to implement advanced methods and technologies. This financial pressure may lead to compromises in study design and execution, ultimately affecting the quality of outcomes. As noted by Max Baumann from Tree Hill Partners, the crowded health end-markets in biotech present fundamental business model challenges that can intensify these financial constraints. Bioaccess™ provides strategic project management and reporting services to assist Medtech companies in effectively optimizing their budgets and resources, while also addressing the challenges in clinical trial design specific to Colombia. This requires a strategic approach that considers the unique characteristics of the Colombian healthcare system and the specific needs of the patient population. By leveraging local expertise and fostering cooperation with stakeholders, such as the alliance between bioaccess™ and Caribbean Health Group, Medtech companies can enhance the efficiency and effectiveness of their research in Colombia. Furthermore, emphasizing the improvement of result assessments in medical studies, particularly through standardized outcome metrics, is crucial for supporting medical guidelines and advancing the evaluation of innovative treatments, as highlighted in recent case analyses. As Ariel Katz, CEO of H1, points out, regulatory changes can significantly influence the number of new medications progressing through evaluations and reaching the market, ultimately limiting treatment options for individuals.
Patient-Level Barriers: Recruitment and Retention Issues
Recruitment and retention of participants are paramount for the success of research studies, particularly in Colombia, where various obstacles can impede these processes.
-
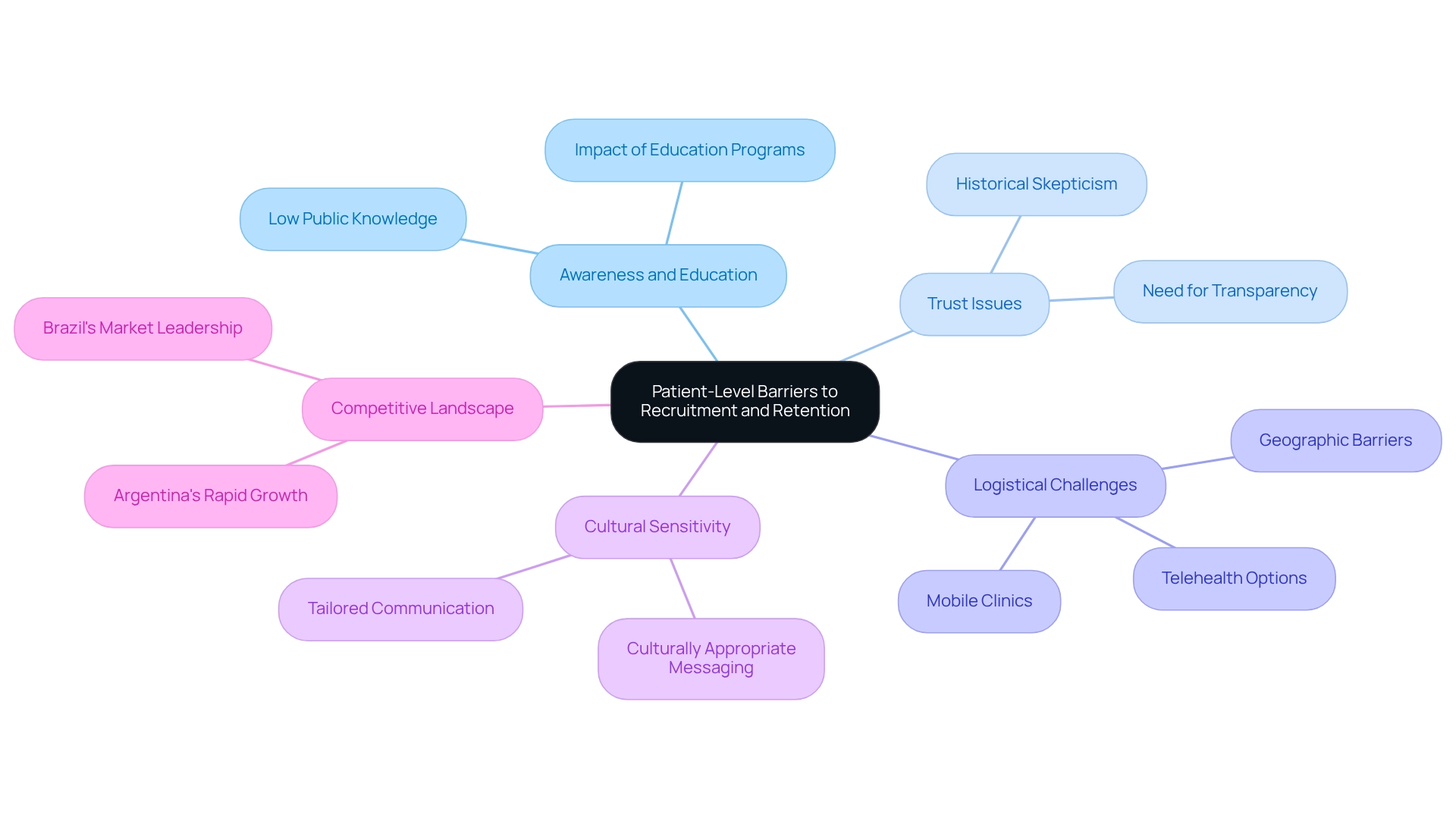
Awareness and Education: A significant challenge lies in the lack of awareness among potential participants regarding research studies and their objectives. Many individuals may not fully comprehend the benefits or implications of participation, contributing to low enrollment rates. Statistics reveal that knowledge of medical studies among the general public remains alarmingly low, underscoring the necessity for impactful educational programs.
-
Trust Issues: Historical skepticism surrounding medical research, stemming from past unethical conduct, continues to deter individuals from engaging in studies. This skepticism is exacerbated by a lack of transparency in clinical studies, as illustrated by initiatives like the All Trials petition, which advocates for the registration and reporting of all clinical studies. Establishing trust through transparent communication and community involvement is essential. As David Vizcaya noted, the accessibility of secondary data sources for real-world evidence (RWE) studies in the country is poorly documented, complicating recruitment efforts further.
-
Logistical Challenges: Geographic barriers and transportation issues can significantly hinder patient attendance at study visits. Numerous prospective participants reside in remote regions, making it challenging to access study locations. Addressing these logistical challenges through mobile clinics or telehealth options can enhance participation rates.
-
Cultural Sensitivity: Tailoring communication and engagement strategies to resonate with local populations is crucial for fostering trust and participation. Understanding cultural nuances and employing culturally appropriate messaging can significantly enhance outreach initiatives and participant involvement.
The competitive landscape in Latin America, with Brazil expected to lead the research market and Argentina experiencing rapid growth, emphasizes the need for effective recruitment strategies in the region. To effectively tackle the challenges in clinical trial design for Colombia, researchers should implement targeted outreach programs that prioritize education and awareness, utilize community engagement strategies to build trust, and ensure that informed consent processes are clear and culturally appropriate. By adopting these strategies, research studies in the country can enhance participant recruitment and retention, ultimately leading to more successful research outcomes.
Furthermore, bioaccess® aims to expedite the advancement of medical devices through expertise in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). This customized approach is vital for overcoming recruitment and retention challenges. Additionally, understanding the regulatory landscape and the role of INVIMA in overseeing research studies is crucial for navigating the complexities of conducting investigations in Colombia. The impact of Medtech research studies on local economies, including job creation and healthcare enhancement, further underscores the significance of efficient recruitment and retention strategies.
Clinician and Healthcare System Barriers to Effective Trials
The success of medical studies hinges not only on patient involvement but also significantly on clinician participation and the healthcare system's resources. Several key barriers impede progress in this area:
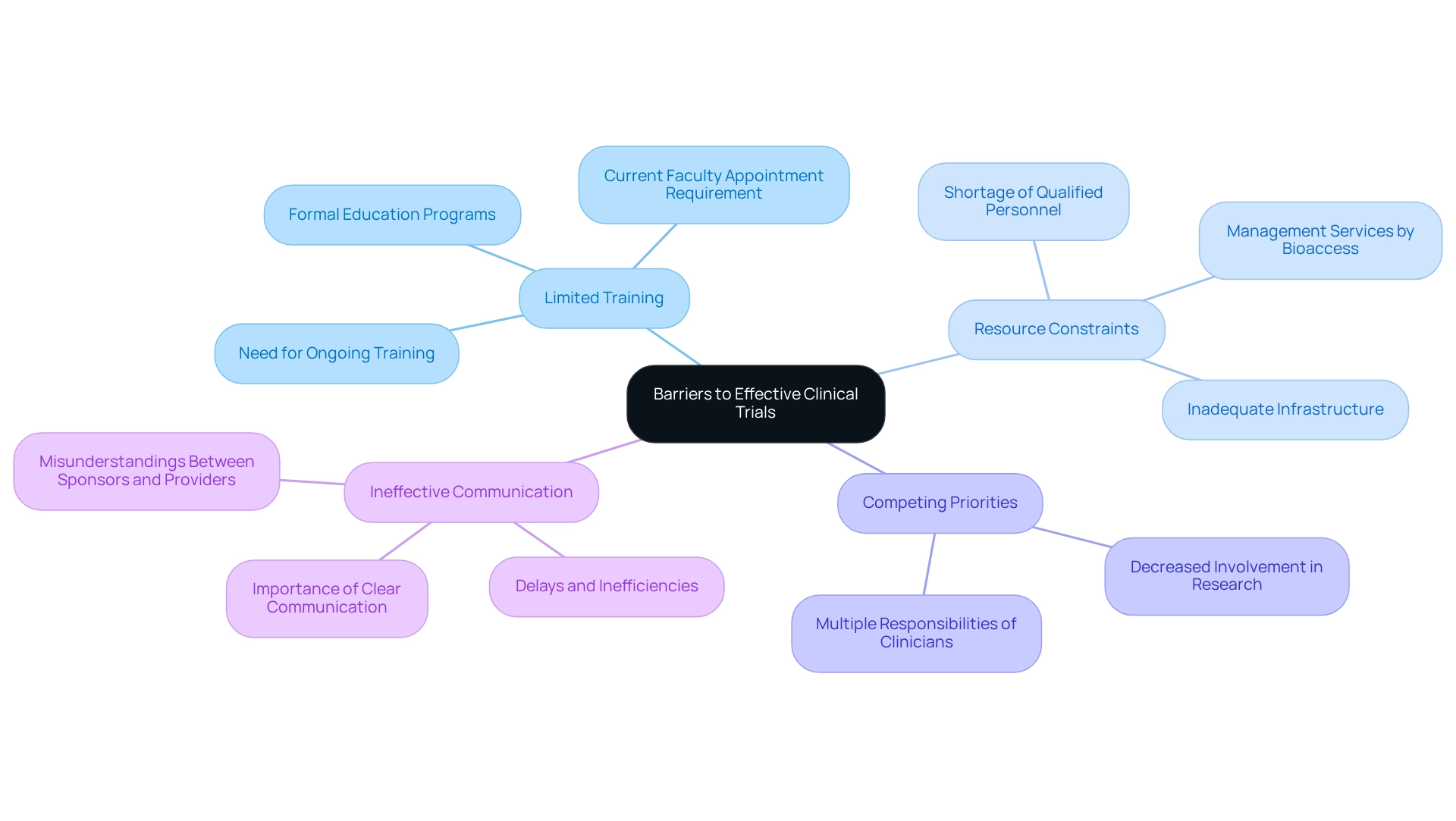
- Limited Training: A notable number of clinicians may not receive adequate training in clinical trial protocols, resulting in inconsistent implementation and adherence to study guidelines. This gap in knowledge can undermine the integrity of the experiments. In fact, applicants for research positions must have a current faculty appointment within 15 years from their first faculty appointment, underscoring the need for ongoing training and development.
- Challenges in clinical trial design for Colombia arise from resource constraints, as numerous healthcare establishments encounter difficulties due to inadequate resources, such as a shortage of qualified personnel and essential infrastructure, which are vital for the efficient implementation of medical studies. Bioaccess addresses these concerns by offering extensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, and reporting on serious and non-serious adverse events, ensuring that facilities are prepared to conduct studies efficiently.
- Clinicians face challenges in clinical trial design for Colombia due to competing priorities, as their multiple responsibilities can detract from their focus on clinical research. This juggling act can result in decreased involvement in studies and impede the overall advancement of research initiatives.
- Ineffective communication between study sponsors and healthcare providers highlights the challenges in clinical trial design for Colombia, leading to misunderstandings, delays, and inefficiencies that can ultimately affect study outcomes. Bioaccess emphasizes the importance of clear communication and project management in its services, which helps streamline operations and enhance collaboration.
To tackle the challenges in clinical trial design for Colombia, it is essential to establish thorough training programs for healthcare professionals, ensuring they are well-informed about research protocols. For instance, CRM students receive formal training in skills essential for designing and analyzing research studies involving human subjects, which emphasizes the importance of training in overcoming these challenges. Additionally, the Advanced Clinical Research Training Institute in Latin America provides ongoing training and mentorship to address the challenges in clinical trial design for Colombia, focusing on skill enhancement through workshops and one-on-one faculty meetings, which can help bridge the training gap.
Ultimately, encouraging open lines of communication among all parties involved in the process will help bridge gaps and enhance operations. As K. Kimberly McCleary, Founder & CEO of The Kith Collective, observes, "Effective communication and training are essential for improving clinician involvement in research studies." This method, backed by Bioaccess's dedication to enabling medical device studies in Latin America, will result in more successful research outcomes in that region.
Navigating Regulatory Challenges in Clinical Trials
Challenges in clinical trial design for Colombia create substantial regulatory barriers in the clinical research environment, significantly impacting the efficiency and success of Medtech firms. Key issues include:
-
Approval Timelines: The approval process in Colombia can be notably lengthy, with the median time for regulatory approval reported at 236 days during the ALTTO study, highlighting a range that can extend from 21 to 257 days. Such delays can hinder trial initiation, ultimately affecting project timelines and budgets. Significantly, this nation currently ranks fourth in Latin America for clinical studies per million people, underscoring the growing importance of the region for clinical research.
-
Documentation Requirements: INVIMA, the regulatory authority in Colombia, mandates extensive documentation that can be daunting, especially for researchers unfamiliar with the local market. This complexity necessitates a thorough understanding of the specific requirements to ensure compliance and expedite the approval process. bioaccess® offers comprehensive support in reviewing and providing feedback on study documents to comply with country requirements, significantly easing this burden.
-
Changing Regulations: The regulatory environment is dynamic, with frequent updates that can introduce uncertainty. Researchers must remain alert and flexible, as these changes may necessitate continuous modifications to study protocols. As Evandro de Azambuja from the Clinical Trials Support Unit at the Jules Bordet Institute notes, "Navigating the regulatory landscape requires not only knowledge but also a proactive approach to adapt to changes." bioaccess®'s expertise in study preparation, start-up, and approval processes ensures that Medtech firms are well-equipped to manage these evolving regulations.
To effectively navigate the challenges in clinical trial design for Colombia, researchers must engage with regulatory experts who possess in-depth knowledge of the Colombian landscape. Staying informed about regulatory changes and preparing comprehensive documentation can significantly streamline the approval process. Moreover, involvement in research studies provides individuals access to cutting-edge therapies and enhanced healthcare experiences. Case studies illustrate that research studies offer substantial health alternatives for individuals, particularly in fields such as diabetes and oncology.
Overcoming these regulatory hurdles is essential to advance medical technology in the region. bioaccess®'s expertise in feasibility studies, site selection, import permits, project management, and customized approaches can help Medtech companies bring their devices to market sooner.
Strategies for Overcoming Challenges in Clinical Trial Design
To effectively address the challenges encountered in clinical study design in Colombia, stakeholders can adopt several strategic approaches:
- Engage Local Experts: Collaborating with local clinical research organizations (CROs) like bioaccess™ is crucial. These specialists provide essential perspectives on the regulatory environment and participant demographics, greatly improving study design and implementation. As noted by the Head of Clinical Information Engineering, 'Historically, information management was delegated to our CRO vendor partners,' emphasizing the significance of local expertise.
- Utilize Technology: Employing digital tools for participant recruitment and data management streamlines processes, enhances efficiency, and enables real-time monitoring of study progress. This is particularly relevant in Colombia, where the healthcare system is ranked among the best globally, ensuring high-quality data collection and management.
- Foster Community Relationships: Establishing trust within communities through outreach and education initiatives leads to improved patient recruitment and retention rates. Interacting with local communities clarifies research studies and promotes involvement, particularly in a nation where approximately 95 percent of the population is covered by universal healthcare.
- Adapt Protocols: Tailoring research protocols to correspond with local customs and cultural standards is crucial. This adjustment not only improves practicality but also boosts participant involvement, addressing the challenges in clinical trial design for Colombia and making studies more pertinent to the local context.
Alongside these approaches, bioaccess™ provides thorough services including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting, which are essential for successful research. Furthermore, this country offers attractive R&D tax incentives, such as a 100% tax deduction for investments in science, technology, and innovation projects, making it an appealing destination for Medtech companies. The partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a prominent location for medical studies in Latin America, backed by the Colombian Minister of Health.
Moreover, the recruitment challenge in the U.S. has resulted in heightened foreign investment in medical research in Latin America, rendering the nation an even more appealing option.
The Role of Collaboration in Enhancing Clinical Trials
Cooperation is crucial for overcoming the challenges in clinical trial design for Colombia and enhancing the efficiency of medical studies in that country. Key components include:
-
Partnerships with Local Institutions: Collaborating with local hospitals and research centers is vital for facilitating patient recruitment and accessing necessary resources. The country features 135 accredited research facilities and 76 approved ethics committees, offering a strong structure for performing medical studies. A notable example of the challenges in clinical trial design for Colombia is the recruitment process for survey participants, where a new database was created to compile emails of individuals linked to various health and life sciences institutions. This initiative successfully engaged a diverse range of participants, ensuring comprehensive representation in the survey results. By leveraging these local institutions, Medtech companies can enhance their operational efficiency and ensure compliance with ethical standards. The partnership between bioaccess™ and Caribbean Health Group illustrates this strategy, seeking to establish Barranquilla as a premier location for research in Latin America, backed by the Minister of Health of the country.
-
Engagement with Regulatory Bodies: Establishing open lines of communication with INVIMA and other regulatory authorities is crucial for navigating the approval process. As emphasized by Julio G. Martinez-Clark, CEO, "The government of Colombia appears to be the sole nation in Latin America with a proactive effort to draw more research studies as part of its strategy to transform into a knowledge-based economy by 2031." This proactive approach highlights the significance of working together with regulatory entities to simplify procedures and cultivate a supportive atmosphere for research involving patients, particularly in light of the challenges in clinical trial design for Colombia.
-
Involvement of Advocacy Organizations: Collaborating with advocacy groups can greatly enhance awareness and confidence in clinical studies, resulting in higher participation rates. By engaging these groups, researchers can gain improved insights into patient needs and concerns, which is essential for overcoming the challenges in clinical trial design for Colombia and enhancing the implementation of studies.
-
Cross-Disciplinary Collaboration: Promoting cooperation among different fields nurtures innovation and enhances study design. This method not only improves the quality of research but also addresses the challenges in clinical trial design for Colombia by guaranteeing that varied viewpoints are taken into account, resulting in more thorough and impactful studies. The insights shared by industry leaders, including Dushyanth Surakanti during bioaccess®'s initial human study, emphasize the importance of such collaborations.
By encouraging cooperation among all parties involved, including local organizations, regulatory entities, advocacy groups, and interdisciplinary teams, we can effectively address the challenges in clinical trial design for Colombia, allowing research studies in the country to be planned and carried out more efficiently. This collaborative effort aligns with the roadmap for public policy in health and STI in Colombia over the next 20 years, ultimately resulting in improved patient outcomes. Furthermore, bioaccess™ provides extensive services such as feasibility studies, site selection, compliance assessments, setup for experiments, import permits, project management, and reporting, which are essential for the success of research studies.
The partnership was officially revealed on March 29, 2019, signifying an important advancement in improving medical research in Barranquilla.
Conclusion: Moving Forward with Improved Clinical Trial Design
Successfully navigating the challenges in clinical trial design for Colombia necessitates a comprehensive strategy that addresses various dimensions of the medical research landscape. This approach requires a profound understanding of local dynamics, tackling both patient-level and systemic barriers, and adeptly managing regulatory complexities. Furthermore, cooperation among stakeholders is vital to create an atmosphere conducive to efficient research studies.
bioaccess offers a range of thorough research management services that significantly aid in this process. Their capabilities encompass:
- Feasibility studies
- Site selection
- Compliance evaluations
- Contributions to study documents to meet country requirements
- Experiment setup
- Import permits
- Project management
- Comprehensive reporting on study status and adverse events
By leveraging these services, researchers can streamline their research processes and enhance adherence to local regulations.
Recent data reveals that inter-trial intervals have markedly improved, now averaging 17 months of total development time, a reduction from a peak of 32 months in 2022. This decrease underscores the ongoing efforts to simplify research study processes, which is essential for enhancing the effectiveness of research initiatives.
While Colombia's research market represents a mere 0.2% of worldwide revenue in 2023, it holds significant potential for expansion. The opportunity for groundbreaking research strategies to thrive in the region is underscored by Brazil's anticipated leadership in the regional market by 2030 and Argentina's emergence as the fastest-growing market, with projections to reach USD 506.1 million by the same year. These trends suggest that, despite its modest share, the market in Colombia has substantial room for growth.
To improve research design in the country, investigators should consider strategies that leverage local knowledge and resources. Engaging with local regulatory bodies early in the process can facilitate smoother approvals and enhance compliance. Additionally, adopting patient-centric approaches can boost recruitment and retention rates, ultimately leading to more robust data collection.
Expert opinions emphasize that optimizing the development journeys of medical assets is crucial not only for securing regulatory approval but also for ensuring commercial success. As Max Baumann, Head of Execution, stated, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." By concentrating on these strategies, researchers can significantly enhance the efficiency of their studies, thereby advancing medical knowledge and improving patient outcomes in the country.
Moreover, the biotech sector faces critical business model challenges as medical markets become saturated and innovative breakthrough medications are scarce. Addressing the obstacles in clinical trial design for Colombia is essential for fostering a sustainable clinical trial environment.
Conclusion
Successfully navigating the complexities of clinical trial design in Colombia necessitates a multifaceted approach that addresses the unique challenges inherent to the region. Stakeholders must cultivate a profound understanding of local healthcare dynamics while tackling barriers at both the patient and systemic levels. Furthermore, regulatory complexities demand collaboration among all involved parties to create an environment that fosters effective clinical research.
The services offered by bioaccess play a pivotal role in facilitating this process. With expertise in feasibility studies, compliance reviews, and project management, bioaccess equips researchers with the essential tools to streamline clinical trials and ensure adherence to local regulations. Recent improvements in inter-trial intervals underscore the progress being made, significantly reducing development times and enhancing the overall efficiency of research initiatives.
While Colombia's clinical trials market currently represents a small fraction of global revenue, the potential for growth is substantial. With neighboring countries like Brazil and Argentina poised to lead the regional market, Colombia stands at the threshold of expanding its clinical trial capabilities. By harnessing local expertise and resources, researchers can enhance their trial designs, streamline regulatory approvals, and adopt patient-centric strategies to improve recruitment and retention.
As the biotech industry faces increasing challenges, optimizing the clinical development journey becomes critical not only for regulatory success but also for commercial viability. By embracing these strategies, researchers in Colombia can significantly advance medical knowledge and improve patient outcomes, ultimately contributing to a more sustainable clinical trial environment in the region.
Frequently Asked Questions
What is Colombia's role in the medical technology research landscape?
Colombia is a crucial participant in the research landscape, particularly in medical technology, and is designated as an upper-middle-income nation by the World Bank, influencing its healthcare research environment.
What factors contribute to the research environment in Colombia?
Key factors include a diverse population for study recruitment, a regulatory system overseen by INVIMA, and recent regulatory reforms that streamline research approvals.
What is INVIMA's role in clinical trials in Colombia?
INVIMA oversees studies to ensure they meet safety and efficacy standards, facilitating a smoother approval process and providing comprehensive research management services.
How does the patient population in Colombia benefit clinical trials?
The rich diversity of Colombia's population enhances recruitment potential, allowing for a wide range of demographic representation, which is crucial for first-in-human studies.
What financial incentives does Colombia offer for research and development?
Colombia offers significant financial incentives, including a 100% tax deduction on investments in science, technology, and innovation projects, a 25% tax discount, and a 50% future tax credit.
What are the main challenges in clinical trial design in Colombia?
Challenges include regulatory delays, limited infrastructure, cultural and linguistic barriers, and financial constraints affecting research trials.
How long does the approval process for medical studies typically take in Colombia?
The approval process can exceed six months due to bureaucratic complexities, posing challenges for timely data access.
What is the role of bioaccess™ in navigating the Colombian research landscape?
Bioaccess™ provides extensive assistance in overcoming regulatory hurdles, enhancing infrastructure, and offering strategic project management to optimize budgets and resources for Medtech companies.
Why is cultural understanding important in Colombian clinical trials?
Cultural nuances and linguistic differences can influence participant recruitment and retention, necessitating a culturally sensitive approach to ensure compliance and data integrity.
What is the projected growth of the research support services market in Colombia?
The research support services market is projected to grow from USD 79.7 million in 2024 to USD 126.7 million by 2030, with a compound annual growth rate of 8.1% from 2025 to 2030.




