Introduction
The 510(k) submission process, governed by the U.S. Food and Drug Administration (FDA), is a cornerstone of regulatory compliance for medical device manufacturers. This pathway is essential for demonstrating that new devices are safe and effective by establishing substantial equivalence to existing products on the market.
As the medical device landscape continues to evolve, understanding the intricacies of this process becomes increasingly critical for manufacturers seeking to innovate while ensuring patient safety. From navigating various submission types to addressing the challenges posed by regulatory expectations, a comprehensive grasp of the 510(k) process is vital.
This article delves into the key components of the 510(k) submission, providing insights into:
- Best practices
- Common pitfalls
- The importance of thorough documentation in achieving successful market entry.
Introduction to the 510(k) Submission Process
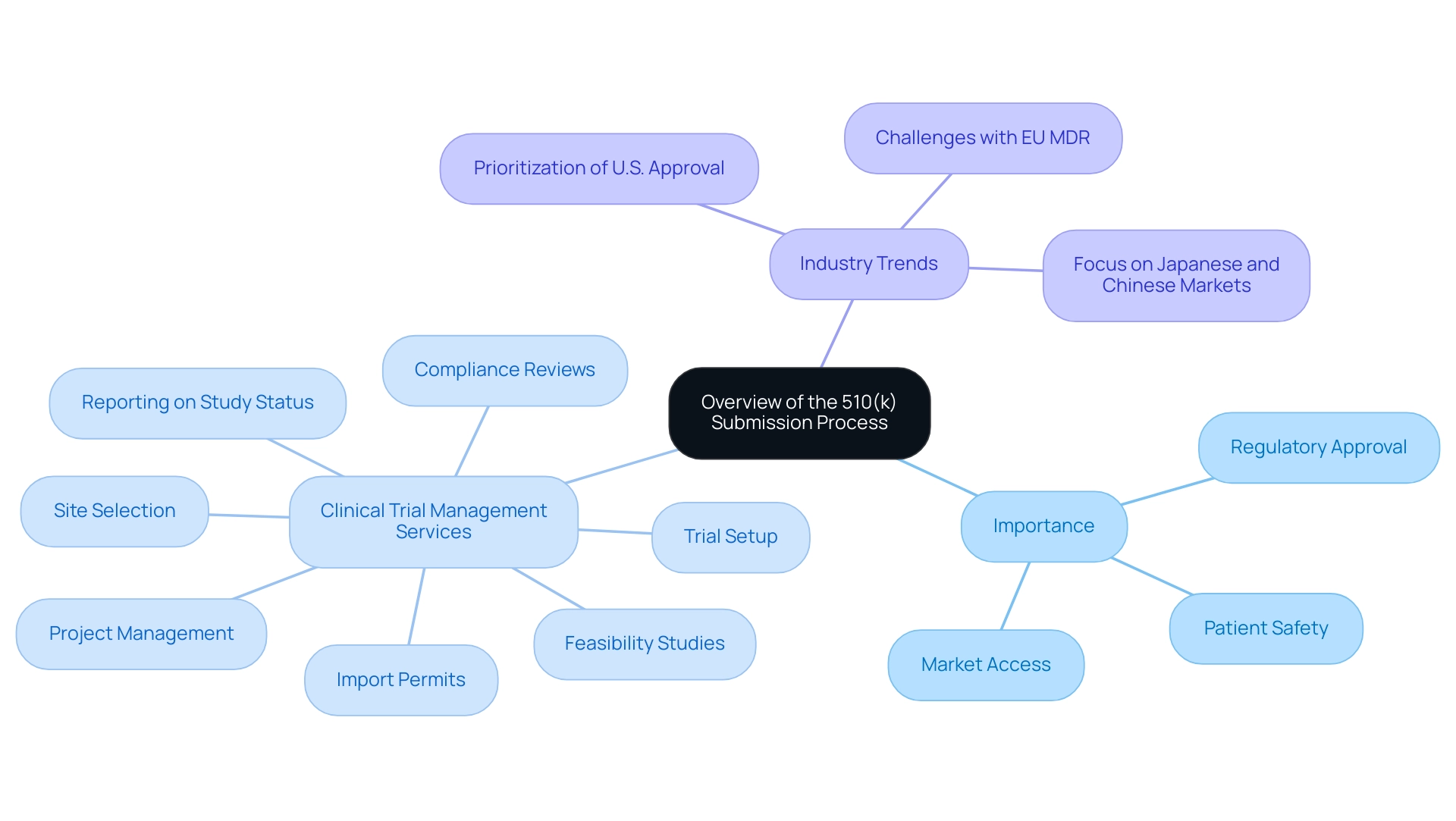
What is 510 k refers to the submission process established by the U.S. Food and Drug Administration (FDA), which serves as a vital pathway for medical product manufacturers to demonstrate the safety and efficacy of their offerings. By demonstrating substantial equivalence to an already marketed product, manufacturers can ease the introduction of new items into the market, ensuring that patient safety remains a top priority. This process not only streamlines market access but also fosters innovation in the medical equipment sector.
Our comprehensive clinical trial management services, led by Katherine Ruiz—an expert in Regulatory Affairs for medical products and in vitro diagnostics in Colombia—encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Thorough reporting on study status, inventory, and adverse events
According to the FDA, the estimated end-of-year FY 2023 operating reserves of carryover user fees is $30,019,132, reflecting the financial stability of the FDA in managing these contributions. Olufunmilayo Ariyo from the Office of Financial Management notes, 'For questions relating to this notice, please reach out to our office for guidance.'
Furthermore, a recent survey of medical equipment industry leaders revealed that almost 90 percent plan to prioritize U.S. regulatory approval over the EU due to the challenges posed by the new MDR, highlighting what is 510 k's importance in the current market environment. For manufacturers, a thorough understanding of what is 510 k is crucial for effectively navigating the complex regulatory landscape, allowing them to respond promptly to the evolving standards and expectations set forth by the FDA.
Types of 510(k) Submissions: Traditional, Special, and Abbreviated
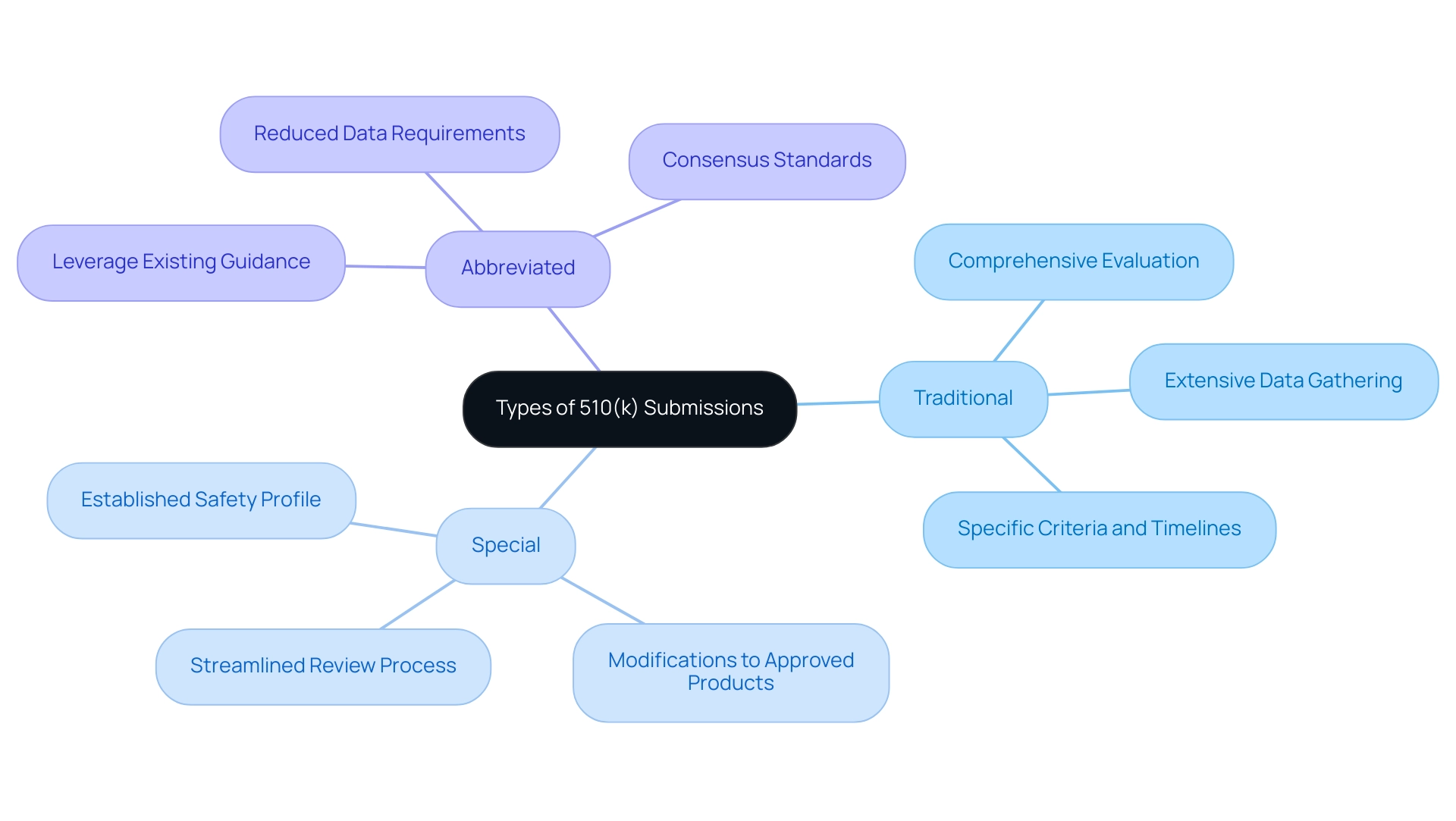
The FDA classifies applications related to what is 510(k) into three main types:
- Traditional
- Special
- Abbreviated
The Traditional 510(k) pathway is the most common method, requiring a comprehensive evaluation of a product's safety and effectiveness, which is essential for securing a product's market entry and often entails extensive data gathering. Conversely, the Special 510(k) application is designed for modifications to products that have previously received approval, allowing for a more streamlined review process due to the established safety profile of the product.
The Abbreviated 510(k) is designed for instances where a manufacturer can leverage existing guidance documents or recognized consensus standards, significantly diminishing the volume of data necessary for review. Each type of submission comes with specific criteria and timelines, which play a pivotal role in shaping the overall submission strategy. With nearly half of all medical instruments utilized daily in the United States associated with what is 510(k) as of 2024, its significance within the industry cannot be overstated.
Experts like Ana Criado, Director of Regulatory Affairs and a professor in biomedical engineering, emphasize the importance of understanding these nuances for effective navigation of the regulatory landscape. Ana notes, 'Navigating the FDA 510(k) process requires a deep understanding of both the regulatory requirements and the specific features of the product in question.' Furthermore, the 'Evidentiary Expectations for 510(k) Implant Devices' serves as a primary resource for manufacturers.
Additionally, to claim a preamendments product, a firm must demonstrate that the item was marketed for a specific intended use that has not changed, adding another layer of consideration for manufacturers. Ana's extensive experience as a consultant for global companies such as General Electric and her academic background in health economics further inform her insights on this critical regulatory process.
Understanding Substantial Equivalence in 510(k) Submissions
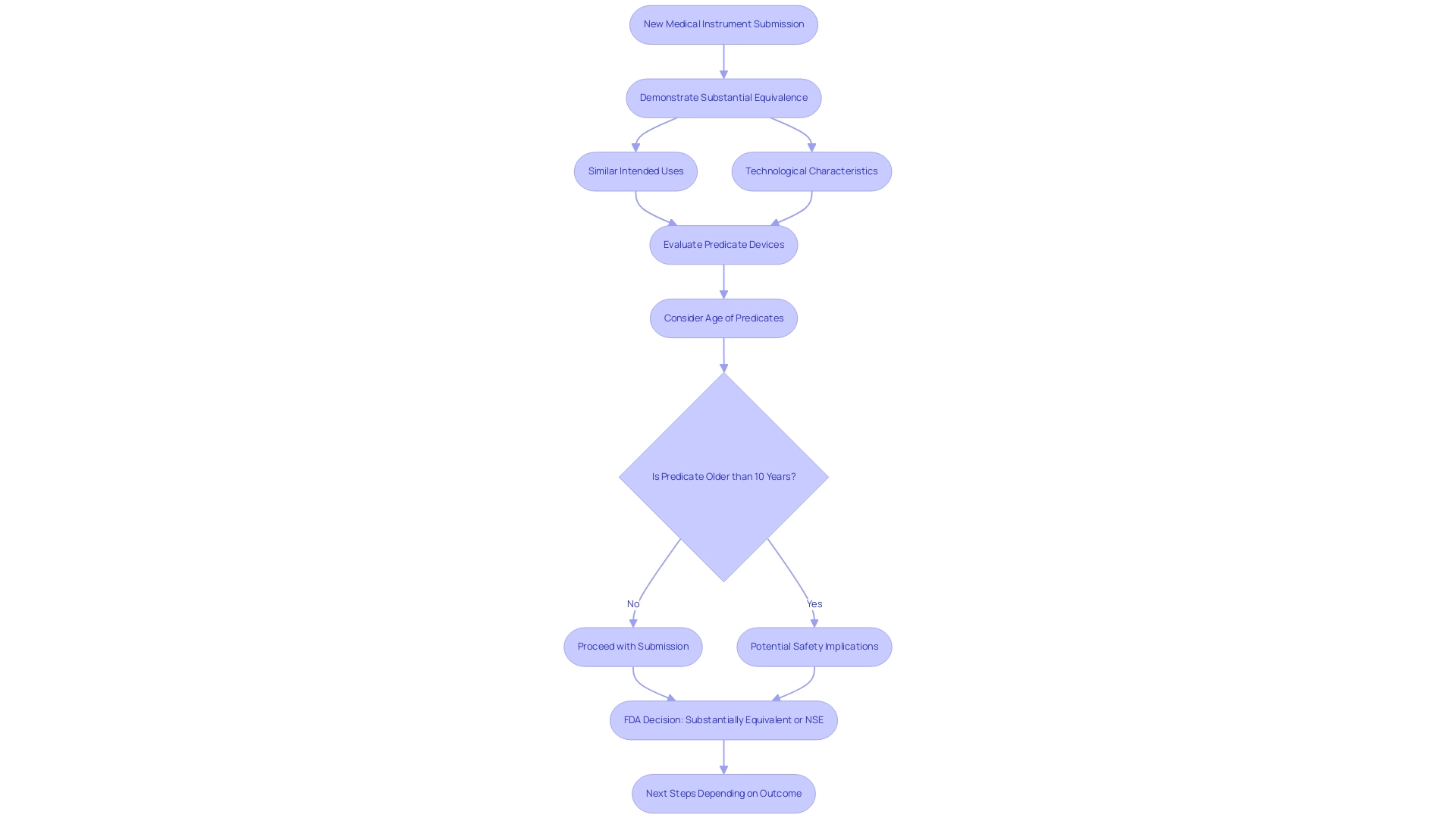
Substantial equivalence is a critical concept in understanding what is 510 k, defined as a determination that a new medical instrument is as safe and effective as one already legally marketed. To substantiate this claim, manufacturers must present compelling evidence showing that their product shares similar intended uses and technological characteristics with existing predicates. Notably, if there are any differences between the equipment, these must not introduce new questions regarding safety and effectiveness.
This understanding is crucial for manufacturers, as it significantly impacts what is 510 k submission success. Recent findings indicate that 27.0% of instruments cited at least one predicate item that is 10 years or older, underscoring the importance of evaluating the relevance and safety implications of predicate choices. The case study titled 'Regulatory Submission Characteristics and Device Safety' explored how the reliance on older predicates for demonstrating safety may be insufficient, revealing that products citing older predicates experienced fewer recalls, which contradicts the FDA's intent to phase out outdated predicates.
Furthermore, Jon Speer, Founder and VP of QA/RA at Greenlight Guru, emphasizes that the reliance on older predicates can lead to unintended consequences for product safety. As the FDA prepares to host a webinar on October 26, 2023, to discuss draft guidances and solicit comments until December 6, 2023, it is essential for industry stakeholders to stay informed about ongoing discussions regarding substantial equivalence determinations and to understand what is 510 k in relation to the implications of proposed changes in the program. Additionally, the FDA will address options for manufacturers if a product is deemed Not Substantially Equivalent (NSE), which could have significant consequences for their regulatory strategy.
Preparing Your 510(k) Submission: Documentation and Best Practices
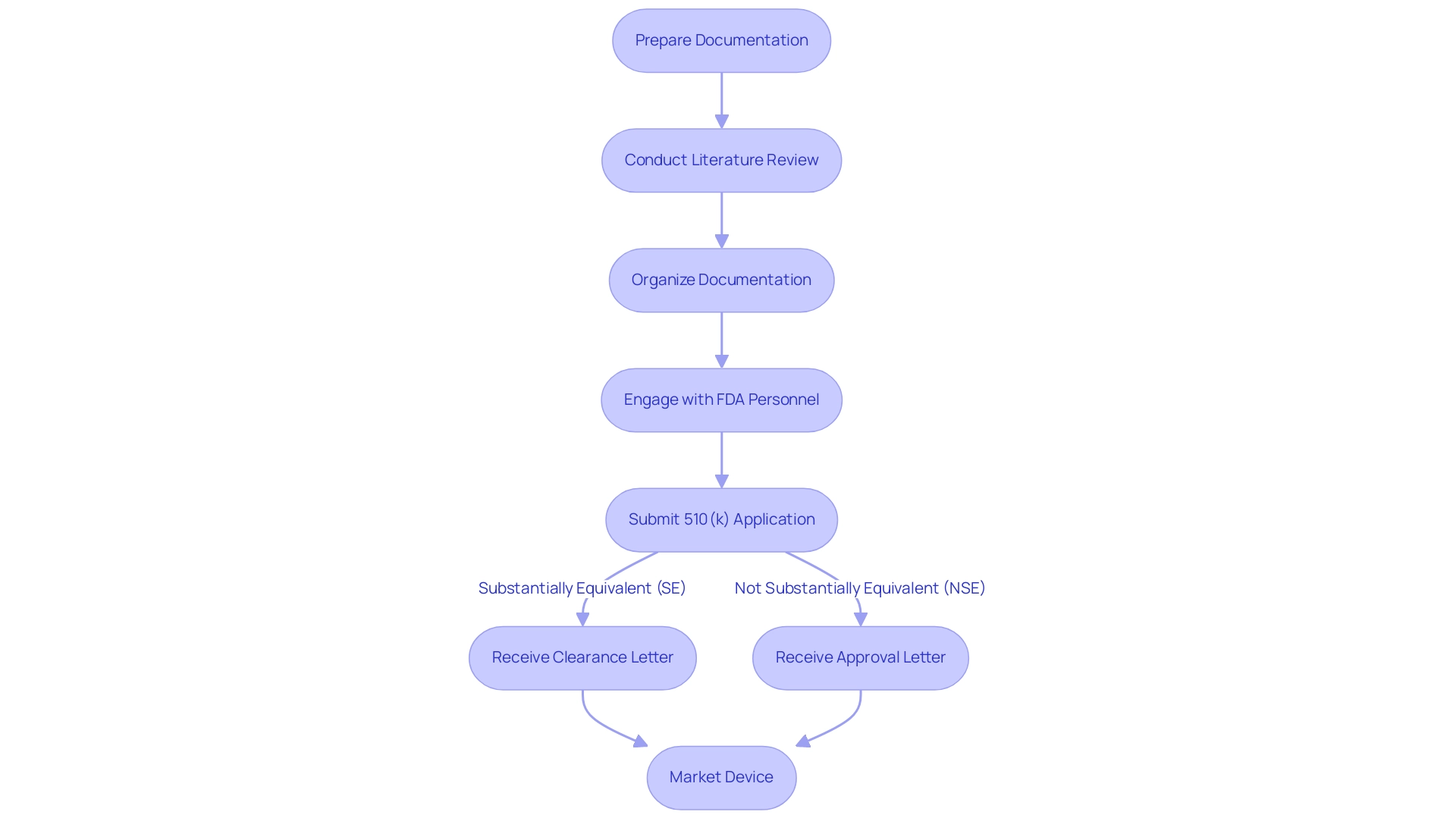
Preparing a 510(k) application requires meticulous attention to detail and comprehensive documentation to understand what is 510 k. Essential elements include a thorough description of the device, its intended use, labeling, and performance data. Best practices for creating a successful proposal involve:
- Conducting a thorough literature review to substantiate claims of substantial equivalence.
- Ensuring that the documentation is organized and aligns with FDA guidelines.
Engaging with FDA personnel early in the process is crucial, as it can provide valuable insights and clarify expectations, which ultimately contributes to a more streamlined review process. As emphasized by Kelliann Payne, if you have any inquiries regarding the new draft guidance documents, or on premarket proposals or Estar requirements more generally, please reach out to any of the authors of this alert or the Hogan Lovells attorney you typically collaborate with. This proactive approach not only enhances communication but also helps in avoiding common documentation errors.
Understanding the implications of receiving a substantially equivalent (SE) letter versus a not substantially equivalent (NSE) letter is essential; while the FDA 'clears' products, it is important to recognize that:
- A clearance letter indicates that the product is deemed substantially equivalent to an already marketed item.
- An approval means the product has undergone a more rigorous premarket approval process.
Furthermore, it is important to consider that if an AI request is submitted, the entry may be placed on hold for up to 180 days, which can significantly impact timelines. Additionally, changing the prevalence of the condition in the study population can alter agreement measures without changing test performance, which highlights the importance of accurate documentation.
The case study outlining what is 510 k review cycle highlights that companies equipped with an understanding of this review process can navigate their entries more efficiently, ultimately aiding market access for their products once approved by the FDA.
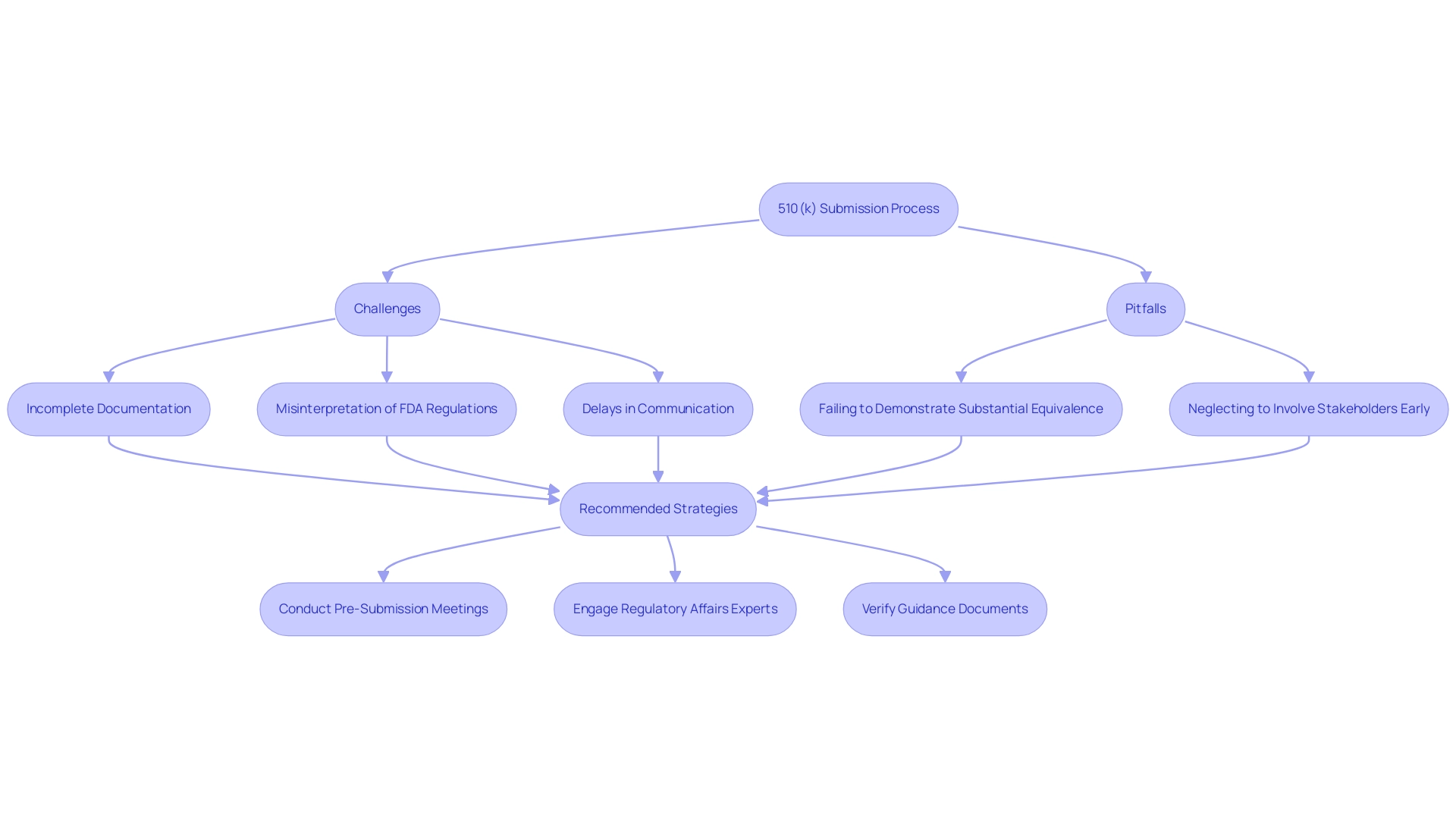
Navigating Challenges in the 510(k) Submission Process
Understanding what is 510 k, the application process is filled with obstacles that can impede the advancement of medical equipment manufacturers. Key issues often arise from:
- Incomplete documentation
- Misinterpretation of FDA regulations
- Delays in communication with regulatory bodies
Notably, manufacturers frequently encounter pitfalls such as:
- Failing to adequately demonstrate substantial equivalence
- Neglecting to involve stakeholders early in the process
A significant study analyzing 35,176 medical devices approved by the FDA between 2003 and 2018 highlighted that referencing predicate devices with ongoing recalls significantly increased the probability of recalls for applicant devices, underscoring the critical importance of thorough due diligence in applications. To navigate these hurdles effectively, manufacturers are encouraged to conduct comprehensive pre-submission meetings with the FDA, ensuring that documentation is complete and reflects the latest guidance. Engaging experts in Regulatory Affairs, such as Ana Criado, Director of Regulatory Affairs and a consultant with extensive experience, can also be invaluable.
Ana's expertise includes comprehensive clinical trial management services, from feasibility studies and site selection to compliance reviews and trial setup. Her insights into project management and her role in guiding organizations through the regulatory landscape are crucial for successfully understanding what is 510 k and navigating the application process. It is essential to verify that guidance documents referenced in their research are still in effect, as draft guidance documents may still be applicable.
The FDA emphasizes a 'reasonable assurance of effectiveness,' meaning the use of the product must yield clinically significant results in a significant portion of the target population. Maintaining open lines of communication with all parties involved further enhances the likelihood of a successful submission. Adopting these proactive strategies not only mitigates common pitfalls but also fosters a more collaborative relationship with regulatory authorities.
Conclusion
The 510(k) submission process is a critical element in the regulatory framework for medical device manufacturers, serving as a gateway to ensuring both safety and efficacy in new products. Understanding the nuances of this process, including the various submission types—Traditional, Special, and Abbreviated—enables manufacturers to choose the most appropriate pathway for their devices. Each submission type comes with its own set of criteria and expectations, underscoring the importance of a tailored approach to navigate this complex landscape effectively.
Central to the success of a 510(k) submission is the concept of substantial equivalence. Manufacturers must provide compelling evidence that their device is as safe and effective as an already marketed counterpart, with careful consideration of predicate choices. The reliance on older predicate devices can pose risks, making it essential for manufacturers to stay informed about ongoing FDA discussions and potential changes to the program.
Thorough documentation and adherence to best practices are paramount in preparing a successful submission. Engaging with FDA personnel early in the process and conducting comprehensive pre-submission meetings can significantly enhance the likelihood of a favorable outcome. By avoiding common pitfalls, such as incomplete documentation and misinterpretation of regulations, manufacturers can streamline their path to market entry.
In conclusion, a deep understanding of the 510(k) submission process not only facilitates regulatory compliance but also supports innovation within the medical device industry. As the landscape continues to evolve, manufacturers who prioritize thorough preparation, proactive communication, and adherence to FDA guidelines will be better positioned to succeed in bringing safe and effective medical devices to market.
Frequently Asked Questions
What is the 510(k) submission process?
The 510(k) submission process is established by the U.S. Food and Drug Administration (FDA) to allow medical product manufacturers to demonstrate the safety and efficacy of their products by showing substantial equivalence to already marketed products.
Why is the 510(k) process important for manufacturers?
The 510(k) process streamlines market access for new medical products, fosters innovation in the medical equipment sector, and ensures patient safety.
What services are included in clinical trial management related to 510(k)?
Clinical trial management services encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and thorough reporting on study status, inventory, and adverse events.
What are the types of 510(k) submissions classified by the FDA?
The FDA classifies 510(k) submissions into three main types: Traditional, Special, and Abbreviated.
What is the Traditional 510(k) pathway?
The Traditional 510(k) pathway is the most common method requiring a comprehensive evaluation of a product's safety and effectiveness, often involving extensive data gathering.
What is a Special 510(k) application?
A Special 510(k) application is for modifications to previously approved products, allowing for a more streamlined review process due to the established safety profile of the product.
What is an Abbreviated 510(k)?
An Abbreviated 510(k) is designed for cases where a manufacturer can use existing guidance documents or recognized consensus standards, significantly reducing the volume of data required for review.
Why is understanding the 510(k) process critical for manufacturers?
A thorough understanding of the 510(k) process is crucial for effectively navigating the complex regulatory landscape and responding to evolving standards set by the FDA.
What must a firm demonstrate to claim a preamendments product?
A firm must demonstrate that the preamendments product was marketed for a specific intended use that has not changed.
What role does expert insight play in navigating the 510(k) process?
Experts emphasize the importance of understanding regulatory requirements and product features, which are essential for effective navigation of the FDA 510(k) process.




