Introduction
The regulatory landscape governing computerized systems in clinical investigations is undergoing a profound evolution, shaped by stringent guidelines from authorities such as the Food and Drug Administration (FDA) and the International Conference on Harmonisation (ICH).
Central to this framework is 21 CFR Part 11, which establishes critical requirements for electronic records and signatures, ensuring data integrity and security.
As medical device startups navigate these complex regulations, understanding the implications of compliance becomes paramount for successful clinical trials.
This article delves into the multifaceted world of computerized systems in clinical investigations, exploring their types, benefits, challenges, and future trends.
It highlights how these systems not only enhance research efficiency and data accuracy but also play a pivotal role in shaping the future of clinical trials amidst evolving technological advancements.
Regulatory Framework for Computerized Systems in Clinical Investigations
The regulatory landscape for computerized systems used in clinical investigations is chiefly defined by the guidelines established by the Food and Drug Administration (FDA) and the International Conference on Harmonization (ICH). Central to these regulations is 21 CFR Part 11, which outlines the requirements for electronic records and electronic signatures, ensuring the integrity, security, and confidentiality of information. Compliance with this regulation is critical, particularly for medical device startups facing regulatory hurdles and financial constraints.
Adherence to 21 CFR Part 11 significantly influences the overall success of studies, as it is a key factor in maintaining regulatory compliance across investigations. Notably, the collections of information in 21 CFR part 812 have been approved under OMB control number 0910-0078, underscoring the importance of structured data management. Moreover, adhering to Good Clinical Practice (GCP) guidelines is vital, providing a strong framework that guarantees studies are conducted ethically and scientifically.
As Lauren K. Roth, Associate Commissioner for Policy, highlighted in her recent address, the significance of comprehending these regulations cannot be overstated, as they provide researchers with the essential knowledge to navigate the complexities of using computerized systems used in clinical investigations while remaining compliant with legal mandates. Moreover, professionals such as Ana Criado, Director of Regulatory Affairs at Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for medical devices, emphasize the essential role of informed consent and compliance reviews in promoting medical device studies. Once a child reaches the legal age of consent, they must provide their own informed consent for any further research interventions, further demonstrating the evolving regulatory requirements.
Efficient record-keeping methods, as emphasized in a case study on investigational products, illustrate the importance of careful documentation concerning the receipt, shipment, and disposition of investigational materials, which is essential for both accountability and adherence to 21 CFR Part 11 in research studies. Our services also include study set-up, import permits, and comprehensive project management to ensure seamless execution and adherence throughout the investigation process. For more information, feel free to schedule a meeting with us to discuss how we can assist you in advancing your medical device studies.
Types of Computerized Systems and Their Applications in Clinical Trials
In the field of medical studies, computerized systems used in clinical investigations can be categorized into various unique types, each fulfilling a crucial role in enhancing research effectiveness and ensuring information integrity. Computerized systems used in clinical investigations, such as Electronic Data Capture (EDC) systems, are essential in gathering and managing research information, significantly simplifying the information entry process and enhancing accuracy. These computerized systems used in clinical investigations have become essential tools for researchers, ensuring that the data collected is both reliable and consistent.
Clinical Experiment Management Systems (CTMS) are examples of computerized systems used in clinical investigations that enhance this by enabling thorough planning, tracking, and oversight of research studies. Our service capabilities encompass:
- Feasibility studies
- Selection of research locations and principal investigators (PIs)
- Review and feedback on study documents to meet country requirements
- Setup, initiation, and approval processes involving ethics committees and health ministries
Significantly, user-friendly interfaces in computerized systems used in clinical investigations ensure that even individuals with limited technical abilities can effectively navigate and utilize the software, making management of research studies more accessible.
For instance, Medrio provides flexible clinical trial technology suitable for traditional, virtual, or hybrid trials, offering a suite of solutions including EDC and electronic consent. Additionally, our services include obtaining import permits and nationalization of investigational devices, as well as comprehensive project management and monitoring, which encompasses detailed reporting on study status, inventory, and both serious and non-serious adverse events. Furthermore, computerized systems used in clinical investigations, including electronic patient-reported outcomes (ePRO) systems, empower participants to report their health status directly, thereby improving the efficiency of information collection and enhancing participant engagement.
The incorporation of digital health technologies—like wearable devices and mobile health applications—has also gained traction, allowing real-time information collection and monitoring of crucial patient health metrics. As stressed by Jonathan Boel, a CSV specialist at the QbD Group Clinical Software Services & Solutions Pharma, 'Regulatory agencies such as the FDA, EMA, and others mandate that information gathered during research studies is precise, comprehensive, and consistent.' Combined, the computerized systems used in clinical investigations not only improve the quality of information gathered but also encourage more knowledgeable decision-making throughout the research process, ultimately aiding the progress of medical studies while supporting job creation and economic development within local communities.
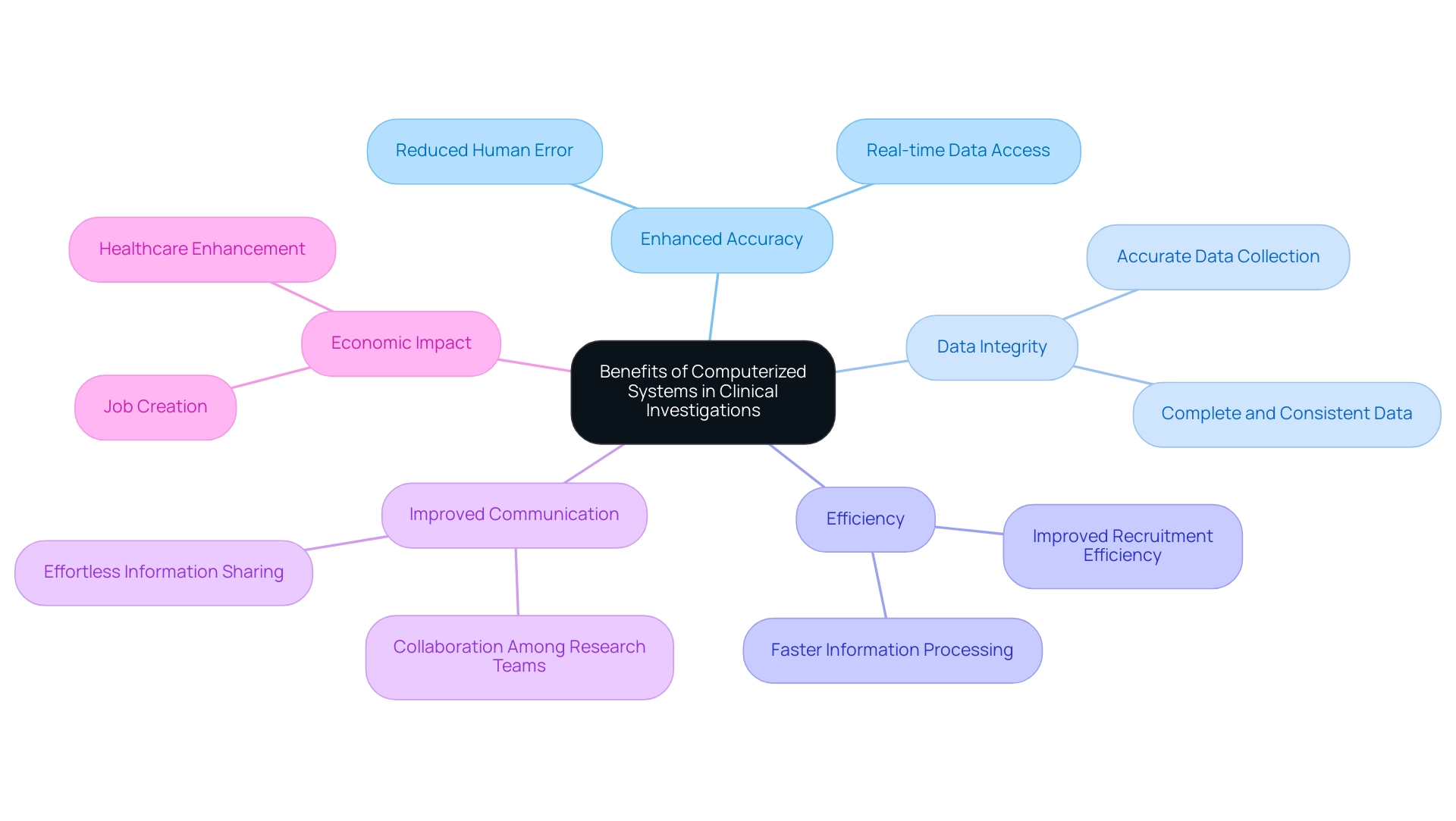
Benefits of Implementing Computerized Systems in Clinical Investigations
The implementation of computerized systems used in clinical investigations provides a variety of important benefits, especially in extensive research management services. These systems enhance accuracy by significantly reducing the likelihood of human error related to manual entry. Ensuring data integrity is vital, as it assures that the information gathered during research is accurate, complete, consistent, and trustworthy, ultimately enhancing results and participant safety.
Significantly, the partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for medical research in Latin America, endorsed by Colombia's Minister of Health. This initiative includes various service capabilities, such as:
- Feasibility studies
- Site selection
- Project management
These are essential for successful execution. Furthermore, advancements in Electronic Data Capture Systems (EDCS) enhance patient recruitment and outcome assessment in clinical trials, enabling randomization during patient visits and allowing for prescreening of eligibility to improve recruitment efficiency.
The use of computerized systems used in clinical investigations expedites information processing and analysis, decreasing the time needed to produce actionable results, while ensuring compliance with regulatory standards through built-in audit trails and documentation features. Moreover, case analyses demonstrate that Clinical Trial Management Systems (CTMS) outputs, such as real-time data access and analytics, promote improved communication and collaboration among research teams, enabling effortless information sharing and real-time updates. Ultimately, the use of computerized systems used in clinical investigations not only contributes to enhanced patient safety but also improves overall results in trials, as shown by successful applications in various research.
The effect of Medtech research on local economies, including job creation and healthcare enhancement, further emphasizes the significance of these initiatives in promoting international cooperation and economic development.
Challenges in the Adoption of Computerized Systems
The adoption of computerized systems used in clinical investigations offers numerous advantages, yet it also presents several challenges. A primary concern is the initial investment required for implementation, which can pose a significant barrier for smaller research organizations. These entities often struggle to allocate the necessary funds, making it difficult to integrate advanced technologies effectively.
For instance, Orbis Medicines recently secured €90 million in Series A funding to develop oral competitors of biologics, highlighting the financial support essential for technology adoption in research settings. Vesta Marciulioniene, Director of Regulatory Affairs at ICON, emphasizes the necessity of thorough preparation in these contexts, stating, 'This clarification was sought and allowed us to ensure we had considered the entire scope expected by the EMA, ensuring we were driving towards inspection-readiness.' Furthermore, the extensive services involved in research management, such as:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup—including obtaining import permits and nationalization of investigational devices
- Strong project management
play a crucial role in tackling these challenges.
Resistance to change from staff accustomed to traditional methods can also hinder progress, underscoring the need for extensive training and ongoing support. Data security and privacy are paramount considerations; breaches can jeopardize patient confidentiality and lead to severe regulatory consequences. Furthermore, the pilot observational cohort research titled 'Smartphone-Derived Location Data in Critical Illness' exemplifies the real-world challenges encountered when integrating computerized systems used in clinical investigations into trials, particularly in evaluating patient involvement and recovery after critical illness.
To navigate these hurdles, organizations must:
- Engage stakeholders early in the process
- Develop tailored training programs
- Establish stringent security protocols to safeguard sensitive information
Moreover, the reporting aspect—monitoring progress and documenting serious and non-serious adverse events—is vital for compliance and transparency. The impact of Medtech research studies on local economies—through job creation, economic growth, healthcare enhancement, and international collaboration—further emphasizes the significance of overcoming these barriers.
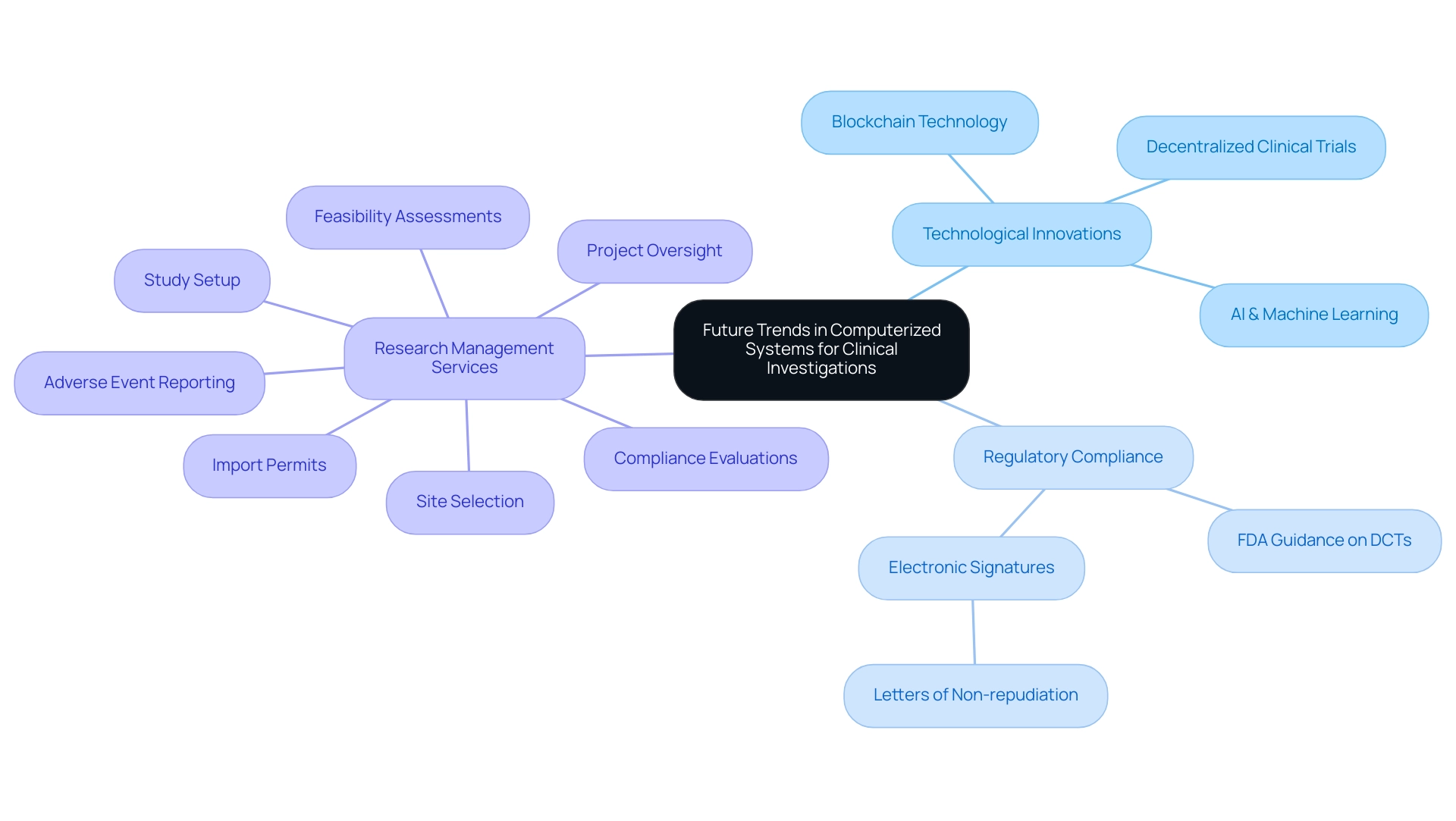
Future Trends in Computerized Systems for Clinical Investigations
The landscape of computerized systems used in clinical investigations is on the brink of significant transformation, primarily fueled by technological innovation and advanced data analytics. In 2023, the U.S. FDA emphasized the significance of decentralized clinical studies (DCTs) by providing guidance on the design and implementation, reflecting the increasing trend toward remote methodologies. According to the FDA, users of electronic signatures must present letters of non-repudiation to confirm that an electronic signature is the legally binding equivalent of a traditional handwritten signature, emphasizing the need for regulatory compliance in these studies.
Artificial intelligence (AI) and machine learning are set to be pivotal in this evolution, enabling researchers to extract insights with greater speed and precision. IQVIA Technologies, which combine data science, technology, and analytics driven by AI, support efficiencies and business insights, streamlining data analysis and enhancing the overall research process through predictive analytics and improved decision-making.
Furthermore, our extensive research study management services include essential elements such as:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting on both serious and non-serious adverse events
This not only guarantees that tests are carried out efficiently but also aids in job creation, economic growth, and healthcare enhancement within local economies through international collaboration. The integration of blockchain technology is anticipated to enhance security and traceability, addressing critical concerns regarding integrity in clinical trials.
For instance, blockchain can offer a tamper-proof record of alterations, ensuring that all modifications are transparent and verifiable. As organizations increasingly adopt these sophisticated systems, they can ensure a higher level of accountability and transparency in their information management practices. Case studies have demonstrated how North America, possessing over 33% of the global research software market share, utilizes its existing infrastructure and government backing to excel in this field.
The rise of remote and decentralized trials is further influencing the design and functionality of computerized systems used in clinical investigations, which facilitate essential features such as remote patient monitoring and efficient data collection. As these trends continue to develop, they promise to reshape clinical research, enhancing operational efficiencies and ultimately improving patient outcomes.
Conclusion
The evolution of computerized systems in clinical investigations marks a significant shift in the regulatory landscape, driven by the imperative to ensure data integrity and compliance with established guidelines. Central to this transformation is 21 CFR Part 11, which establishes the framework for electronic records and signatures, underscoring its critical role in the success of clinical trials. Medical device startups must navigate these complex regulations, as adherence not only fosters compliance but also enhances the overall quality and reliability of clinical data.
The various types of computerized systems, including Electronic Data Capture (EDC) and Clinical Trial Management Systems (CTMS), have demonstrated their value in improving research efficiency and data accuracy. These systems facilitate better planning, tracking, and management of clinical trials, ultimately leading to enhanced patient safety and more informed decision-making. The integration of innovative technologies, such as artificial intelligence and blockchain, further supports advancements in clinical research while addressing concerns about data security and traceability.
Despite the clear advantages, challenges remain in the adoption of these systems, particularly for smaller organizations facing financial constraints and resistance to change. However, with the right strategies—such as tailored training and stakeholder engagement—these barriers can be overcome, paving the way for more effective clinical trials.
As the regulatory environment continues to evolve, the future of computerized systems in clinical investigations looks promising. Decentralized clinical trials and advanced data analytics are set to redefine the landscape, promoting operational efficiencies and improved patient outcomes. Embracing these trends is essential for stakeholders looking to thrive in the dynamic world of clinical research.
Frequently Asked Questions
What are the main regulatory guidelines for computerized systems used in clinical investigations?
The main regulatory guidelines are established by the Food and Drug Administration (FDA) and the International Conference on Harmonization (ICH), with 21 CFR Part 11 being central to these regulations.
What does 21 CFR Part 11 cover?
21 CFR Part 11 outlines the requirements for electronic records and electronic signatures, ensuring the integrity, security, and confidentiality of information in clinical investigations.
Why is compliance with 21 CFR Part 11 important for medical device startups?
Compliance is critical for medical device startups as it helps them navigate regulatory hurdles and financial constraints, significantly influencing the overall success of their studies.
What are Good Clinical Practice (GCP) guidelines, and why are they important?
Good Clinical Practice (GCP) guidelines provide a framework to ensure that studies are conducted ethically and scientifically, which is vital for maintaining regulatory compliance.
What role does informed consent play in medical device studies?
Informed consent is essential for promoting medical device studies, and once a child reaches the legal age of consent, they must provide their own informed consent for further research interventions.
How does efficient record-keeping contribute to compliance with 21 CFR Part 11?
Efficient record-keeping methods ensure careful documentation regarding the receipt, shipment, and disposition of investigational materials, which is essential for accountability and adherence to regulations.
What services are offered to assist with medical device studies?
Services include study set-up, import permits, project management, and comprehensive support to ensure seamless execution and adherence throughout the investigation process.
What types of computerized systems are used in clinical investigations?
Examples include Electronic Data Capture (EDC) systems and Clinical Trial Management Systems (CTMS), which enhance research effectiveness and ensure data integrity.
How do computerized systems improve the research process?
They simplify the information entry process, enhance accuracy, enable thorough planning and tracking of studies, and empower participants to report health status directly.
What is the significance of digital health technologies in clinical investigations?
Digital health technologies, like wearable devices and mobile health applications, allow for real-time information collection and monitoring of crucial patient health metrics, improving the efficiency of research.




