Overview
The primary focus of this article is mastering clinical trial oversight in Argentina, highlighting the critical need for understanding regulatory frameworks and implementing effective project management strategies. This discussion is supported by an examination of ANMAT's role in overseeing clinical trials, the necessity of adhering to Good Clinical Practice guidelines, and the imperative for ongoing staff training to ensure compliance and elevate the quality of research studies. By addressing these elements, the article underscores the significance of collaboration and the next steps necessary for success in the clinical research landscape.
Introduction
In the rapidly evolving landscape of clinical trials, understanding the regulatory frameworks is paramount, particularly in regions like Argentina.
With the National Administration of Drugs, Food and Medical Technology (ANMAT) at the helm, navigating the complexities of Good Clinical Practice (GCP) standards is essential for contract research organizations (CROs).
As Argentina emerges as a leading hub for clinical research in Latin America, organizations must remain vigilant regarding:
- Regulatory changes
- Recruitment challenges
- Effective project management strategies
to ensure compliance and success.
This article delves into the key aspects of conducting clinical trials in Argentina, underscoring the critical importance of robust training and development to uphold the integrity of research while leveraging the country's potential for groundbreaking medical advancements.
Understand Regulatory Frameworks for Clinical Trials in Argentina
In Argentina, clinical trial oversight is primarily managed by the National Administration of Drugs, Food and Medical Technology (ANMAT). Understanding ANMAT's guidelines, particularly the Good Clinical Practice (GCP) standards, is crucial for any contract research organization (CRO) involved in clinical trial oversight. Key regulations mandate ethical approval from local ethics committees and adherence to international standards that prioritize patient safety and data integrity, essential components of clinical trial oversight. Organizations must ensure that all documentation is meticulously prepared and submitted to ANMAT, as this diligence can significantly influence the approval timeline. The significance of GCP standards is paramount for clinical trial oversight in Argentina, serving as the foundation for conducting ethical and scientifically valid studies. In 2025, adherence to these guidelines will be crucial for ensuring compliance and enabling the successful implementation of research studies related to clinical trial oversight in Argentina.
Recent developments, including the introduction of new medical device regulations, underscore the importance for CROs to remain updated on regulatory changes. For instance, the case study on controlled substances regulation illustrates the rigorous control measures in place that ensure public safety and mitigate risks associated with misuse while allowing for legitimate medical use under regulated conditions. This attentiveness is essential to prevent setbacks in study commencement and to guarantee that all medical activities conform with the evolving regulatory environment.
bioaccess® focuses on overseeing a variety of medical research, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies (PMCF). With over 20 years of experience in Medtech, their expertise ensures that organizations can navigate the complexities of research trials effectively, leveraging Argentina's potential as a prime location for studies.
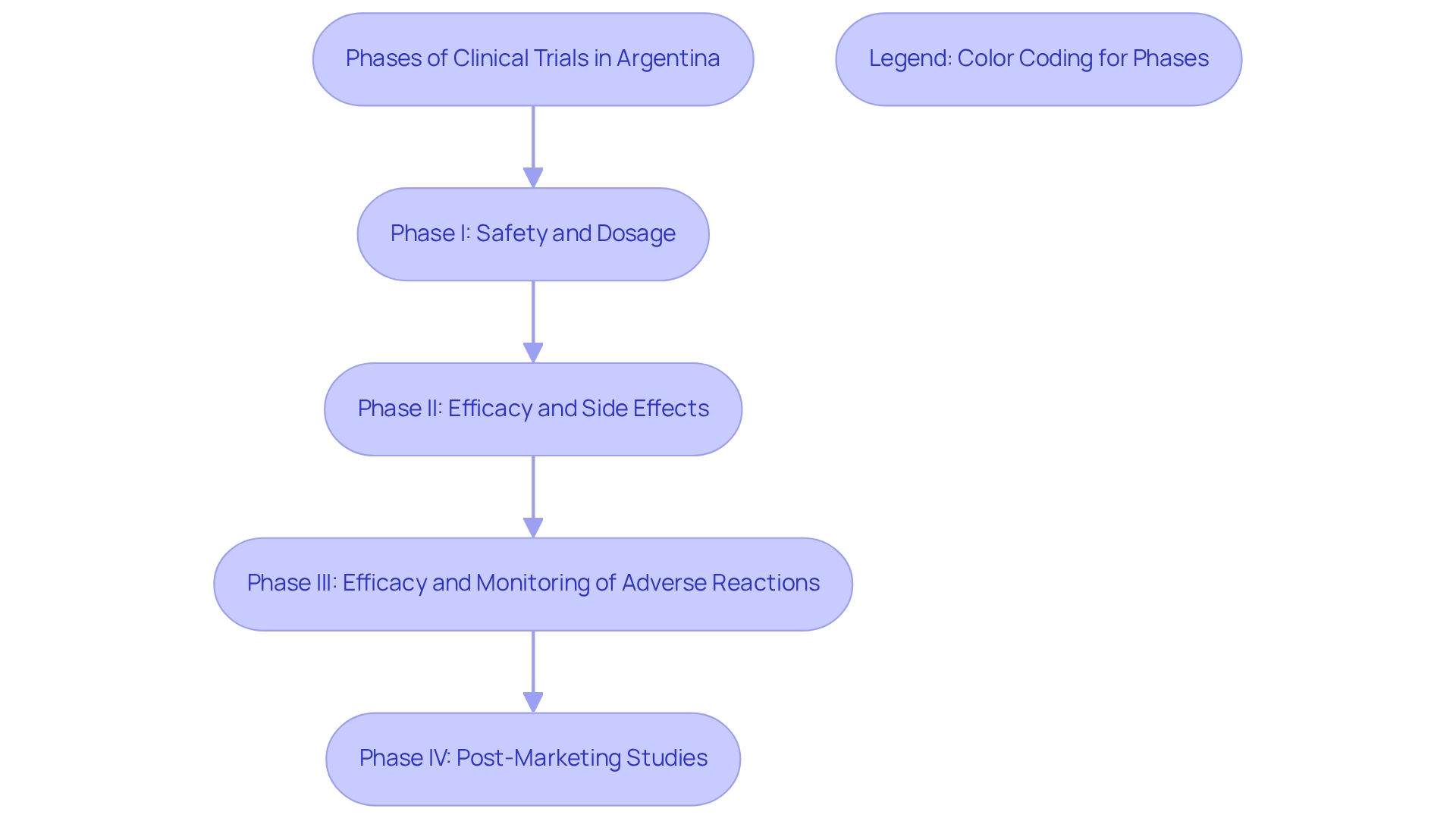
Research studies in Argentina are categorized by phase, which includes:
- Phase I: Safety and dosage
- Phase II: Efficacy and side effects
- Phase III: Efficacy and monitoring of adverse reactions
- Phase IV: Post-marketing studies
Argentina's position as the fastest-growing market for trials in Latin America further underscores the importance of robust regulatory compliance, as the country is projected to reach USD 506.1 million by 2030. By effectively navigating the regulatory complexities, organizations can leverage Argentina's potential as a prime site for medical research, particularly through clinical trial oversight. However, CROs must be aware of common pitfalls, such as failing to keep up with regulatory updates or misinterpreting guidelines, which can hinder their progress. While the number of registered research studies in Argentina as of December 2024 is currently unspecified, recognizing this gap in data is essential for maintaining transparency. Additionally, bioaccess® is committed to ensuring information security and addressing client concerns through established grievance and data protection procedures, reinforcing their dedication to compliance and client trust.
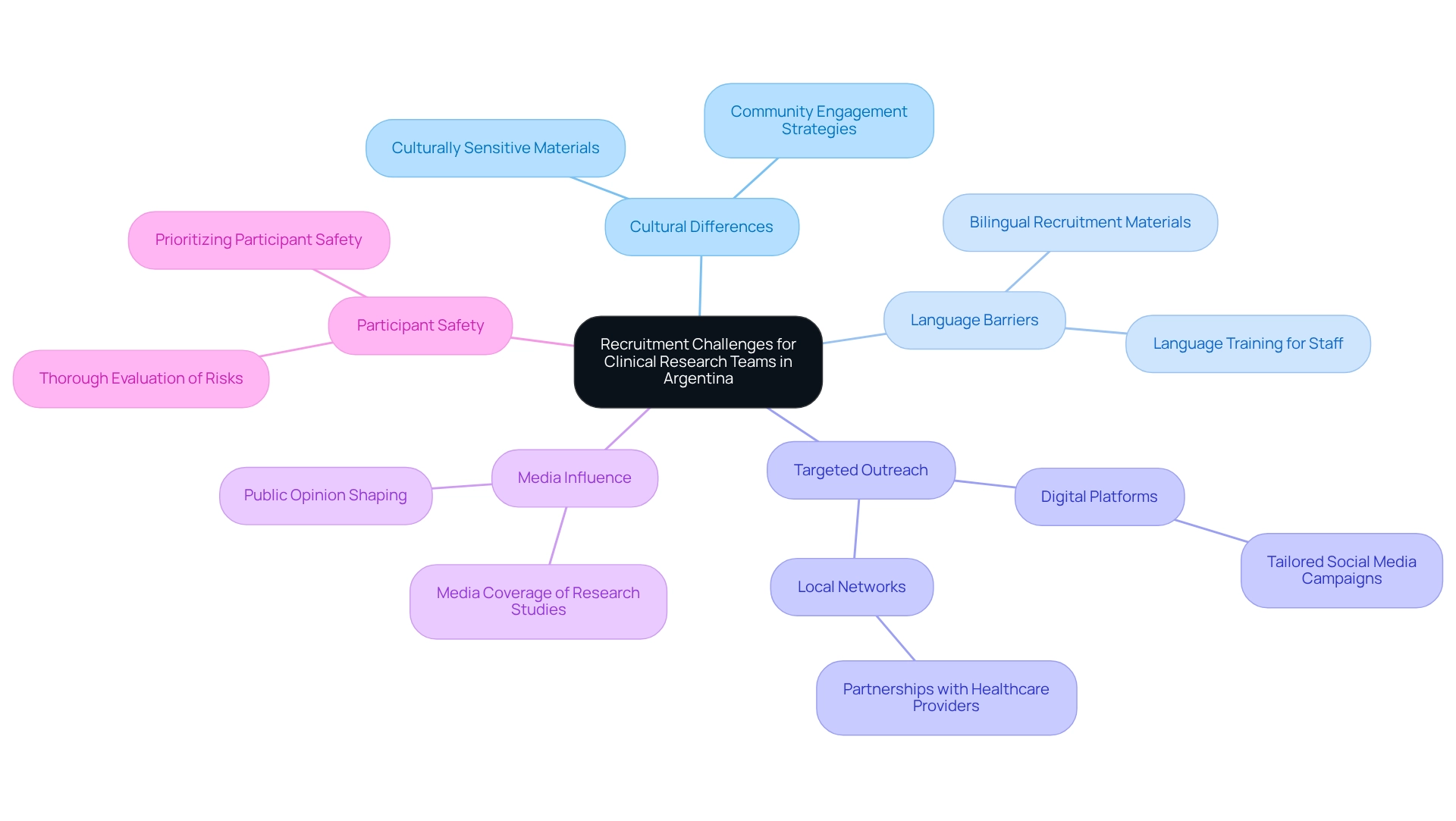
Address Recruitment Challenges for Clinical Research Teams
Recruitment challenges in Argentina stem from a multitude of factors, including cultural differences, language barriers, and the need for targeted outreach. To effectively enhance recruitment efforts, research teams must leverage local networks and implement community engagement strategies. Digital platforms can significantly broaden outreach; for example, tailored social media campaigns aimed at specific demographics can heighten awareness and stimulate curiosity about research studies.
Moreover, establishing partnerships with local healthcare providers can facilitate referrals and build participant trust. It is essential that recruitment materials are culturally sensitive and available in both Spanish and English, as this approach can markedly improve engagement and participation rates.
In 2025, statistics indicate that recruitment challenges remain prevalent, with 16% of total assessments categorized as 'Other,' underscoring the difficulties many studies face in meeting enrollment targets. As Samruddhi Yardi observes, "There can be risks, such as potential side effects of the treatment being tested, but these are thoroughly evaluated, and participant safety is a top priority."
Additionally, media coverage of research studies in Latin America, particularly by platforms such as Clinical Leader, plays a significant role in shaping public opinion and can enhance recruitment efforts. By adopting these strategies and harnessing media influence, research teams can more effectively navigate the complexities of recruitment in Argentina, which is vital for clinical trial oversight and ultimately contributes to the local economy through job creation and healthcare advancements.
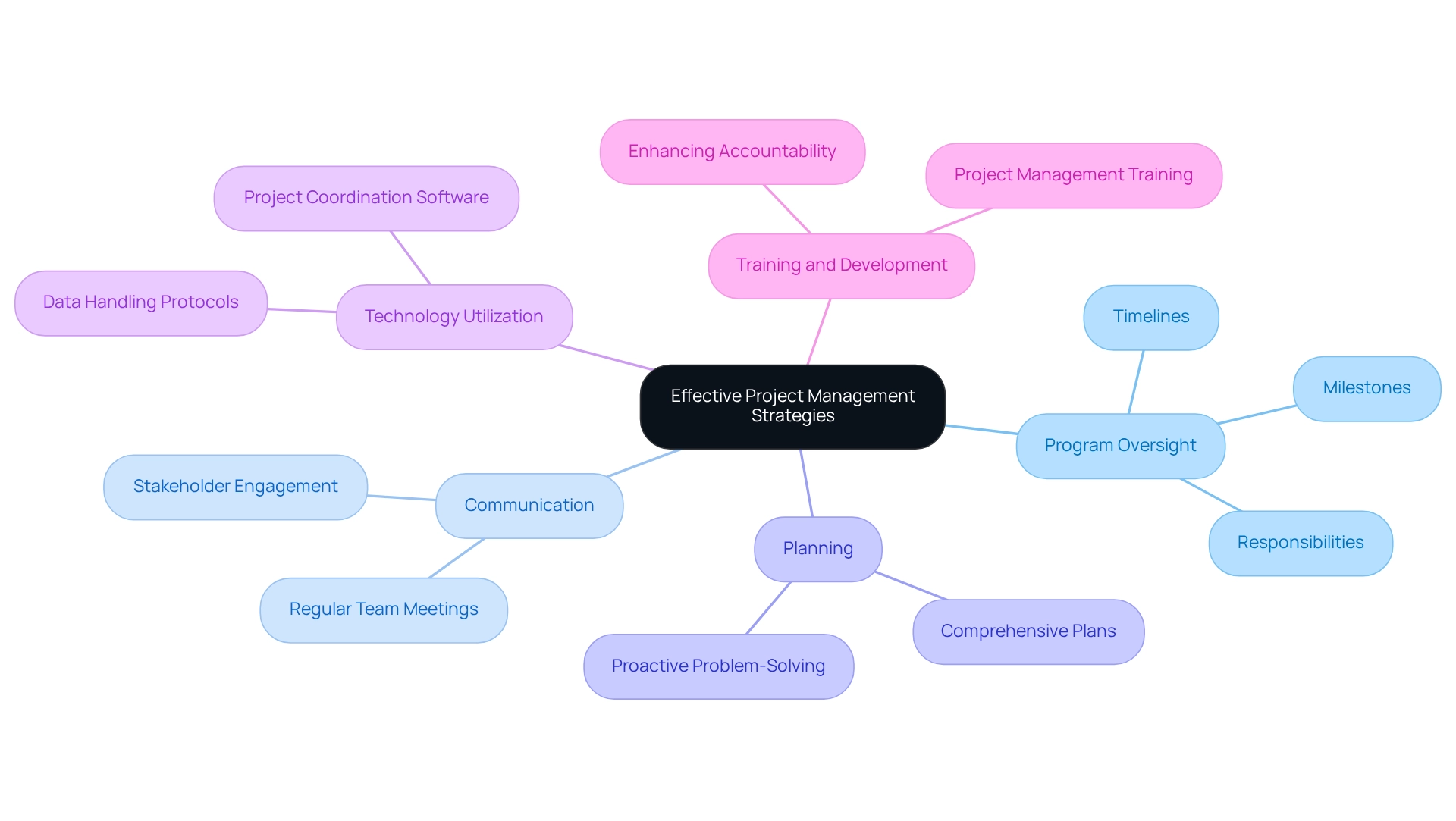
Implement Effective Project Management Strategies
Applying effective program oversight strategies is essential for the success of clinical trial oversight in Argentina, particularly in the evolving landscape of Latin America. A comprehensive plan must be created, detailing timelines, milestones, and responsibilities for each team member. The implementation of project coordination software can significantly enhance communication and facilitate progress tracking, ensuring that all stakeholders remain informed and engaged. Regular team meetings are crucial for addressing challenges and making necessary adjustments to timelines, thereby fostering a proactive approach to problem-solving.
At bioaccess®, we recognize the unique challenges of conducting research studies in Latin America, including regulatory compliance and resource allocation. Establishing clear protocols for data handling and reporting not only enhances transparency but also strengthens accountability among team members. In a landscape where 41% of research professionals struggle to illustrate the added value of offices overseeing initiatives (PMOs), effective strategies can directly address this challenge by highlighting tangible benefits. As Stephen Covey wisely remarked, "Technology and tools are beneficial and strong when they serve you and not control you," underscoring the importance of utilizing technology as a supportive resource in overseeing tasks.
Moreover, the impact of program coordination software on clinical trial success rates cannot be overstated. Effective executions have shown that organized task oversight procedures can greatly minimize waste and enhance results, as evidenced by case studies examining task oversight performance statistics. These studies demonstrate that organizations with established management practices achieve higher success rates, emphasizing the necessity of careful planning and execution.
In Argentina, where the clinical research landscape is rapidly evolving, organizations like bioaccess® are well-positioned to leverage their expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, thereby contributing to effective clinical trial oversight. By fostering a cooperative atmosphere and encouraging transparent communication, managers can align their teams efficiently, ensuring a shared focus on achieving the goals of the experiment. Furthermore, with only 34% of underperformers offering similar training, investing in training and development for project management is crucial to circumvent common pitfalls and enhance overall project success.
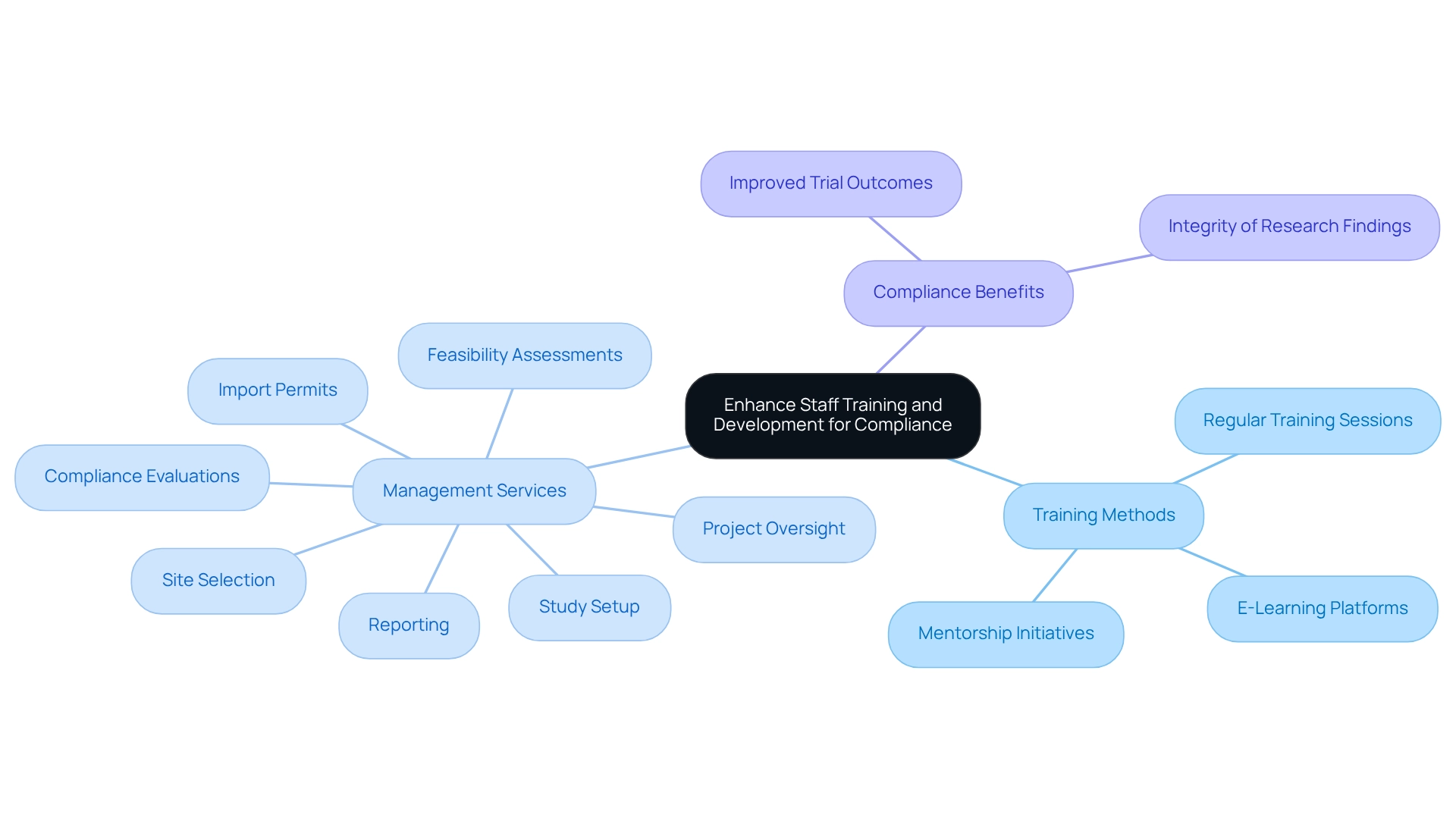
Enhance Staff Training and Development for Compliance
Investing in employee training and development is essential for maintaining compliance and ensuring the integrity of medical studies. Regular training sessions are vital to keep team members informed about the latest regulatory changes, Good Clinical Practice (GCP) guidelines, and ethical considerations. Statistics indicate that effective GCP training can lead to a significant reduction in protocol deviations, thereby enhancing the overall quality of research studies. E-learning platforms provide flexible training options that can accommodate various schedules, facilitating continuous education for staff. Furthermore, establishing mentorship initiatives allows less experienced team members to learn from seasoned experts, promoting a culture of knowledge exchange and collaboration.
At bioaccess, our comprehensive management services for research studies include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
This thorough approach ensures our team is well-equipped to navigate the complexities of research studies, especially in the context of clinical trial oversight in Argentina, where regulatory frameworks are evolving. A case study titled 'Ethics Approval for the Study' illustrates how obtaining ethics approval from the University of Wollongong Human Research Ethics Committee ensured adherence to ethical standards, thereby reinforcing the integrity of research findings.
Prioritizing staff development not only enhances team capabilities but also results in improved trial outcomes and compliance, ultimately benefiting the entire clinical research process. As Lauren Houston emphasizes, it is advisable to further compare what is documented in the literature with current practices at the site level to identify gaps, facilitators, and barriers to implementing data quality management systems. This insight highlights the critical role of comprehensive training programs in addressing compliance challenges.
Conclusion
Navigating clinical trials in Argentina necessitates a profound understanding of the regulatory frameworks set forth by the National Administration of Drugs, Food and Medical Technology (ANMAT). Adherence to Good Clinical Practice (GCP) standards is paramount for ensuring ethical conduct and reliable research outcomes. As Argentina establishes itself as a pivotal player in Latin America's clinical research sector, it is imperative for organizations to remain informed about regulatory updates, address recruitment challenges, and implement effective project management strategies.
Enhancing recruitment can be achieved through culturally sensitive approaches and the utilization of local networks. Leveraging digital platforms and forming partnerships with healthcare providers can significantly elevate participant engagement. Furthermore, robust project management practices streamline trial processes, ensuring clear communication and efficient operations.
Investing in staff training is essential for maintaining compliance and enhancing trial quality. Regular training sessions keep team members abreast of regulatory changes and ethical guidelines, which aids in reducing protocol deviations and bolstering research integrity.
In summary, as Argentina continues to evolve within the global clinical research landscape, organizations must prioritize regulatory compliance, innovative recruitment methods, and strong project management. By adopting these strategies, they can capitalize on the country's potential for groundbreaking medical advancements while upholding high standards of patient safety and research reliability. This commitment will not only facilitate successful clinical trials but also contribute to the overall advancement of healthcare in the region.
Frequently Asked Questions
What organization manages clinical trial oversight in Argentina?
Clinical trial oversight in Argentina is primarily managed by the National Administration of Drugs, Food and Medical Technology (ANMAT).
Why are ANMAT's guidelines important for contract research organizations (CROs)?
ANMAT's guidelines, particularly the Good Clinical Practice (GCP) standards, are crucial for CROs as they ensure ethical approval, patient safety, and data integrity in clinical trials.
What are the key components required for clinical trial oversight in Argentina?
Key components include ethical approval from local ethics committees, adherence to international standards, meticulous documentation preparation, and submission to ANMAT.
What is the significance of Good Clinical Practice (GCP) standards in Argentina?
GCP standards serve as the foundation for conducting ethical and scientifically valid studies, and adherence to these guidelines will be essential for compliance and successful research implementation by 2025.
What recent developments should CROs in Argentina be aware of?
CROs should stay updated on new medical device regulations and the rigorous control measures surrounding the regulation of controlled substances to prevent setbacks in study commencement.
What types of medical research does bioaccess® oversee?
bioaccess® oversees a variety of medical research, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Medical Follow-Up Studies (PMCF).
How are clinical research studies in Argentina categorized?
Research studies in Argentina are categorized by phase: Phase I: Safety and dosage; Phase II: Efficacy and side effects; Phase III: Efficacy and monitoring of adverse reactions; Phase IV: Post-marketing studies.
What is Argentina's market projection for clinical trials by 2030?
Argentina is projected to reach USD 506.1 million by 2030, making it the fastest-growing market for trials in Latin America.
What common pitfalls should CROs avoid in Argentina?
CROs should avoid failing to keep up with regulatory updates and misinterpreting guidelines, as these can hinder their progress in clinical trial oversight.
How does bioaccess® ensure information security and client trust?
bioaccess® is committed to ensuring information security and addressing client concerns through established grievance and data protection procedures.




