Overview
The key elements of clinical trial design are pivotal in defining research questions, selecting appropriate study methodologies, and ensuring compliance with regulatory standards. These components are crucial for the success and integrity of clinical studies. A well-structured design framework significantly enhances the reliability of results, facilitates effective patient recruitment, and ultimately leads to improved outcomes in medical research. This article underscores the necessity of these elements, positioning them as foundational to advancing clinical research endeavors.
Introduction
In the rapidly evolving landscape of clinical research, the design of clinical trials stands as a cornerstone for ensuring efficacy and safety in medical advancements. As the industry approaches 2025, the importance of a meticulously structured trial framework becomes increasingly clear, particularly with the anticipated rise in innovative methodologies utilizing human cells and tissues. This article delves into the multifaceted elements of clinical trial design, from the foundational protocols that guide study execution to the critical feasibility assessments that determine a trial's viability.
By examining the latest trends, regulatory challenges, and effective recruitment strategies, it highlights how a robust design framework not only enhances scientific integrity but also aligns clinical objectives with market needs. Ultimately, this alignment paves the way for breakthroughs that can transform patient care and drive economic growth in healthcare sectors.
Understanding the Framework of Clinical Trial Design
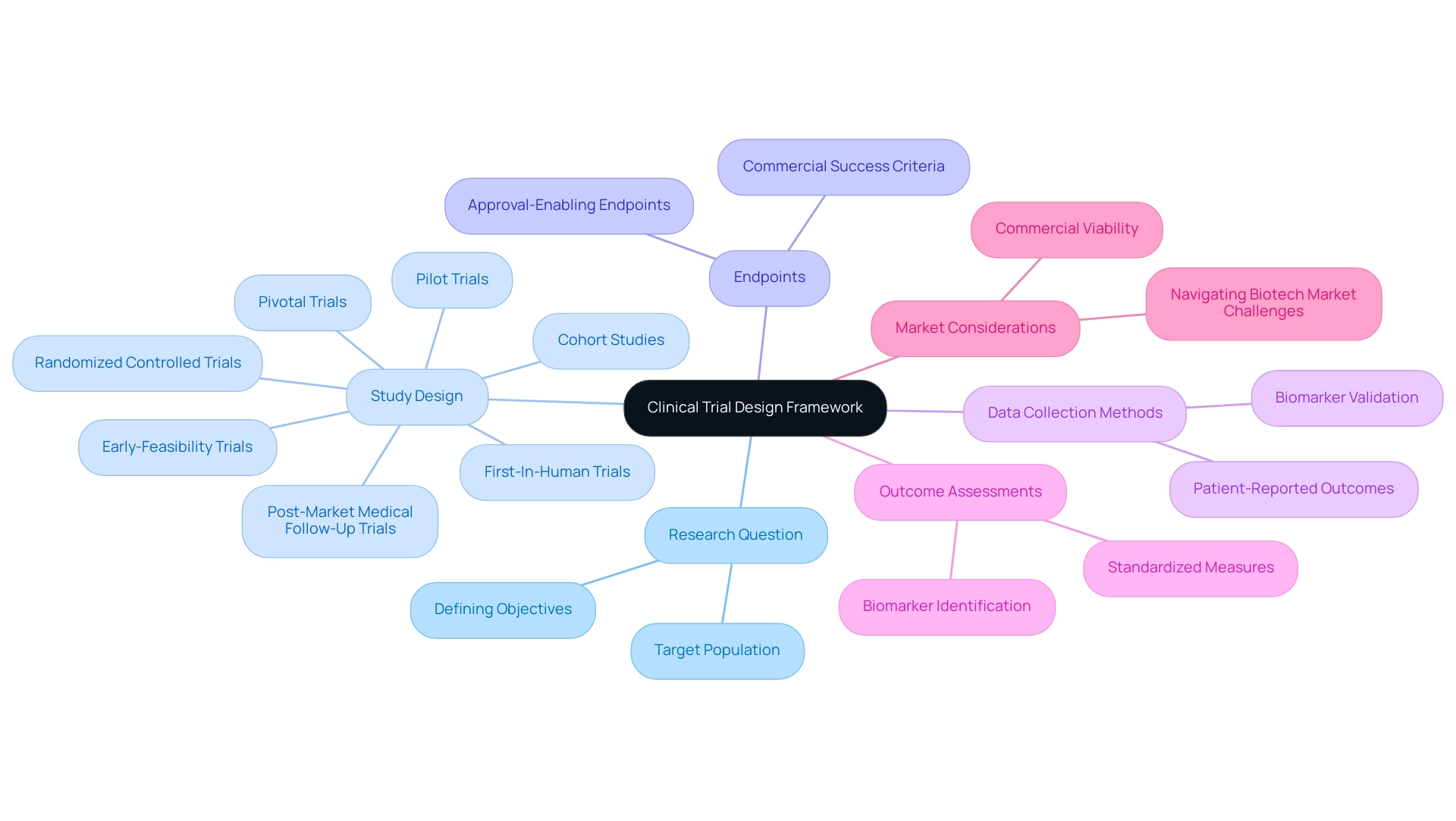
Clinical research design represents a meticulously structured method that outlines the execution of an investigation. This framework encompasses defining the research question, selecting an appropriate study design—such as randomized controlled trials or cohort studies—and determining the endpoints. By 2025, the significance of a structured research design is underscored by the anticipated broader application of methodologies utilizing human cells and tissues for drug discovery and development, necessitating a robust framework to ensure these innovative approaches are effectively implemented.
A clearly defined research framework is indispensable, directing the entire process while ensuring scientific validity and ethical integrity. Within this framework, the study's objectives, target population, and methods for data collection and analysis emerge as key elements of clinical trial design. Recent advancements indicate a growing emphasis on improving outcome assessment in research studies, particularly through patient-reported outcomes and biomarker validation.
Looking ahead to 2025, there is optimism for standardized outcome measures across studies, which will facilitate biomarker identification and therapeutic evaluation, particularly for conditions like hereditary angioedema. These enhancements are intricately linked to the organized research study design framework, emphasizing the essential components of clinical trial design, as they rely on clear definitions and methodologies for effective integration into studies.
Expert opinions highlight the necessity of optimizing research study frameworks to achieve not only approval-enabling endpoints but also to ensure commercial viability. As Max Baumann from Treehill Partners articulates, early-stage developers must prioritize commercial outcomes within their strategies to successfully navigate the increasingly crowded biotech market. This underscores the importance of a systematic approach that aligns medical objectives with market demands.
Successful research design examples illustrate the efficacy of a structured method. For instance, the research study for a new hereditary angioedema therapy utilized a comprehensive framework that incorporated rigorous patient-reported outcomes and biomarker validation, resulting in enhanced reliability of results and adherence to regulatory standards. At bioaccess®, we specialize in overseeing a diverse range of medical trials, including Early-Feasibility Trials, First-In-Human Trials, Pilot Trials, Pivotal Trials, and Post-Market Medical Follow-Up Trials.
Our extensive research management services encompass feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. As the landscape of medical research evolves, the importance of a well-defined study design framework remains vital, paving the way for advancements in medical technology and ultimately benefiting patient care while driving economic growth and healthcare enhancement in local communities.
The Importance of Protocol Design in Clinical Trials
The clinical study protocol serves as the foundational blueprint for any research, meticulously detailing the key elements of clinical trial design, including the study's objectives and the methodologies for data collection and analysis. Key elements of clinical trial design encompass outlining eligibility criteria, treatment regimens, and safety monitoring procedures, all of which are crucial for maintaining the integrity of the study. A well-organized protocol that incorporates these elements significantly reduces variability and bias, thereby enhancing the replicability of the study and the credibility of its results.
Furthermore, the key elements of clinical trial design are indispensable for securing regulatory approvals and serve as a guiding document for the research team throughout the study's duration.
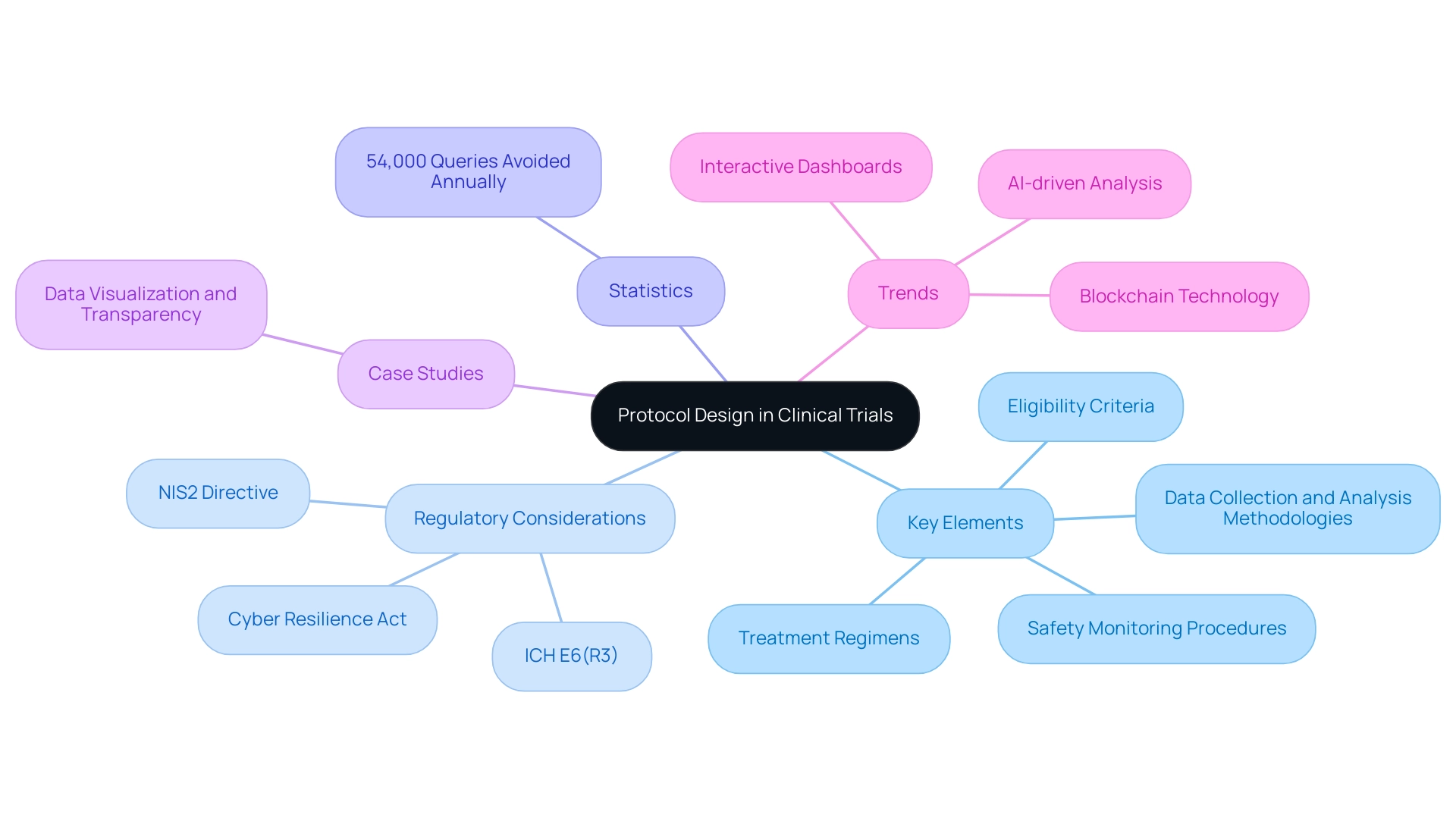
In 2025, the significance of research protocol design is underscored by new EU regulations, such as the NIS2 Directive and the Cyber Resilience Act, which aim to strengthen cybersecurity in studies. These regulations highlight the necessity for protocols that include key elements of clinical trial design, ensuring they are not only comprehensive but also adaptable to evolving standards and technologies. Regular updates and amendments to the protocol are key elements of clinical trial design that are often required as new information emerges, ensuring that the study remains relevant and compliant.
As Max Baumann from Treehill Partners observed, "Entering 2025, we still observe biotech encountering fundamental business model challenges as end-markets become increasingly crowded," highlighting the necessity for early-stage developers to concentrate on commercial outcomes in their strategies.
Statistics reveal that improving system functionality by enhancing protocol design, which is one of the key elements of clinical trial design, can prevent an estimated 54,000 queries annually, such as eliminating the need for users to input future visit dates. This efficiency not only streamlines the testing process but also contributes to better data integrity and transparency, which are key elements of clinical trial design.
At bioaccess, our extensive research management services include feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. These services are intended to assist the effective implementation of research studies while promoting global cooperation and advancement in Medtech. For example, our viability assessments utilize advanced analytics to identify optimal research locations, while our project management team employs agile methodologies to adapt to evolving project dynamics.
Case studies, such as the application of advanced information visualization techniques in research studies, demonstrate the positive impact of the key elements of clinical trial design. The introduction of new regulatory standards, such as ICH E6(R3), underscores the key elements of clinical trial design, emphasizing the need for compliance and transparency in governance while fostering a culture of openness and accountability. Key trends, including AI-driven analysis and interactive dashboards, further illustrate how key elements of clinical trial design can enhance data integrity and clarity, ultimately leading to improved study success rates.
Conducting Feasibility Assessments for Successful Trials
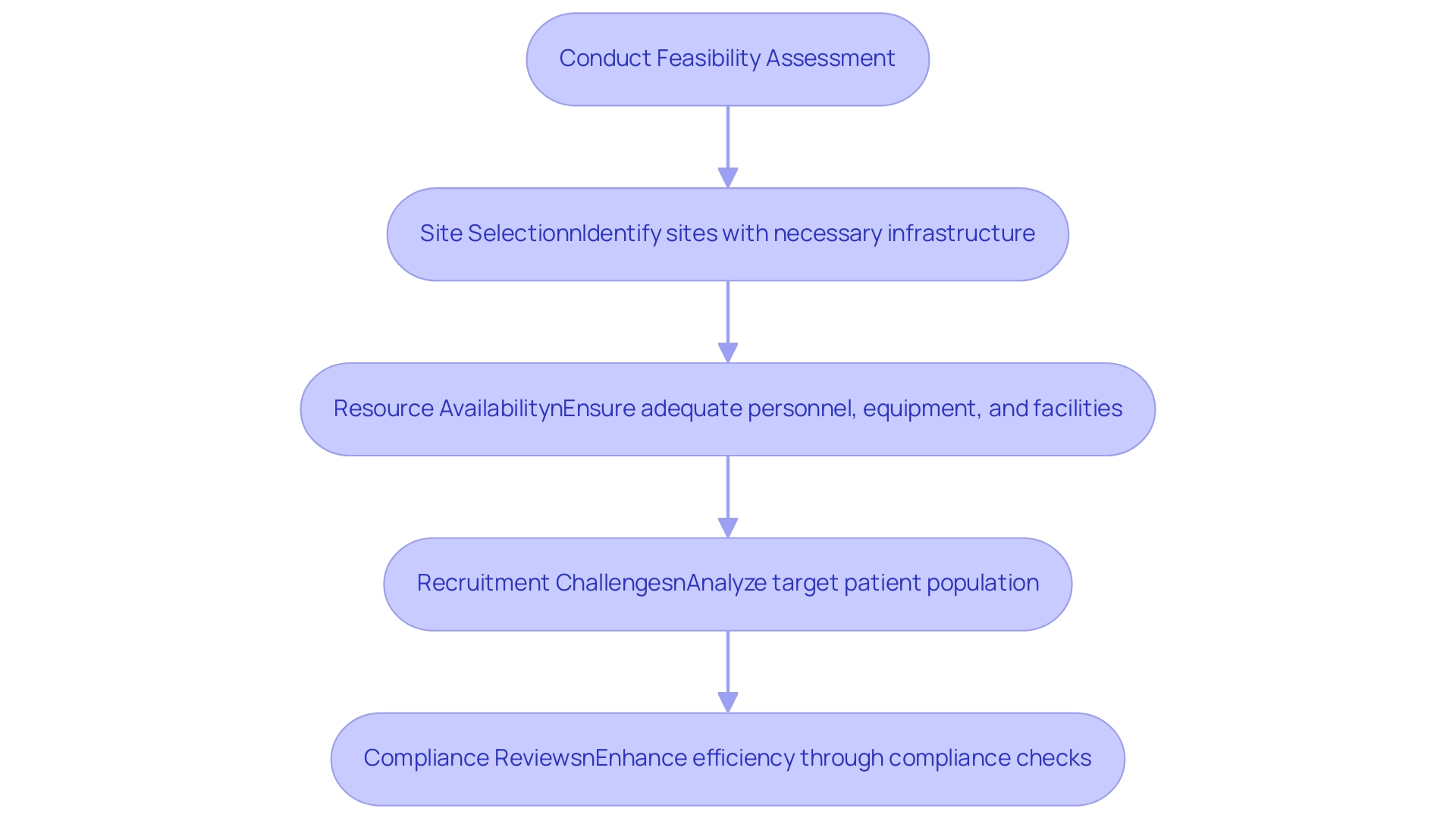
Feasibility assessments are essential in clinical studies, serving as pivotal components of clinical trial design. They encompass a comprehensive evaluation of factors such as site capabilities, patient population availability, and logistical considerations. This meticulous process is crucial for determining whether the key elements of clinical trial design can be realistically executed within designated timeframe and budget constraints.
Key components of a feasibility assessment include:
- Site Selection: Identifying sites with the necessary infrastructure and expertise to support the trial is vital. For instance, ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain in Colombia exemplifies this necessity. The capabilities of chosen locations significantly influence the overall success of medical studies.
- Resource Availability: Ensuring that adequate personnel, equipment, and facilities are accessible is a hallmark of bioaccess®'s comprehensive clinical research management services.
- Recruitment Challenges: Analyzing the target patient population is essential to anticipate potential recruitment hurdles. Bioaccess® has effectively addressed these challenges, achieving over a 50% reduction in recruitment time and 95% retention rates in their research.
In addition to these elements, bioaccess® employs specific approaches in conducting feasibility evaluations, including compliance reviews and setup, which further enhance the efficiency of their services.
Conducting thorough feasibility assessments is one of the key elements of clinical trial design, allowing researchers to proactively identify and mitigate risks early in the planning process. This strategic foresight is vital; research indicates that comprehensive feasibility evaluations correlate with significantly higher success rates. Successful feasibility studies have revealed that up to 70% of the population lives two hours or more from an academic Medical Center, underscoring the importance of selecting sites that effectively reach diverse patient demographics.
Expert insights emphasize that the capabilities of chosen locations are crucial to the overall success of research studies. The expertise and tailored approach of organizations like bioaccess® are instrumental in advancing medical devices more swiftly for companies in the medtech industry. Innovations aimed at improving site experiences focus on simplifying information entry processes and reducing administrative burdens. This not only enhances site performance but also elevates patient care, resulting in improved study outcomes and increased patient satisfaction.
As we look towards 2025, the landscape of clinical trials continues to evolve, with AI-driven analytics revolutionizing patient recruitment, retention, and endpoint tracking. These advancements render the research process more adaptive, agile, and cost-effective, further underscoring that robust feasibility assessments are key elements of clinical trial design. As the Head of Clinical Information Engineering noted, "Traditionally, information management was outsourced to our CRO vendor partners."
A part of the initiative is to bring all research in-house, enabling internal teams to engage directly. By prioritizing the key elements of clinical trial design, they can implement research in-house, take control of their data, and deliver high-quality outcomes for their patients, ensuring that their studies are well-positioned for success from the outset.
Navigating Regulatory Requirements and Compliance
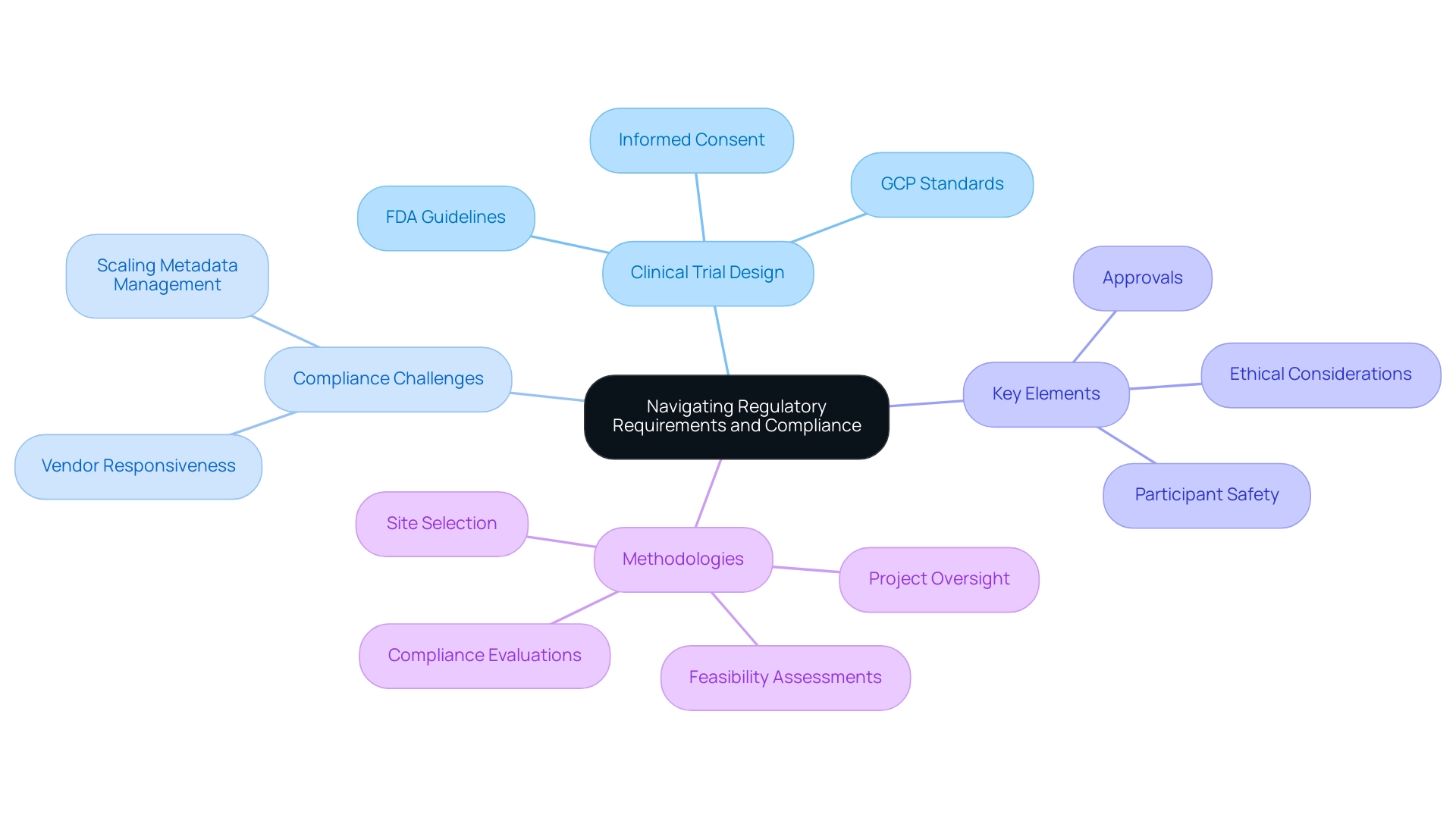
Navigating the regulatory landscape is crucial in study design. Understanding the key elements of clinical trial design—such as compliance with guidelines set by regulatory bodies like the FDA and EMA—is vital for successful execution. This process involves obtaining necessary approvals, ensuring informed consent, and strictly following Good Clinical Practice (GCP) standards. A robust understanding of these regulations not only aids in designing studies that prioritize participant safety and ethical considerations but also enhances the overall integrity of the research.
In 2025, the significance of adherence in trial design cannot be overstated. Current statistics reveal that 77% of corporate risk and compliance professionals recognize the necessity of staying updated on Environmental, Social, and Governance (ESG) developments, which directly impacts clinical research practices. This highlights a growing awareness of the complexities involved in regulatory compliance, particularly as 58% of compliance teams cite gauging vendor responsiveness as their biggest challenge in managing third-party risk.
The challenge of scaling metadata management remains a significant hurdle for many organizations, emphasizing the need for effective MDR solutions that integrate research design, data collection, analysis, and submission.
Moreover, navigating FDA regulations effectively is crucial for study success. For instance, a recent case analysis illustrated how a Medtech startup successfully aligned its clinical trial design with FDA guidelines, resulting in expedited approval processes and enhanced participant recruitment strategies. Such examples underscore the key elements of clinical trial design, particularly the importance of incorporating regulatory requirements from the outset.
At bioaccess, our extensive research management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
We utilize specific methodologies such as thorough document reviews and feedback processes to ensure compliance with country requirements. Our reporting includes detailed study status updates, inventory management, and tracking of serious and non-serious adverse events.
With experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, we ensure that our clients navigate the regulatory landscape with confidence and expertise.
Regular training and updates on regulatory changes are vital for maintaining compliance. Experts stress that anything that detracts from patient engagement can be a significant pain point for healthcare sites. As Vivienne van der Walle, Founder and Medical Director, aptly noted, "Anything that takes away time from patients is a pain point for a site, and anyone who resolves that is helping patient care."
This perspective reinforces the need for streamlined processes that prioritize patient involvement while adhering to regulatory standards, particularly in light of the challenges compliance teams face in gauging vendor responsiveness.
In summary, comprehending and maneuvering through the regulatory requirements in research studies is not merely a compliance necessity; it is a strategic imperative that involves understanding key elements of clinical trial design, which can greatly impact the success of medical research initiatives.
Effective Strategies for Participant Recruitment
Key elements of clinical trial design encompass effective participant recruitment strategies, which are vital for the success of clinical studies. To optimize recruitment, researchers must first identify their target population and develop tailored plans that specifically address potential barriers to participation. Successful examples include:
- Community outreach initiatives
- Strategic partnerships with healthcare providers
- Utilization of social media platforms to raise awareness and engage potential participants
In 2025, leveraging technology such as artificial intelligence and machine learning can significantly enhance recruitment efforts. These innovations have been demonstrated to reduce study timelines by up to 30% and costs by as much as 20%, while also improving patient recruitment and retention rates, as highlighted by the Tufts Center for the Study of Drug Development. Continuous monitoring of recruitment strategies is essential, allowing for timely adjustments to ensure enrollment goals are met without compromising participant quality.
Moreover, simplifying the enrollment process and providing clear, accessible information about the study can greatly enhance participant engagement. Statistics indicate that products developed with positive patient experiences are more likely to succeed, underscoring the importance of a participant-centered approach. Additionally, with the FDA proposing a rule to mandate single IRB review for FDA-regulated research, regulatory preparedness becomes increasingly important in recruitment strategies.
As Dipanwita Das, CEO & co-founder, notes, "Regulations are getting more complex and more prescriptive and more demanding... staying current with international regulations, so that you have a very smooth commercialization process, as well as data privacy."
At bioaccess, our extensive research management services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
By concentrating on efficient methods for participant recruitment, including community outreach and personalized communication, researchers can greatly enhance the key elements of clinical trial design and increase their likelihood of conducting successful studies. This approach not only enhances the quality of the assessments but also contributes to job creation, economic growth, and healthcare improvement in local economies, ultimately transforming lives in Latin America through advanced medtech.
Additionally, our approaches guarantee that every stage of the study is meticulously organized and performed, resulting in anticipated outcomes such as enhanced participant retention and improved information accuracy—key elements of clinical trial design essential for the success of research.
Implementing Strong Data Management Practices
Robust information management practices are vital for ensuring the accuracy and integrity of information collected during clinical trials, as they are key elements of clinical trial design. Implementing standardized procedures for information collection, entry, and validation is essential. The integration of electronic information capture (EDC) systems has revolutionized management processes, significantly reducing errors and streamlining workflows.
Research suggests that using EDC can improve information integrity, with predictive analytics expected to reduce testing expenses by 15-25%, aiding both sponsors and contract research organizations (CROs). Furthermore, integrating AI into clinical trials could reduce study timelines by up to 20%, showcasing the potential of advanced technologies in improving efficiency.
To uphold information integrity and adhere to regulatory standards, regular audits and quality checks are essential. As the regulatory landscape becomes increasingly stringent, with new rules emphasizing data privacy and patient safety, it is crucial for researchers to adopt advanced data management solutions that align with evolving regulations such as GDPR and HIPAA. As the Head of Clinical Data Engineering noted, "Part of the initiative is to bring all our research in-house so that our internal teams can start working on it. They can be more hands-on, and we implement research in-house and we are able to take control of our data, and we deliver for our patients with high quality."
In addition to these practices, bioaccess offers comprehensive trial management services that include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
These services not only improve information management but also support the local economy through job creation and healthcare enhancement. Successful instances of information management in medical research emphasize the significance of these practices.
Organizations that have embraced large information sets and predictive analytics have observed enhanced anticipation of patient reactions and treatment effectiveness, reshaping their clinical information management strategies. The case analysis titled "Regulatory Changes and Information Compliance in Clinical Research" illustrates the evolving regulatory landscape and its impact on information management practices, emphasizing the need for adherence to new regulations. Furthermore, the focus on the key elements of clinical trial design for enhancing study design and information collection reflects current trends and challenges in the industry.
As we enter 2025, the focus on key elements of clinical trial design, such as robust data management practices and extensive research study management services from bioaccess, will remain a fundamental aspect of ensuring that the integrity of data is maintained and that studies can be executed efficiently and ethically.
Exploring Future Trends in Clinical Trial Design
The landscape of medical experimentation design is undergoing a significant transformation, driven by pivotal trends that are reshaping the essential components of clinical trial design for the future of research. Decentralized clinical studies are particularly noteworthy, as they facilitate remote participation and broaden accessibility for a diverse array of participants. This evolution is especially crucial in 2025, as sponsors are increasingly expected to comply with a core outcome set across various research designs, streamlining processes and improving data comparability.
Key elements of clinical trial design encompass:
- Adaptive design approaches, which are on the rise, allowing researchers to modify protocols based on interim findings.
- Enhanced project trajectories and optimized resource allocation, which is vital in addressing the challenges faced by the biopharma sector, including high dropout rates and extended recruitment timelines that can inflate study costs.
- A professionalized approach to the key elements of clinical trial design, exemplified by bioaccess®'s comprehensive services—feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting—is crucial for improving recruitment and retention, ultimately accelerating study delivery times.
The recruitment challenges underscore the necessity for this professionalization, as evidenced by the substantial impact of dropout rates on overall trial expenses. Furthermore, the integration of real-world information and patient-centric methodologies is becoming increasingly essential within the key elements of clinical trial design. As sponsors pursue greater data ownership and transparency, there is a rising demand for technology that enables direct access to live data, transitioning towards fully insourced models. This trend not only empowers sponsors but also fosters a collaborative environment between research organizations and their clients.
In 2025, the influence of decentralized studies on participant recruitment is expected to be profound, highlighting key elements of clinical trial design, with statistics indicating improved engagement and retention rates. Notably, partnerships like that of bioaccess™ and Caribbean Health Group aim to position Barranquilla as a leading hub for medical research in Latin America, supported by Colombia's Minister of Health. This collaboration is anticipated to bolster local economies through job creation and enhanced healthcare opportunities.
As Max Baumann, Head of Execution at Treehill Partners, observes, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." By remaining attuned to these evolving trends and harnessing innovative technologies, researchers can improve the efficiency and effectiveness of medical studies—key elements of clinical trial design—ultimately paving the way for successful outcomes in the Medtech sector. bioaccess® specializes in a range of study types, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
This showcases their expertise in managing medical device clinical trials.
Conclusion
The evolving landscape of clinical trial design is pivotal for the advancement of medical research and patient care. A robust clinical trial framework is essential for guiding study execution, ensuring scientific integrity, and meeting regulatory requirements. The significance of well-structured protocols, comprehensive feasibility assessments, and effective participant recruitment strategies cannot be overstated. Collectively, these elements enhance the reliability of trial results and facilitate successful navigation of the complex regulatory landscape.
Looking ahead to 2025, emerging trends such as decentralized trials and adaptive designs are poised to transform how clinical research is conducted. Embracing innovative technologies and methodologies will not only improve participant engagement and retention but also streamline processes, ultimately driving down costs and enhancing data integrity. Insights shared by industry experts underscore the necessity of aligning clinical objectives with market needs while emphasizing compliance's significance in achieving successful outcomes.
In conclusion, the future of clinical trial design hinges on a commitment to meticulous planning, adaptability, and a patient-centered approach. By prioritizing these strategies, researchers and organizations can significantly contribute to breakthroughs that enhance healthcare delivery and foster economic growth within local communities. As the industry progresses, aligning innovative practices with regulatory standards will be crucial in shaping the next generation of clinical trials, ultimately transforming patient care for the better.
Frequently Asked Questions
What is clinical research design?
Clinical research design is a structured method that outlines the execution of an investigation, including defining the research question, selecting an appropriate study design (e.g., randomized controlled trials or cohort studies), and determining endpoints.
Why is a structured research design important by 2025?
By 2025, a structured research design is crucial due to the anticipated broader application of methodologies using human cells and tissues for drug discovery and development, which necessitates a robust framework for effective implementation.
What are the key elements of clinical trial design?
Key elements of clinical trial design include the study's objectives, target population, methods for data collection and analysis, eligibility criteria, treatment regimens, and safety monitoring procedures.
How does a well-defined research framework benefit clinical trials?
A well-defined research framework directs the entire process, ensures scientific validity and ethical integrity, reduces variability and bias, enhances replicability, and serves as a guiding document for the research team.
What advancements are expected in clinical trial design by 2025?
There is optimism for standardized outcome measures across studies, facilitating biomarker identification and therapeutic evaluation, particularly for conditions like hereditary angioedema.
What role do expert opinions play in optimizing research study frameworks?
Expert opinions emphasize the necessity of optimizing research study frameworks to achieve approval-enabling endpoints and ensure commercial viability, aligning medical objectives with market demands.
Can you provide an example of successful research design?
An example of successful research design is a study for a new hereditary angioedema therapy that utilized a comprehensive framework incorporating rigorous patient-reported outcomes and biomarker validation, resulting in reliable results and adherence to regulatory standards.
What services does bioaccess offer in research management?
Bioaccess offers extensive research management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
How do new EU regulations affect clinical trial protocol design by 2025?
New EU regulations, such as the NIS2 Directive and the Cyber Resilience Act, highlight the need for comprehensive and adaptable protocols that include key elements of clinical trial design to strengthen cybersecurity in studies.
What are the benefits of improved protocol design in clinical trials?
Improved protocol design can prevent an estimated 54,000 queries annually, streamline the testing process, and contribute to better data integrity and transparency.
How do case studies illustrate the impact of clinical trial design?
Case studies demonstrate the positive impact of key elements of clinical trial design, such as the application of advanced information visualization techniques and the introduction of new regulatory standards like ICH E6(R3), which emphasize compliance and transparency.

