Introduction
In the highly regulated world of medical devices, the Abbreviated 510(k) submission process stands out as a pivotal mechanism for manufacturers aiming to achieve FDA clearance efficiently. This streamlined pathway allows for the demonstration of substantial equivalence to existing devices, significantly reducing the documentation burden typically associated with traditional submissions.
As the landscape of medical device regulation evolves, understanding the nuances of the Abbreviated 510(k) process becomes essential for manufacturers. With recent statistics indicating a surge in applications and ongoing challenges such as increased panel track wait times, navigating this process effectively can mean the difference between timely market entry and costly delays.
This article delves into the intricacies of the Abbreviated 510(k) submission, providing a comprehensive guide to its preparation, eligibility criteria, benefits, and common challenges faced by manufacturers.
Understanding the Abbreviated 510(k) Submission Process
The abbreviated 510 k procedure provides a simplified route for certain medical instruments to obtain FDA approval. This approach enables manufacturers to demonstrate substantial equivalence to an existing, legally marketed product, referred to as a predicate. In contrast to conventional 510(k) filings, which require extensive documentation, the abbreviated 510 k is intended to streamline the requirements, thus significantly accelerating the review.
According to recent statistics, 737 different 510(k) product codes were applied for in 2019, reflecting the ongoing trend of manufacturers seeking efficient pathways to market. It is important to note that devices cleared through the 510(k) pathway are not marketed as 'approved by FDA,' which is a crucial distinction in understanding the regulatory landscape. Furthermore, recent news shows that panel track wait times have risen from an average of 285.80 days in 2023 to 289.62 days in 2024, emphasizing ongoing challenges in the submission system.
Considering these factors, grasping the nuances of the abbreviated 510 k procedure is essential for manufacturers. It not only facilitates timely market entry but also ensures adherence to FDA regulations, ultimately supporting the goal of increasing the number of 510(k) applications in the U.S. by a projected 50% by the end of 2020, a notable leap from the 4% baseline anticipated at the program's 2011 inception. Additionally, the situation with Artivion, which encountered interruptions in its shipping operations due to a ransomware attack, highlights the weaknesses in operational capabilities within the medical equipment sector.
Professionals such as Ana Criado, with her vast experience in regulatory matters and biomedical engineering, and Katherine Ruiz, who focuses on Regulatory Affairs for medical products and in vitro diagnostics, can offer essential perspectives on maneuvering through the intricacies of the 510(k) application process. Their expertise not only enhances understanding but also assists manufacturers in ensuring compliance and improving operational capabilities as the landscape of medical submissions evolves.
Step-by-Step Guide to Preparing Your Abbreviated 510(k) Submission
- Identify Your Predicate Instrument: Begin by conducting thorough research to select a legally marketed item that closely resembles your product. This predicate instrument is crucial as it establishes substantial equivalence, a key requirement in the 510(k) process. Successful predicate identification is vital, as it significantly influences the result of your work. In fact, the success rates of abbreviated 510(k) submissions can vary by product type, highlighting the importance of strategic selection.
- Gather Required Documentation: Assemble all necessary documentation, which should include comprehensive device descriptions, intended use statements, labeling, and performance testing data. It's essential to ensure that this information is complete and accurate, as any gaps could delay the review process. Significantly, the Medical Device User Fee Cover Sheet must be included, as it is necessary for processing abbreviated 510(k) applications, excluding third-party reviews. This cover sheet is critical for ensuring that your entry is processed efficiently.
- Prepare a Summary of Safety and Effectiveness: Develop a thorough summary that articulates how your device meets safety and effectiveness standards in comparison to the chosen predicate. This document is critical for demonstrating compliance with FDA expectations and should be meticulously crafted to reflect all necessary details. Ana Criado, our Director of Regulatory Affairs with extensive experience in regulatory agencies, emphasizes that clear comparisons are essential for regulatory success.
- Complete the Application Form: Accurately fill out the FDA's 510(k) application form. Ensure that all sections are completed with the required information. Pay special attention to the 510(k) Statement, which necessitates leaving the space for the 510(k) number blank until it is received in the acknowledgment letter, as per updated guidance. This step is crucial for avoiding any delays in processing your application.
- Submit the Application: Compile all documentation and send your application through the FDA’s electronic gateway. Adherence to guidelines for handing in work is paramount; any deviations could result in rejection or delays in processing. Notably, statistics indicate that in 2022, 30% of 510(k) applications were not accepted for initial review, underscoring the importance of meticulous preparation.
- Respond to FDA Queries: Be prepared to engage with the FDA regarding any questions or requests for additional information that may arise during the review process. Timely and clear communication can significantly influence the approval timeline and result of your proposal. Furthermore, keep in mind that all medical instruments, even those exempt from 510(k) filings, must comply with the Quality System Regulation (QSR) detailed in 21 CFR 820. Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, emphasizes the significance of strong communication strategies during this phase.
Eligibility Criteria for Abbreviated 510(k) Submissions
To qualify for the Abbreviated 510(k) submission, a medical instrument must adhere to several essential criteria:
- Substantial Equivalence: The equipment must demonstrate substantial equivalence to a predicate product that has already received FDA clearance. This foundational requirement is crucial for ensuring that the new apparatus can be safely and effectively used in a clinical setting.
- Device Type: The equipment must belong to a specific category eligible for the abbreviated 510(k) pathway, predominantly encompassing those classified as low to moderate risk. This categorization helps streamline the approval process while maintaining safety standards. In fact, almost half of all medical instruments used in the United States every day have passed through the 510(k) route, highlighting its significance in the medical instrument landscape.
- Guidance Documents: Manufacturers are encouraged to consult FDA guidance documents that delineate specific eligibility requirements pertinent to their device type. These documents offer clarity on the criteria that must be fulfilled for a successful entry. It's important to note that specification developers are responsible for the 510(k) filing even if production is outsourced to a contract manufacturer, ensuring accountability throughout the process.
Professionals in the field, such as Ana Criado, a Director of Regulatory Affairs and a professor with expertise in biomedical engineering and health economics, emphasize the importance of adhering to these guidelines to navigate the regulatory framework effectively. Ana's extensive experience in Regulatory Affairs, including her work with global companies and her role as a consultant, underscores the practical implications of these criteria for manufacturers. If all these criteria are met, manufacturers can confidently proceed with preparing their application, thus contributing to the regulatory environment that ensures the safety and effectiveness of medical products in everyday use.
As Brian J Miller noted, potential policy improvements to the third-party review program are designed to improve the efficiency of FDA medical device regulation, thus freeing up internal agency resources for complex product reviews and over-the-horizon regulatory efforts targeting emerging complex product categories.
Benefits of the Abbreviated 510(k) Submission Pathway
The abbreviated 510 k process presents multiple advantages that are pivotal for manufacturers aiming to efficiently navigate the regulatory landscape. Key benefits include:
-
Reduced Documentation: This pathway requires fewer documentation necessities than the conventional 510(k) filing, thereby streamlining the procedure and lessening administrative burdens.
-
Faster Review Times: The structured nature of the abbreviated 510 k often leads to expedited FDA review times, facilitating quicker access to the market. Recent reports indicate that 41% of De Novo decisions have successfully met the current MDUFA review goals, reflecting the FDA's commitment to improving timeliness. However, the 510(k) program faces challenges such as increasing review volume and the need for thoughtful reforms to maintain this pace.
-
Cost Savings: By minimizing the extent of required testing and documentation, manufacturers can achieve significant cost reductions. The 3Q FY2022 MDUFA report emphasized an 80% approval rate for 510(k) applications, reinforcing the economic benefits of thorough preparation in the application procedure. This high approval rate highlights the significance of an effective strategy for proposals.
-
Enhanced Focus on Innovation: With the regulatory burden lessened, manufacturers can allocate more resources toward innovation and product development, utilizing the abbreviated 510 k process, ultimately benefiting the healthcare sector. Quality and compliance expert Alex Pavlovic emphasizes that a transformative culture of quality can ease compliance burdens, allowing companies to prioritize growth and advancement. He argues that by embracing the abbreviated 510 k pathway, manufacturers can not only expedite their market entry but also foster a culture that encourages innovation. Moreover, experts like Katherine Ruiz, a Regulatory Affairs Expert at bioaccess®, bring valuable insights to the table.
With her extensive background in navigating regulatory pathways in Colombia, including her role in streamlining the approval process for over 100 medical products at INVIMA, she supports foreign manufacturers in achieving market clearance for their innovations. Katherine's efforts at bioaccess® have led to a 30% reduction in processing times for clients, showcasing her impact on regulatory efficiency. The abbreviated 510 k pathway not only accelerates time-to-market but also empowers companies to embrace innovative practices while optimizing costs, making it a strategic choice for medical equipment manufacturers.
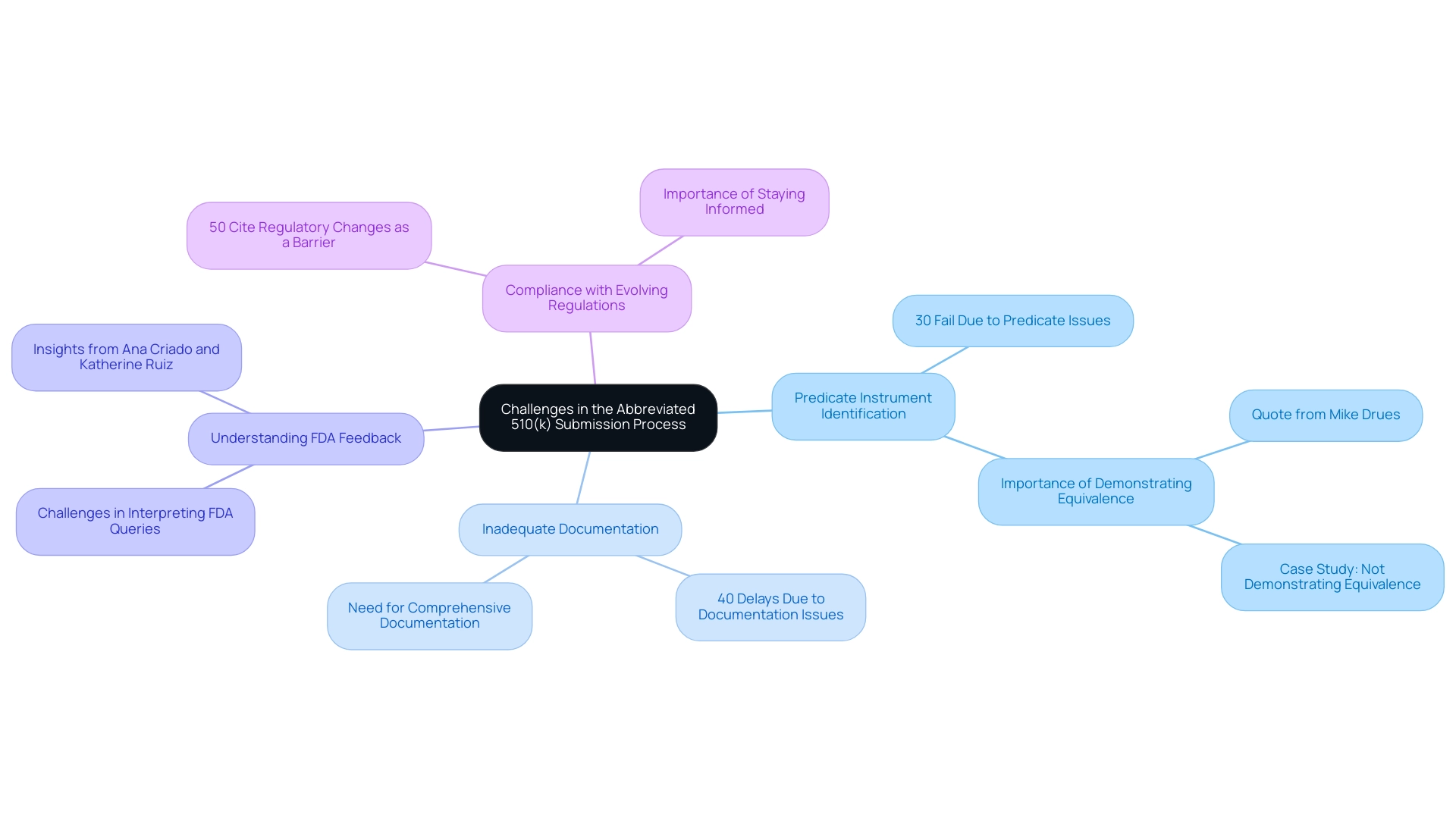
Common Challenges in the Abbreviated 510(k) Submission Process
The abbreviated 510 k submission process presents several hurdles that manufacturers must navigate to ensure compliance and successful approval. Key challenges include:
-
Identifying the Correct Predicate Instrument: A fundamental requirement is demonstrating substantial equivalence to a predicate instrument.
However, manufacturers often encounter difficulties in pinpointing a suitable predicate that aligns closely with their product's intended use and technological characteristics. Research indicates that nearly 30% of entries fail due to issues related to predicate device identification.
-
Inadequate Documentation: The application process demands comprehensive documentation.
Incomplete or insufficient entries can lead to significant delays or outright rejections, with statistics showing that 40% of 510(k) applications experience delays due to documentation issues. This underscores the necessity for meticulous preparation for the abbreviated 510 k.
-
Understanding FDA Feedback: After presenting their materials, manufacturers may struggle with interpreting the FDA's feedback and queries.
Effective communication and comprehension of regulatory expectations are crucial for timely responses and successful outcomes. Regulatory experts like Ana Criado, who serves as the Director of Regulatory Affairs and has extensive experience in this field, emphasize the importance of clarity in responding to FDA inquiries. Katherine Ruiz, another specialist in regulatory matters for medical equipment and in vitro diagnostics in Colombia, also emphasizes the necessity for a comprehensive grasp of the filing procedure to reduce risks.
-
Compliance with Evolving Regulations: The regulatory landscape is continually changing, which can pose compliance challenges for manufacturers.
Keeping informed about these changes is crucial to avoid potential pitfalls that could threaten the approval. A recent survey found that 50% of manufacturers cite regulatory changes as a primary barrier to successful submissions.
To illustrate the importance of demonstrating substantial equivalence, consider the case titled 'Not Demonstrating Equivalence to a Predicate.'
In this instance, the inability to properly show equivalence resulted in the product being regarded as not substantially equivalent, leading to possible reclassification and considerable delays in market entry. To tackle these challenges, manufacturers should engage in extensive research and consider collaborating with regulatory specialists, such as Ana Criado and Katherine Ruiz, who can offer insights into navigating the complexities of the submission. As Mike Drues, President of Vascular Sciences, aptly states,
The whole premise of an abbreviated 510 k is that you are demonstrating substantial equivalents to a predicate product.
Moreover, expert regulatory consultant Jane Smith observes,
A thorough understanding of the predicate landscape is crucial for success in the abbreviated 510 k process.
Therefore, a robust strategy focusing on predicate device identification and documentation will enhance the likelihood of a successful submission.
Conclusion
The Abbreviated 510(k) submission process serves as a crucial pathway for medical device manufacturers seeking efficient FDA clearance. By allowing manufacturers to demonstrate substantial equivalence to existing devices, this streamlined approach significantly reduces the documentation burden and accelerates the review timeline. As highlighted, the importance of selecting an appropriate predicate device cannot be overstated, as it directly influences the success of the submission.
Preparation is key; thorough documentation, a well-crafted summary of safety and effectiveness, and adherence to FDA guidelines are essential for navigating this complex process. Despite the advantages, manufacturers must remain vigilant regarding common challenges, such as identifying the correct predicate and addressing FDA feedback promptly. Engaging with regulatory experts can provide valuable insights that may enhance compliance and operational efficiency.
In conclusion, understanding the nuances of the Abbreviated 510(k) submission process is imperative for manufacturers aiming to bring innovative medical devices to market efficiently. By leveraging the benefits of this pathway while addressing potential challenges, manufacturers can not only achieve timely market entry but also contribute to advancing healthcare through innovation. As the landscape continues to evolve, staying informed and adaptable will be essential for sustained success in the medical device industry.
Frequently Asked Questions
What is the abbreviated 510(k) procedure?
The abbreviated 510(k) procedure provides a simplified route for certain medical instruments to obtain FDA approval by demonstrating substantial equivalence to an existing, legally marketed product, known as a predicate. This approach streamlines the requirements compared to conventional 510(k) filings, significantly accelerating the review process.
How many 510(k) product codes were applied for in 2019?
In 2019, there were 737 different 510(k) product codes applied for, indicating a trend of manufacturers seeking efficient pathways to market.
Are devices cleared through the 510(k) pathway marketed as 'approved by FDA'?
No, devices cleared through the 510(k) pathway are not marketed as 'approved by FDA.' This distinction is crucial for understanding the regulatory landscape.
What are the current panel track wait times for 510(k) submissions?
The average panel track wait times have increased from 285.80 days in 2023 to 289.62 days in 2024, highlighting ongoing challenges in the submission system.
Why is understanding the abbreviated 510(k) procedure important for manufacturers?
Understanding the abbreviated 510(k) procedure is essential for manufacturers as it facilitates timely market entry and ensures adherence to FDA regulations, supporting the goal of increasing 510(k) applications in the U.S. by a projected 50% by the end of 2020.
What challenges did Artivion face related to its operations?
Artivion encountered interruptions in its shipping operations due to a ransomware attack, highlighting weaknesses in operational capabilities within the medical equipment sector.
Who are some professionals that can provide insights into the 510(k) application process?
Professionals like Ana Criado, with experience in regulatory matters and biomedical engineering, and Katherine Ruiz, who focuses on Regulatory Affairs for medical products, can offer essential perspectives on navigating the intricacies of the 510(k) application process.
What is the first step in the abbreviated 510(k) submission process?
The first step is to identify a predicate instrument by conducting thorough research to select a legally marketed item that closely resembles your product, as this establishes substantial equivalence.
What documentation is required for the abbreviated 510(k) submission?
Required documentation includes comprehensive device descriptions, intended use statements, labeling, performance testing data, and the Medical Device User Fee Cover Sheet.
What should be included in the summary of safety and effectiveness?
The summary should articulate how the device meets safety and effectiveness standards in comparison to the chosen predicate, demonstrating compliance with FDA expectations.
What is crucial when completing the FDA's 510(k) application form?
It is crucial to accurately fill out all sections of the application form and leave the space for the 510(k) number blank until it is received in the acknowledgment letter.
How should the application be submitted?
The application should be compiled with all documentation and sent through the FDA’s electronic gateway, adhering to guidelines to avoid rejection or delays.
What should manufacturers be prepared for during the review process?
Manufacturers should be prepared to respond to FDA queries and requests for additional information, as timely communication can significantly influence the approval timeline.
What regulation must all medical instruments comply with, even if exempt from 510(k) filings?
All medical instruments must comply with the Quality System Regulation (QSR) detailed in 21 CFR 820.

