Overview
Partnering successfully with CROs in Latin America involves understanding their critical role in enhancing research efficiency, navigating regulatory compliance, and implementing effective patient recruitment strategies. The article emphasizes that leveraging local expertise and operational capabilities not only accelerates study timelines—demonstrated by a notable reduction in recruitment time and high retention rates—but also ensures adherence to ethical standards, thus fostering improved research outcomes in the region.
Introduction
In the rapidly evolving landscape of clinical research, Contract Research Organizations (CROs) have emerged as indispensable allies, particularly in the diverse and dynamic region of Latin America. These organizations not only streamline the complexities of clinical trials but also bring invaluable local expertise that enhances efficiency and compliance.
With the ability to tap into varied patient populations and navigate intricate regulatory frameworks, CROs are transforming the way clinical studies are conducted. As Latin America positions itself as a hub for innovative research, understanding the role and benefits of CROs becomes essential for stakeholders aiming to harness the region's unique advantages.
Through strategic partnerships and advanced methodologies, CROs are not only accelerating the pace of clinical trials but also contributing to the broader development of healthcare systems across the continent.
Understanding the Role of CROs in Latin America
Contract Research Organizations (CROs) act as essential collaborators in the research landscape, providing a comprehensive range of services that assist in the planning, execution, and management of studies. Partnering with CROs in Latin America allows these organizations to play an essential role by leveraging local expertise, which facilitates access to diverse patient populations and an intricate understanding of regional regulations. The operational capabilities of CROs—including project management, data handling, and adherence to regulatory compliance—are critical for fostering successful collaborations.
Significantly, studies conducted by CROs are finished, on average, 30 days quicker than those overseen by sponsors, emphasizing their efficiency. As Julio G. Martinez-Clark, CEO of Bioaccess, remarks, 'Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.' This vision is additionally reinforced by the partnership between bioaccess™ and the Caribbean Health Group, focused on transforming Barranquilla into the premier location for medical studies in Latin America, particularly through partnering with CROs in Latin America, with the support of Colombia's Minister of Health.
Furthermore, GlobalCare Clinical Trials' collaboration with bioaccess™ has significantly enhanced research ambulatory services in Colombia, attaining over 50% decrease in recruitment time and 95% retention rates. Colombia's highly regarded healthcare system, supported by INVIMA—a Level 4 health authority by PAHO/WHO—ensures that medical research is conducted with rigorous standards and ethical practices, particularly through partnering with CROs in Latin America. INVIMA's regulatory oversight plays a crucial role in maintaining compliance, further enhancing the quality of trials and positioning Colombia as an appealing destination for international studies.
Furthermore, CROs are tasked with ensuring that informed consent is obtained, study monitoring is conducted diligently, and investigator training aligns with Good Clinical Practice (GCP) compliance. This combination of expertise and regulatory integrity positions CROs as key players in enhancing the quality of research outcomes in the region, contributing significantly to both local economies and health systems. The impact of medical studies extends beyond research, fostering job creation, economic growth, and international collaboration, which are vital for the development of Colombia's healthcare landscape, particularly through partnering with CROs in Latin America.
Benefits of Collaborating with Latin American CROs
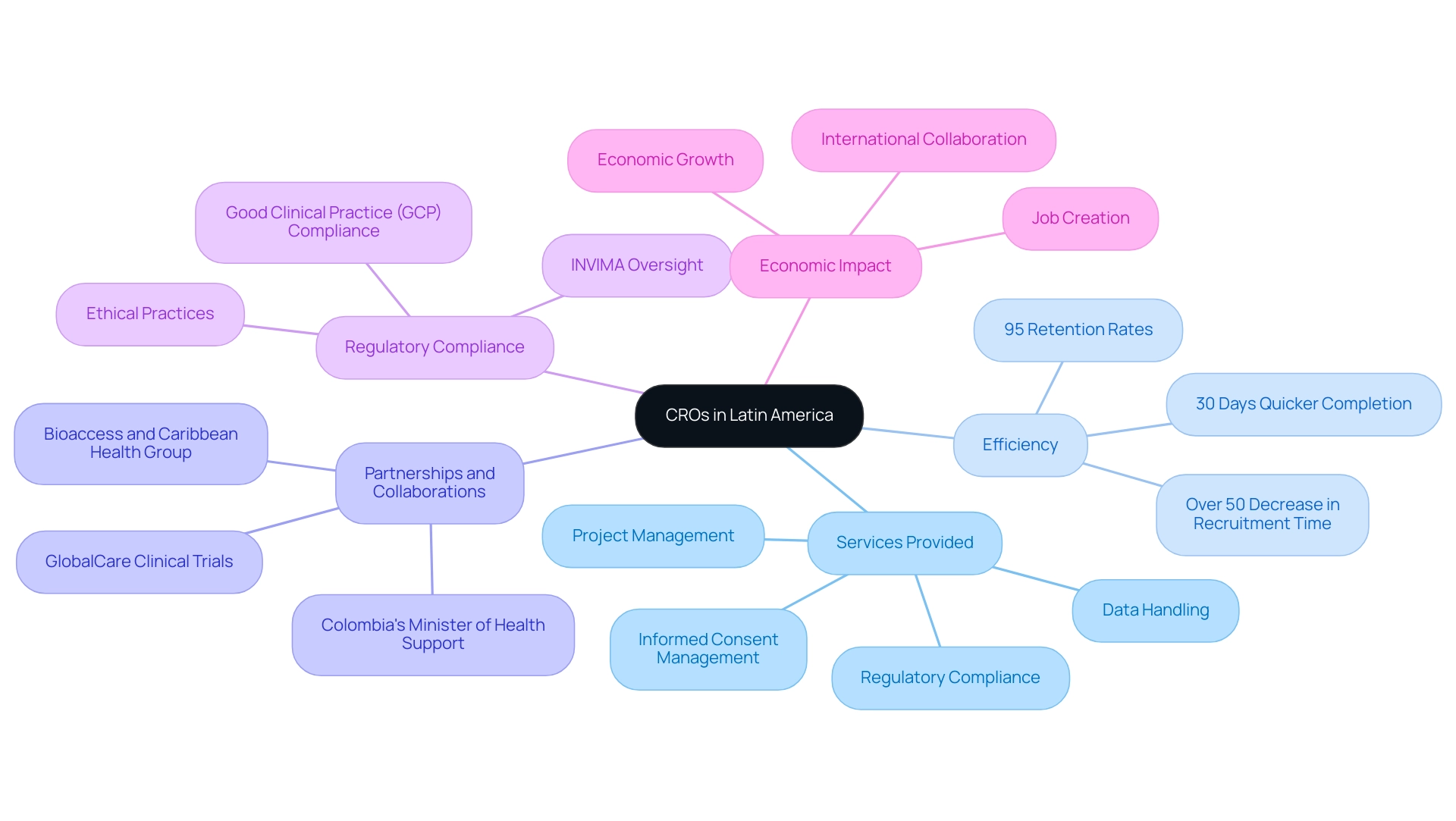
Engaging in partnering with CROs in Latin America presents numerous advantages, particularly in terms of cost efficiency. The average cost per participant in Western Europe can range from $15,000 to $25,000, while Latin America offers a more economical alternative, significantly reducing operational expenses. This financial advantage is enhanced by the region's notable demographic variety, which is essential for clinical studies that aim for thorough representation across different ethnic backgrounds.
Significantly, the occurrence of gallbladder cancer in Peru is said to be high, emphasizing the importance of including diverse groups in research initiatives. Additionally, Latin America's regulatory system is frequently marked by increased flexibility, enabling quicker approvals and reduced timelines for study commencement. For instance, the expedited approval processes in Colombia enable researchers to begin experiments more swiftly compared to many Western countries.
Patricio Ledesma, Head of Clinical Operations and Founder at Sofpromed CRO, emphasizes the strategic advantage that local expertise brings to the table. He observes that grasping cultural subtleties and individual behaviors is crucial for successful engagement and retention in research—an element emphasized by recent partnerships, like the collaboration between bioaccess™ and Caribbean Health Group. This initiative aims to transform Barranquilla into a leading destination for medical research in Latin America, with the support of Colombia's Minister of Health, emphasizing the dedication to advancing research studies in the region.
The unique benefits of this area are further demonstrated by case studies, such as the collaboration with GlobalCare Clinical Studies, which improved medical study ambulatory services in Colombia, resulting in over a 50% decrease in recruitment time and achieving outstanding 95% retention rates. This partnership not only streamlined processes but also improved participant engagement through tailored outreach strategies. Furthermore, the creation of a long-term prognosis estimation model for patients with Acute Coronary Syndromes (ACS) successfully combined UK-specific data with local insights, demonstrating how tailored approaches can lead to improved decision-making in healthcare settings.
Partnering with CROs in Latin America allows sponsors to not only achieve cost savings but also leverage local knowledge to enhance study outcomes and efficiency.
Navigating Regulatory Compliance in Latin America
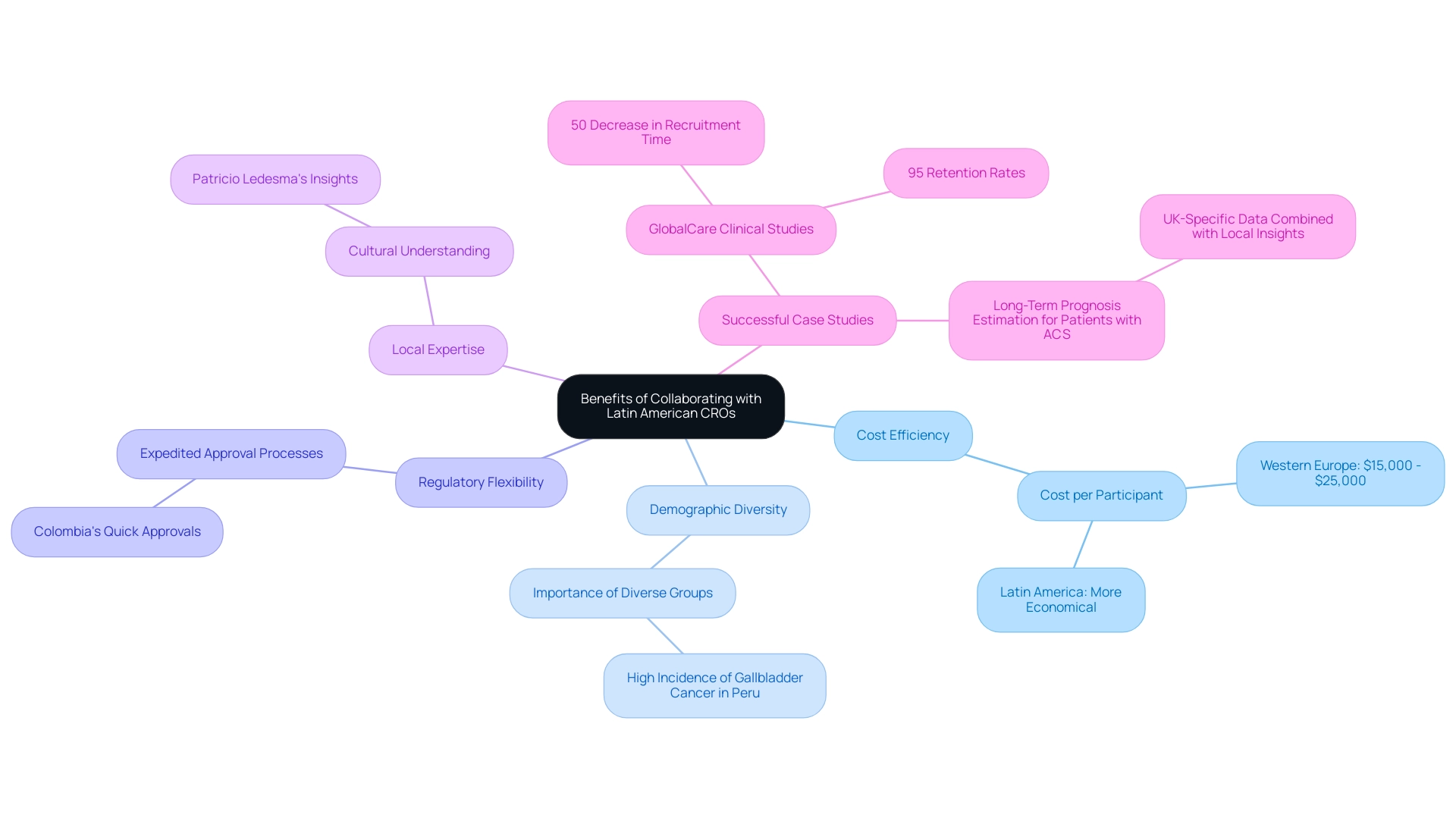
Navigating regulatory compliance in Latin America necessitates a comprehensive understanding of the specific laws and guidelines that govern research across diverse countries. Each country is supervised by its own regulatory body, like ANVISA in Brazil and COFEPRIS in Mexico, responsible for ensuring that research studies comply with established standards. Familiarity with the requirements for ethical approval, informed consent, and data protection is paramount.
Recent developments underscore the urgency for regulations in LATAM to evolve and align with contemporary standards, allowing for more efficient delivery of innovative drugs to patients. For instance, the FDA's 'priority review' program, which results in a review time of just 6 months compared to 10 months for a standard review, highlights the importance of efficient regulatory processes that LATAM countries could aspire to emulate. Partnering with CROs in Latin America, such as bioaccess™, which has established relationships with these regulatory agencies, can significantly streamline the approval and compliance process.
Partnering with CROs in Latin America, the significant partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a leading location for research studies, supported by the backing of Colombia's Minister of Health during a strategic meeting in Miami. This partnership highlights the importance of partnering with CROs in Latin America to implement strategic initiatives essential for improving research operations in the region. Additionally, the collaboration has achieved over a 50% reduction in recruitment time and 95% retention rates, demonstrating its effectiveness.
A case study titled 'Trends in Research Registration Adherence' illustrates the challenges and improvements in registration adherence in LAC, noting that while adherence has improved, many studies still lack adequate registration, emphasizing the need for continued efforts to enhance registration rates across all research characteristics. Furthermore, implementing regular training and updates for your research team on local regulations is advisable, ensuring they remain well-informed about the evolving compliance landscape. As Ludovic Reveiz noted, 'Although there are still many challenges to overcome, the adherence strategies implemented in recent years have proven effective.'
This proactive method not only enhances adherence but also adds to the overall integrity of research in the region.
Effective Patient Recruitment and Retention Strategies
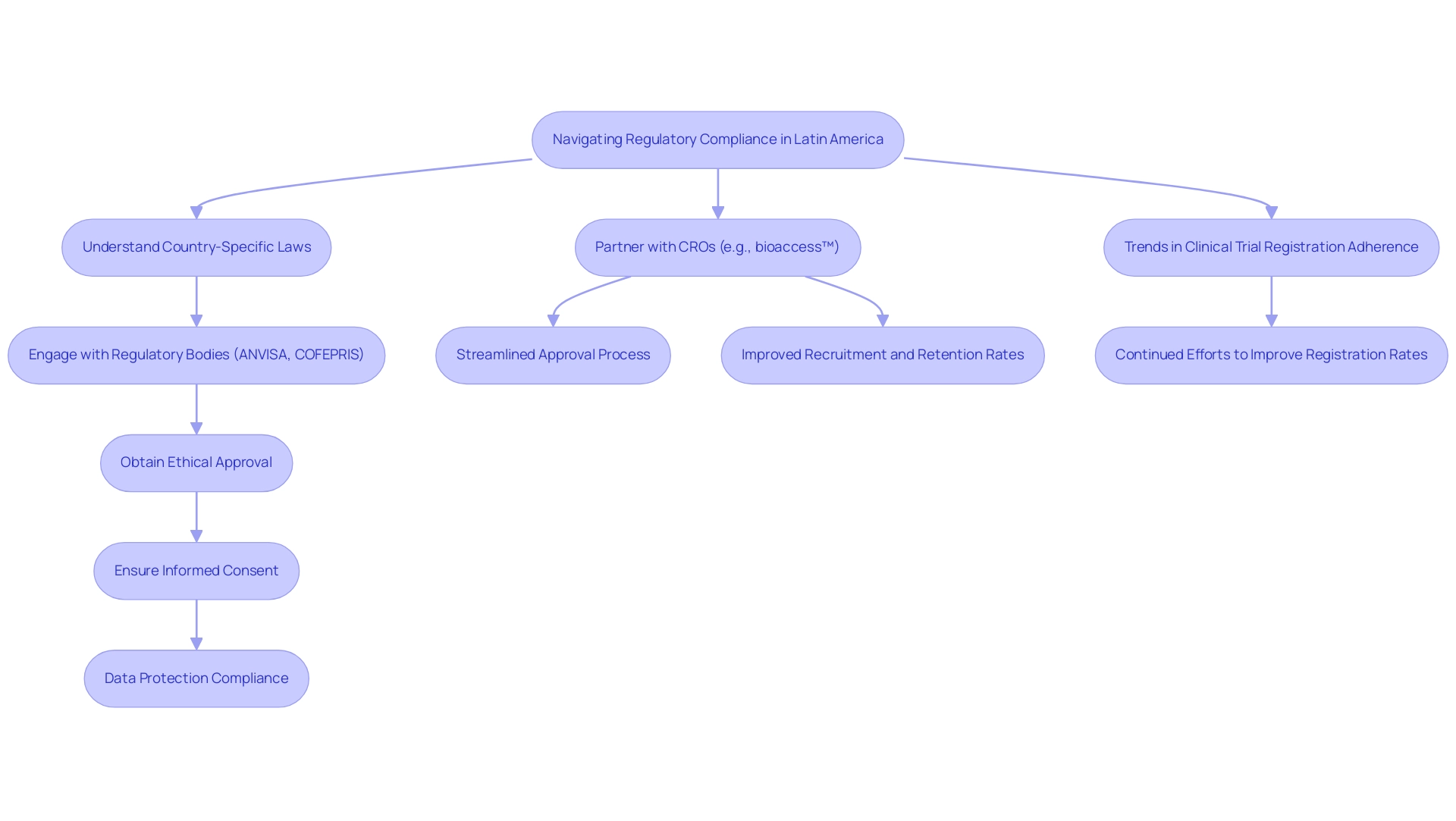
Successful patient enrollment and retention are vital elements for the achievement of clinical studies, especially when partnering with CROs in Latin America. A multifaceted hiring strategy is essential, incorporating:
- Community outreach
- Collaboration with local healthcare providers
- Leveraging digital platforms to maximize reach
For instance, bioaccess®, a leading contract research organization, excels at conducting feasibility studies, site selection, compliance reviews, trial setup, and project management, ensuring that trials are not only compliant but also strategically positioned for success in diverse markets.
A recent analysis highlighted that statistically significant subgroup differences were found in 15 of the top 30 most important features of patient engagement strategies, demonstrating the need for tailored approaches. Customizing hiring materials to reflect local cultures and languages enhances engagement and encourages participation. Furthermore, utilizing technology to reduce site visits can significantly enhance enrollment among under-represented groups, offering a modern solution to recruitment challenges.
Once patients are enrolled, maintaining their commitment to the study is paramount. Consistent communication, updates on progress, and incentives for participation have demonstrated to significantly enhance retention rates. A case study on conversion rates discovered that offline hiring strategies significantly outperformed online methods in converting screened participants to enrolled subjects.
As Rait MA observed concerning a smoking cessation study, while Facebook advertisements can expand reach, offline strategies demonstrate greater cost and time efficiency. Thus, integrating both online and offline methods, while partnering with CROs in Latin America and supported by comprehensive clinical study management services from organizations like bioaccess®, can optimize recruitment and retention efforts in clinical studies. Additionally, bioaccess® navigates regulatory challenges effectively, ensuring compliance with local laws and regulations, which is crucial for successful execution.
This comprehensive approach not only addresses the unique needs of diverse patient populations but also contributes to local economic growth and healthcare improvement.
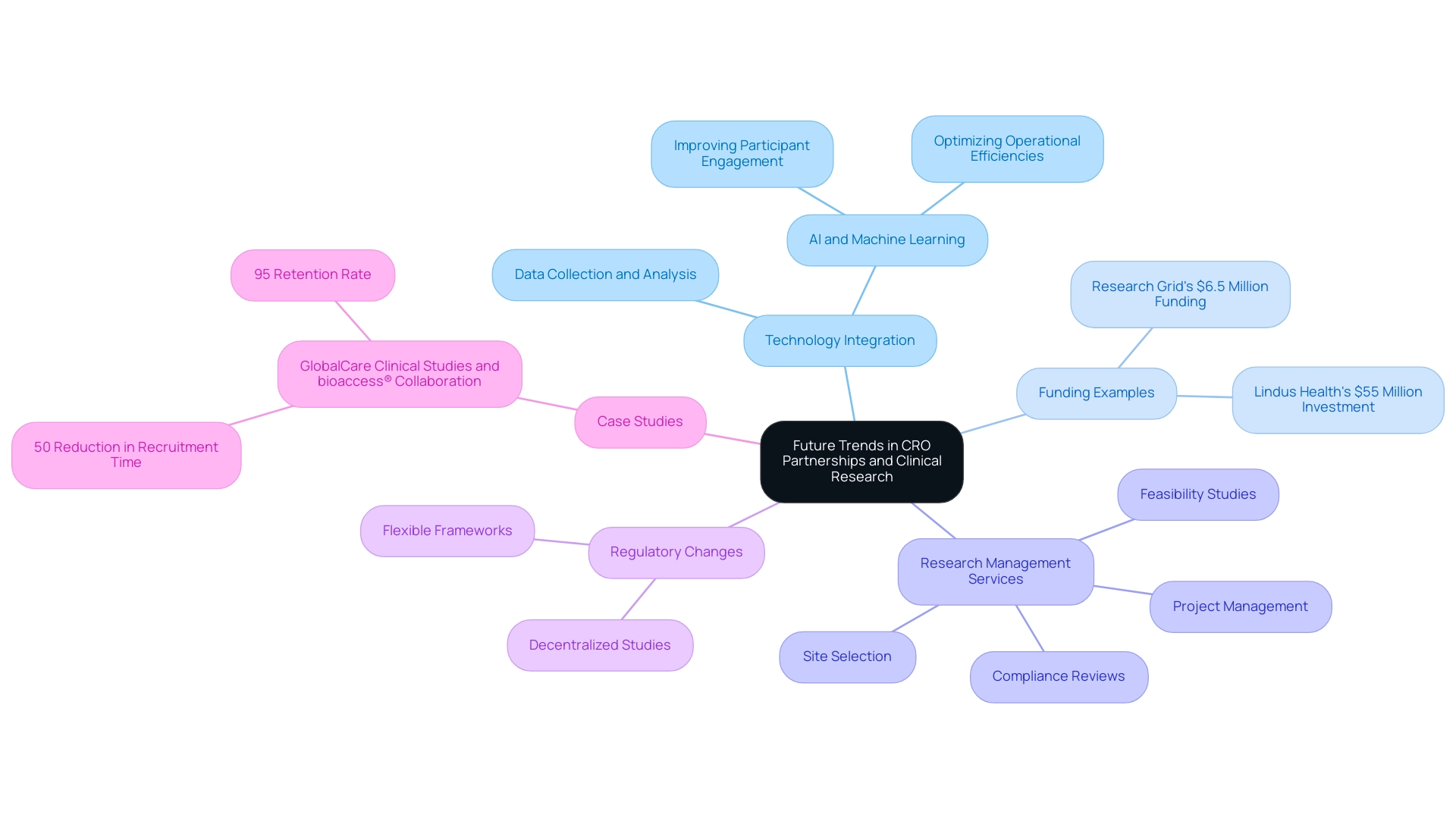
Future Trends in CRO Partnerships and Clinical Research
Partnering with CROs in Latin America is poised for significant evolution, driven by several emerging trends. A key focus will be the integration of technology to enhance data collection and analysis, as evidenced by recent funding rounds, such as:
- Lindus Health's USD 55 million investment aimed at advancing research technology platforms
- Research Grid's USD 6.5 million funding to improve its AI automation engine for studies
Comprehensive research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, are essential as companies navigate these changes.
This includes critical processes like the review and feedback on study documents to ensure compliance with country requirements, as well as obtaining necessary approvals from ethics committees and health ministries. For instance, bioaccess™ serves as a vetted CRO and consulting partner for U.S. medical device companies in Colombia, highlighting the significance of partnering with CROs in Latin America to tackle the unique challenges faced by Medtech startups in the region, such as regulatory hurdles and communication barriers. The use of artificial intelligence and machine learning is poised to enhance research processes, promoting better participant engagement and optimizing operational efficiencies.
In this context, Peyton Howell, CEO of Parexel, notes, 'Partnering with CROs in Latin America is essential as the CRO industry is on the cusp of significant expansion driven by a surge in R&D investment across the pharma and biotech landscapes.' Furthermore, the examination of 297 clinical studies reveals that while most are randomized and blinded, only a small percentage focus on neglected diseases, emphasizing the need for CROs to address these gaps. The growing focus on individual-centered methods will guarantee that CROs prioritize improving the experience of participants throughout the study process.
Regulatory agencies are expected to embrace more flexible frameworks to support innovative study designs, including decentralized studies that broaden access and participation for diverse patient populations. For instance, the collaboration between GlobalCare Clinical Studies and bioaccess® in Colombia led to a remarkable 50% reduction in subject recruitment time and a retention rate of 95%, showcasing the effectiveness of technology in improving trial outcomes. As stakeholders navigate these trends, staying informed will be crucial to maximizing the potential of their collaborations, particularly through partnering with CROs in Latin America.
Conclusion
The pivotal role of Contract Research Organizations (CROs) in Latin America cannot be overstated. They serve as essential partners in the clinical trial ecosystem, leveraging local expertise to navigate complex regulatory landscapes and enhance trial efficiency. By facilitating access to diverse patient populations and streamlining processes, CROs are not only accelerating the pace of clinical research but also contributing significantly to the development of healthcare systems across the region.
Engaging with CROs in Latin America presents a multitude of advantages, including cost savings and faster approval timelines. With the region's demographic diversity, sponsors can achieve more representative and comprehensive clinical trial results. The innovative partnerships being forged, such as those between bioaccess™ and other organizations, demonstrate a commitment to enhancing the clinical trial landscape, ultimately leading to improved patient engagement and retention.
As the regulatory environment evolves, the collaboration between CROs and local authorities is crucial for maintaining compliance while promoting the integrity of clinical research. The effective implementation of patient recruitment and retention strategies further underscores the importance of tailored approaches that respect cultural nuances and local practices.
Looking ahead, the integration of technology and a focus on patient-centric methodologies will drive the future of CRO partnerships in Latin America. As the region continues to position itself as a hub for clinical research, stakeholders must remain proactive in leveraging the unique advantages that CROs offer. By doing so, they can help ensure that clinical trials not only meet regulatory standards but also contribute to the broader development of healthcare solutions that benefit local populations and the global community alike.
Frequently Asked Questions
What are Contract Research Organizations (CROs)?
CROs are essential collaborators in the research landscape that provide a comprehensive range of services to assist in the planning, execution, and management of studies.
How do CROs contribute to research in Latin America?
CROs leverage local expertise, facilitating access to diverse patient populations and understanding regional regulations, which enhances the efficiency and quality of research.
What is the average time advantage of studies conducted by CROs compared to those overseen by sponsors?
Studies conducted by CROs are completed, on average, 30 days quicker than those overseen by sponsors.
What initiatives are being taken in Colombia to enhance medical research?
Colombia has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy and is focusing on transforming Barranquilla into a premier location for medical studies through partnerships with CROs.
What impact has GlobalCare Clinical Trials had on research services in Colombia?
The collaboration with bioaccess™ has significantly improved research ambulatory services, achieving over a 50% decrease in recruitment time and 95% retention rates.
How does INVIMA contribute to medical research in Colombia?
INVIMA, a Level 4 health authority by PAHO/WHO, ensures that medical research is conducted with rigorous standards and ethical practices, enhancing trial quality and compliance.
What responsibilities do CROs have in clinical trials?
CROs ensure informed consent is obtained, conduct diligent study monitoring, and align investigator training with Good Clinical Practice (GCP) compliance.
What are the economic benefits of partnering with CROs in Latin America?
Partnering with CROs offers cost efficiency, as the average cost per participant in Latin America is significantly lower than in Western Europe, alongside enhancing operational efficiency.
What advantages does the demographic variety in Latin America provide for clinical studies?
The region's notable demographic variety is essential for clinical studies aiming for thorough representation across different ethnic backgrounds.
How does the regulatory environment in Latin America benefit research studies?
Latin America often features increased regulatory flexibility, enabling quicker approvals and reduced timelines for study commencement compared to many Western countries.
What specific case studies illustrate the benefits of CRO partnerships in Latin America?
The collaboration with GlobalCare Clinical Studies improved ambulatory services in Colombia, resulting in significant reductions in recruitment time and high retention rates, showcasing enhanced participant engagement and tailored outreach strategies.

