Introduction
Navigating the regulatory landscape for medical devices can be a complex endeavor, particularly for manufacturers seeking to introduce innovative products without a legally marketed predicate. The De Novo classification process, established by the FDA, plays a pivotal role in this journey by providing a streamlined pathway for low to moderate risk devices. This article delves into the intricacies of the De Novo process, outlining essential steps for submission, eligibility criteria, and the critical differences between the De Novo and 510(k) pathways. By understanding the nuances of this classification, manufacturers can better position themselves for success in obtaining marketing authorization while ensuring compliance with rigorous safety and effectiveness standards. Insights from industry experts further illuminate the challenges and opportunities that lie within this regulatory framework, making it imperative for stakeholders to grasp the foundational elements of the De Novo classification process.
Understanding the De Novo Classification Process
The new classification system functions as an essential regulatory route created by the FDA, specifically intended for medical products that pose low to moderate risk and do not have a legally marketed predicate. This pathway not only assists manufacturers in obtaining marketing authorization for innovative products but also ensures that safety and effectiveness are rigorously evaluated. Significantly, the FDA must categorize the product by written order within 120 days under the de novo process, highlighting the effectiveness of this route.
A comprehensive understanding of the de novo process in the new development procedure is essential for manufacturers, as it requires meticulous documentation, strict adherence to FDA regulations, and a thorough review to assess whether the device satisfies the necessary classification criteria. Furthermore, it is important to be aware of potential pitfalls, such as the situation where a new classification request may be regarded as withdrawn if the requester submits a written notice or fails to respond to information requests. This foundational knowledge is essential as it lays the groundwork for the subsequent detailed steps involved in the submission.
As Lauren K. Roth, Associate Commissioner for Policy, noted, 'Understanding the regulatory landscape is crucial for the successful navigation of the new pathway.' With experts like Ana Criado, Director of Regulatory Affairs, who has extensive experience in regulatory consulting for global companies and a robust educational background including a degree in chemical pharmacology, a master's in health economics & pharmacoeconomics, and certifications in clinical epidemiology and good clinical practices, organizations can navigate these complexities more effectively. Furthermore, our extensive clinical trial management services include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Thorough reporting processes
ensuring that all regulatory requirements are met efficiently and in accordance with the new classification procedure.

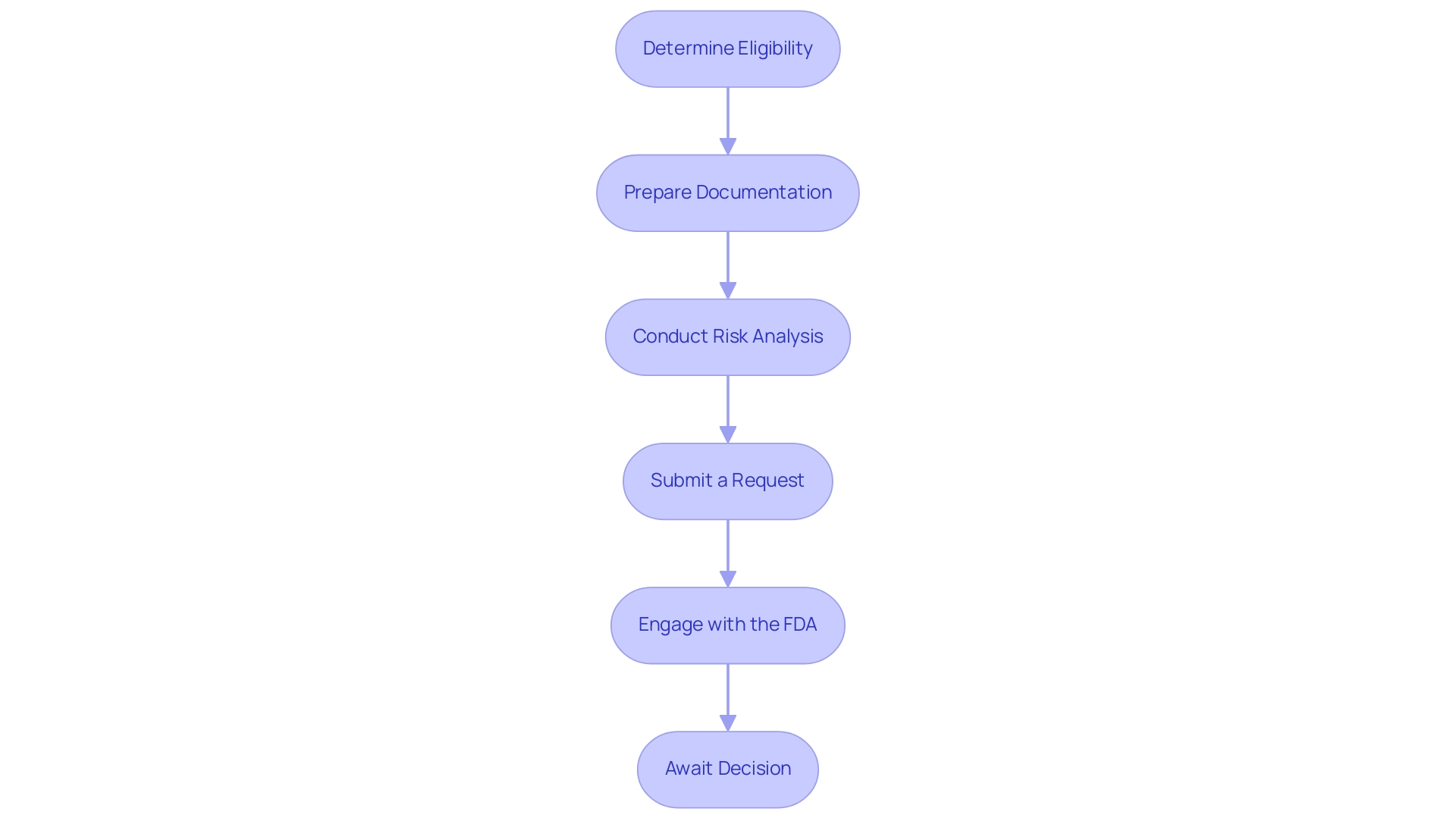
Step-by-Step Guide to Submitting a De Novo Request
- Determine Eligibility: Begin by confirming that your equipment qualifies for De Novo classification. This classification is intended for instruments categorized as low to moderate risk that require a de novo process due to the absence of a suitable predicate item on the market.
- Prepare Documentation: Assemble all necessary documentation meticulously. This should encompass a thorough description of the apparatus, its intended use, and proposed labeling. Accurate and detailed documentation is crucial for the smooth processing of the de novo process, as highlighted by manufacturers who stress its importance. As Michelle E. Tarver, MD, PhD, points out, the FDA is dedicated to inclusivity in assessing medical products, which highlights the significance of comprehensive and representative documentation.
- Conduct Risk Analysis: A thorough risk analysis is essential to identify any potential hazards associated with your device. Outline clear mitigation strategies to address these risks, as this will not only strengthen your application but also demonstrate a proactive approach to safety. The case study titled "Quality Statistical Review for Therapeutic Devices and Best Practices" emphasizes the importance of early collaboration between sponsors and FDA statisticians for successful studies, which can be pivotal during this stage.
- Submit a Request: Complete the request form for the de novo process and ensure it is submitted electronically via the FDA’s submission portal. This digital submission method aligns with the FDA's recent changes aimed at streamlining applications.
- Engage with the FDA: Throughout the evaluation phase, maintain open lines of communication with FDA reviewers. Be prepared to promptly address any inquiries or requests for additional information, as timely responses can facilitate a smoother review.
- Await Decision: After your submission, closely monitor the review timeline. You can expect to receive a letter detailing the FDA's decision within 60 calendar days of submission. Prepare for various potential outcomes, which may range from approval to requests for further information or even denial, ensuring your team is ready for any scenario.
Additionally, leveraging comprehensive clinical trial management services can enhance your submission process. Our capabilities encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, nationalization of investigational equipment, project management, and reporting on study status and adverse events, ensuring your research aligns with both national and international regulatory standards. Katherine Ruiz, a specialist in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, together with INVIMA's supervision, can offer invaluable insights into navigating the complex landscape of medical product approvals.
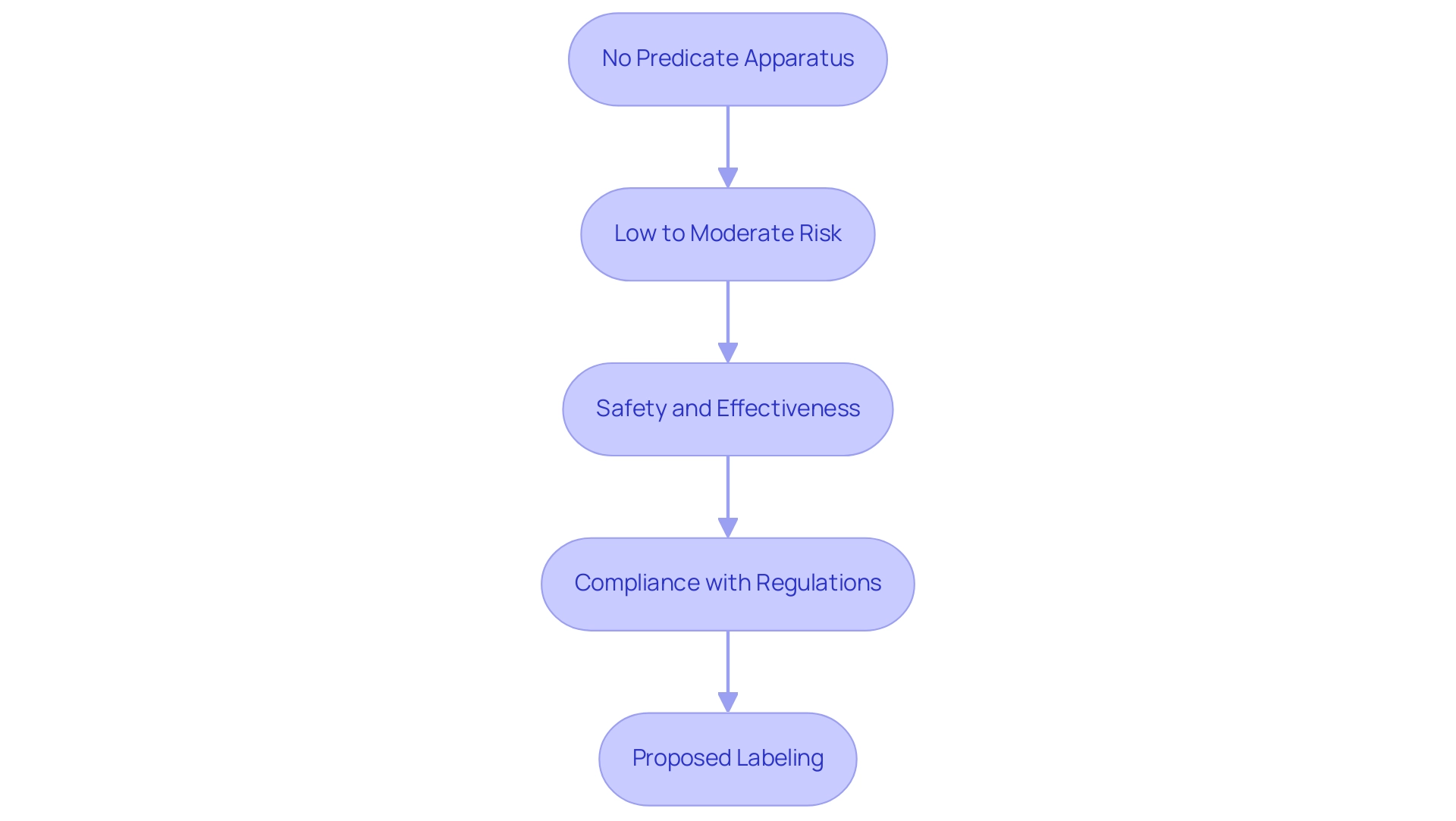
Eligibility Criteria for De Novo Classification
To qualify for De Novo classification, a medical device must adhere to a set of defined criteria:
- No Predicate Apparatus: The apparatus must not have a legally marketed predicate apparatus that is substantially equivalent. This criterion is crucial as it differentiates new innovations from existing products.
- Low to Moderate Risk: The equipment should be categorized as low to moderate risk, which typically involves minimal potential harm to patients. Recent statistics indicate that of the 1,041 products granted Breakthrough Device designation, a significant percentage fall within this risk tier, underscoring the FDA's focus on facilitating access to safer innovations.
- Safety and Effectiveness: Manufacturers are required to provide compelling evidence demonstrating that the item is both safe and effective for its intended use. This includes comprehensive data that supports the performance and risk management strategies of the apparatus.
- Compliance with Regulations: The equipment must comply with all applicable FDA regulations and standards, including quality system regulations (QSR). Adhering to these guidelines is essential for ensuring the reliability and quality of the product.
- Proposed Labeling: Clear and accurate labeling must be provided, detailing the intended use, instructions for use, and any warnings or contraindications. This transparency is vital for both regulatory approval and for informing healthcare providers and patients about the safe application of the product.
In the context of Colombia, regulatory experts such as Ana Criado, Director of Regulatory Affairs at Mahu Pharma, provide invaluable insights into navigating these processes. Ana's extensive background in biomedical engineering and health economics, along with her leadership at Mahu Pharma, positions her as a key contributor to shaping medical equipment policies in Colombia. INVIMA, the Colombian National Food and Drug Surveillance Institute, operates as a Level 4 health authority acknowledged by PAHO/WHO, supervising the regulation of medical instruments and ensuring adherence to international standards.
INVIMA's specific roles include monitoring the safety and effectiveness of medical equipment, suggesting technical standards, and ensuring that products meet health regulations. This underscores the importance of maintaining stringent regulatory practices. In recent updates, the FDA has created a database for New Classification Requests, improving the accessibility of this information for manufacturers and stakeholders.
As noted by the FDA, > We believe this action will also improve patients' access to beneficial innovative products <, reflecting the agency's commitment to advancing healthcare technology while ensuring safety and efficacy. A significant illustration of an apparatus that effectively traversed the de novo process is the INSIGHTEC EXABLATE, which obtained marketing approval under submission number P150038 on 07/11/2016. Furthermore, it is crucial for manufacturers to respond promptly to the FDA's information requests, as this can significantly impact the success of their designation requests.
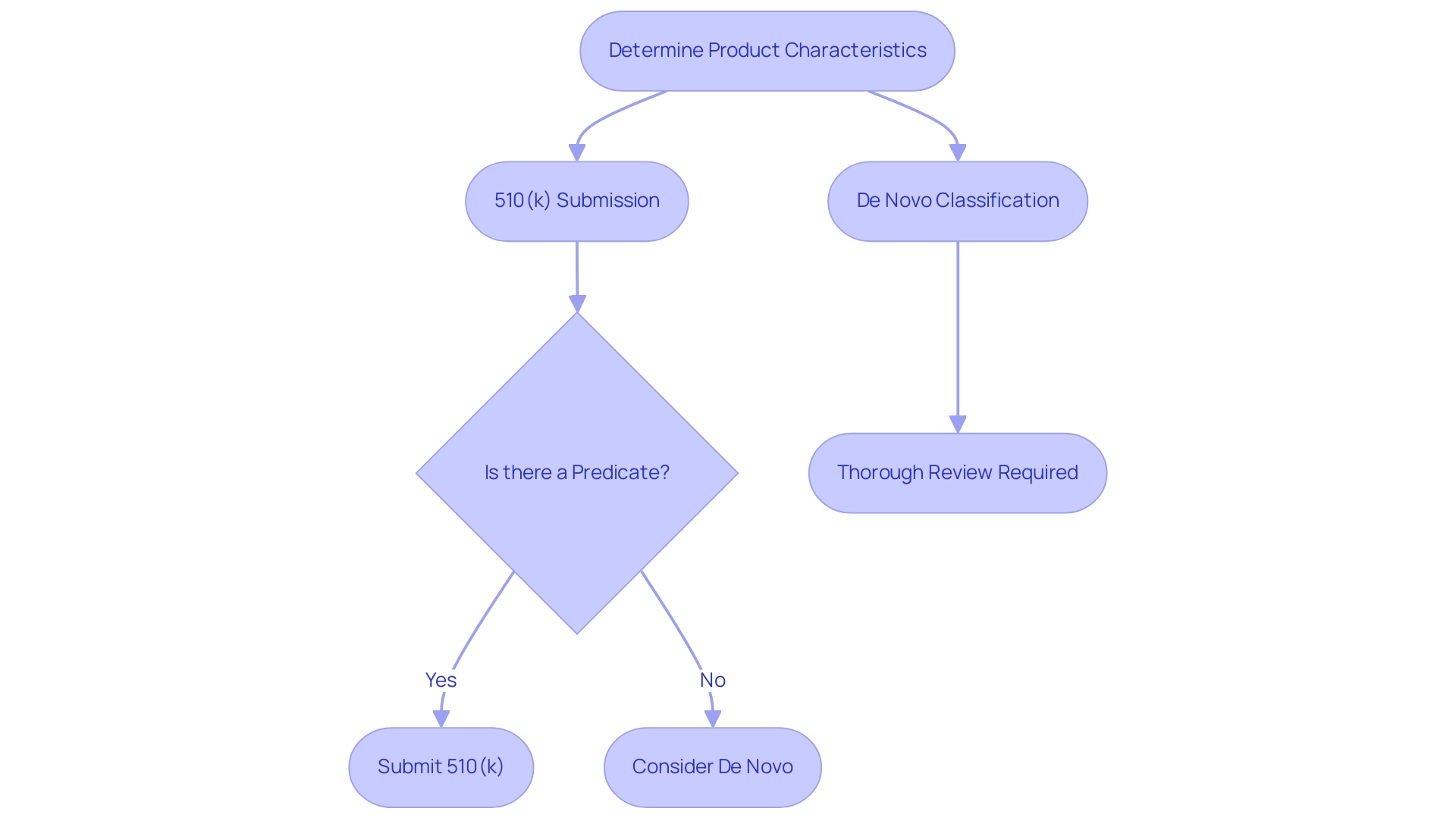
De Novo vs. 510(k): Choosing the Right Pathway
The FDA approval framework offers producers distinct routes for medical product submissions, specifically the De Novo classification method and the 510(k) pathway, each addressing unique requirements:
- 510(k) Submission: This approach is intended for products that can be demonstrated to be substantially equivalent to an existing, legally marketed predicate item. The procedure is generally quicker and requires less extensive documentation, rendering it an appealing choice for numerous producers aiming for faster market access.
- De Novo Classification: Conversely, the de novo process serves cutting-edge products that do not have a predicate. This pathway, which follows a de novo process, necessitates a thorough review, allowing manufacturers to introduce groundbreaking products while ensuring rigorous safety and effectiveness standards are met. This is particularly important considering that over 1.7 million injuries and 83,000 fatalities in the United States over a decade were possibly linked to healthcare instruments, highlighting the necessity for strict safety protocols.
When determining the suitable pathway, manufacturers must carefully evaluate the specifics of their product, the presence of existing predicates, and the associated risk levels. For genuinely innovative products that pose low to moderate risks, the de novo process may be the most appropriate classification choice. Industry experts, including Ana Criado, Director of Regulatory Affairs and a renowned consultant in Colombia’s regulatory landscape, emphasize the importance of this decision.
Ana brings a wealth of knowledge from her roles at INVIMA and her consultancy with global companies, underscoring that the choice of pathway can significantly impact both the approval timeline and market readiness. As highlighted by the Association for the Advancement of Medical Instrumentation (AAMI), following established guidelines is essential in the decision-making framework, particularly concerning the 510(k) and the de novo process. Furthermore, the case study of Blue Goat Cyber illustrates the real-world implications of following AAMI guidelines in medical equipment security and compliance.
By understanding and implementing these guidelines, manufacturers can better navigate the complexities of FDA regulations while ensuring their products meet necessary safety standards.
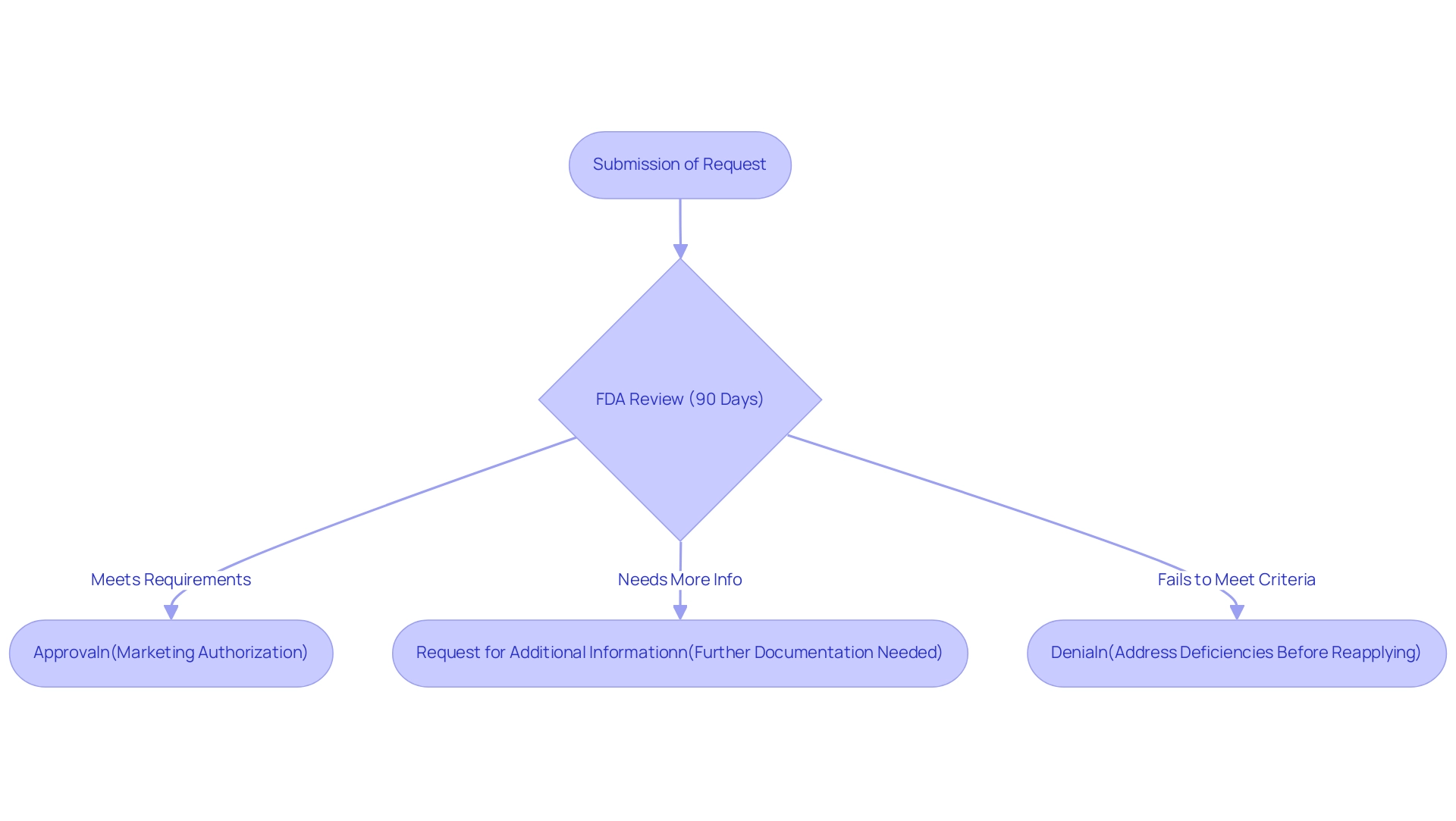
Post-Submission: Understanding FDA Review and Outcomes
Upon submission of a request, the FDA undertakes a comprehensive evaluation as part of the de novo process, which generally spans 90 days. However, this timeline can fluctuate depending on the complexity of the item under consideration. During the review, the FDA may request additional information or clarification to aid in its decision-making.
The possible outcomes of this review process are as follows:
- Approval: Should the apparatus align with all regulatory requirements, it will receive marketing authorization, allowing the manufacturer to proceed with promoting the apparatus. A notable example is the INSIGHTEC EXABLATE, which received marketing authorization with submission number P150038 on 07/11/2016, successfully authorized for marketing.
- Request for Additional Information: The FDA may seek further documentation or data to clarify aspects of the submission before making a final decision.
- Denial: In cases where the device fails to meet the requisite criteria, the request may be denied. Manufacturers will then need to address the identified deficiencies before reapplying. Understanding these potential outcomes is crucial for manufacturers, as it enables them to strategically prepare for each scenario and respond effectively to the FDA's inquiries.
Notably, Michele L. Buenafe highlights the importance of this preparation, stating,
It’s important to note, however, that pushing the envelope for de novo submissions can result in a more intense and lengthy FDA review.
Furthermore, the FDA's commitment to a timely review is underscored by its intention to request any additional information needed for Breakthrough Device designation decisions within 30 days of receiving the request, communicating the final decision within 60 calendar days. In fact, CBER has granted 12 Breakthrough Device designations, demonstrating the effectiveness of the FDA's evaluation.
Additionally, manufacturers can refer to the maintained database for requests related to the de novo process, which serves as a valuable resource in preparing their submissions. This proactive approach is indicative of the FDA's focus on streamlining the review process for innovative medical instruments. In Colombia, similar regulatory oversight is offered by INVIMA, the National Food and Drug Surveillance Institute, which plays a crucial role in ensuring the safety, efficacy, and quality of medical products.
INVIMA is recognized as a Level 4 authority by the Pan American Health Organization and World Health Organization, paralleling the rigorous standards upheld by the FDA. Specifically, the Directorate for Medical Equipment and other Technologies supervises the regulation of medical instruments, ensuring adherence to technical standards and monitoring both pre- and post-market activities. Katherine Ruiz, an expert in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, emphasizes the importance of understanding these regulatory landscapes to navigate the complexities of both markets effectively.
This comparative perspective highlights how INVIMA's functions in medical device oversight resonate with the FDA's de novo process, ensuring that manufacturers are well-informed about the regulatory requirements in both jurisdictions.
Conclusion
Navigating the De Novo classification process is essential for manufacturers aiming to introduce innovative medical devices that do not have a legally marketed predicate. This streamlined pathway allows for the evaluation of low to moderate risk devices, ensuring that safety and effectiveness standards are met while facilitating timely access to the market. A comprehensive understanding of the eligibility criteria, meticulous documentation preparation, and effective risk analysis are pivotal steps that can significantly influence the success of a De Novo submission.
The differences between the De Novo and 510(k) pathways highlight the importance of selecting the appropriate regulatory route based on the unique characteristics of each device. While the 510(k) pathway offers a faster route for devices with existing predicates, the De Novo classification opens doors for truly novel innovations, emphasizing rigorous review processes that prioritize patient safety. As the FDA continues to enhance its review mechanisms, including the establishment of a database for De Novo Classification Requests, manufacturers are encouraged to leverage these resources to navigate the complexities of the submission process effectively.
Ultimately, success in the De Novo classification process hinges on a thorough understanding of both the regulatory landscape and the specific requirements set forth by the FDA. By adhering to established guidelines and maintaining open communication with regulatory bodies, manufacturers can enhance their chances of obtaining marketing authorization for their devices. This proactive approach not only facilitates compliance but also contributes to the advancement of healthcare technology, ensuring that innovative solutions are brought to market in a safe and effective manner.
Frequently Asked Questions
What is the purpose of the new classification system created by the FDA?
The new classification system serves as a regulatory route for medical products that pose low to moderate risk and lack a legally marketed predicate. It helps manufacturers obtain marketing authorization for innovative products while ensuring safety and effectiveness are evaluated.
What is the de novo process and its significance?
The de novo process is a pathway that requires the FDA to categorize a product by written order within 120 days. It is essential for manufacturers as it involves meticulous documentation and strict adherence to FDA regulations to assess whether the device meets the necessary classification criteria.
What are some common pitfalls in the de novo process?
A common pitfall is that a new classification request may be considered withdrawn if the requester submits a written notice or fails to respond to information requests.
Why is understanding the regulatory landscape important for manufacturers?
Understanding the regulatory landscape is crucial for successfully navigating the new pathway, as emphasized by experts in regulatory affairs. It helps manufacturers avoid potential issues and streamline their submission processes.
What are the key steps involved in the de novo process?
The key steps include: 1. Determine eligibility for De Novo classification. 2. Prepare necessary documentation. 3. Conduct a thorough risk analysis. 4. Submit a request electronically via the FDA’s submission portal. 5. Engage with the FDA during the evaluation phase. 6. Await the FDA's decision, which is typically communicated within 60 calendar days.
What kind of documentation is required for the de novo process?
Required documentation includes a detailed description of the device, its intended use, and proposed labeling. Comprehensive and representative documentation is vital for smooth processing.
How can manufacturers enhance their submission process?
Manufacturers can enhance their submission process by leveraging clinical trial management services, which include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and thorough reporting.
What should manufacturers do during the evaluation phase?
Manufacturers should maintain open lines of communication with FDA reviewers and be prepared to promptly address any inquiries or requests for additional information.
What can manufacturers expect after submitting their request?
After submission, manufacturers should monitor the review timeline and expect to receive a letter detailing the FDA's decision within 60 calendar days, which may include approval, requests for further information, or denial.

