Overview
This article delineates the essential steps and strategies necessary for mastering medical device trial design in Argentina, highlighting the critical importance of understanding regulatory requirements and effective recruitment methods. It begins by emphasizing the necessity of familiarizing oneself with ANMAT regulations, preparing the required documentation, and developing targeted recruitment plans.
By detailing these critical steps, the article offers a comprehensive framework that researchers can follow to ensure compliance and achieve success in their clinical studies. Understanding the Medtech landscape and the role of bioaccess is vital in addressing key challenges faced in this field.
Ultimately, the importance of collaboration among stakeholders is underscored, paving the way for actionable next steps in clinical research.
Introduction
Navigating the complex landscape of medical device trials in Argentina demands a keen understanding of regulatory frameworks and strategic planning. With the National Administration of Drugs, Foods, and Medical Devices (ANMAT) at the helm, it is imperative for researchers to familiarize themselves with evolving regulations to ensure compliance and success.
This article explores essential steps for conducting trials, including:
- Comprehending ANMAT guidelines
- Implementing effective recruitment strategies
All while underscoring the critical importance of ethical considerations and participant safety. As the demand for innovative medical solutions escalates, so too does the necessity for rigorous trial design and management, making it essential for stakeholders to remain informed and prepared for the challenges that lie ahead.
Understand the Regulatory Landscape for Medical Device Trials in Argentina
To effectively carry out medical device trial design in Argentina, a thorough comprehension of the framework set by the National Administration of Drugs, Foods, and Medical Devices (ANMAT) is crucial. Here are the critical steps to follow:
-
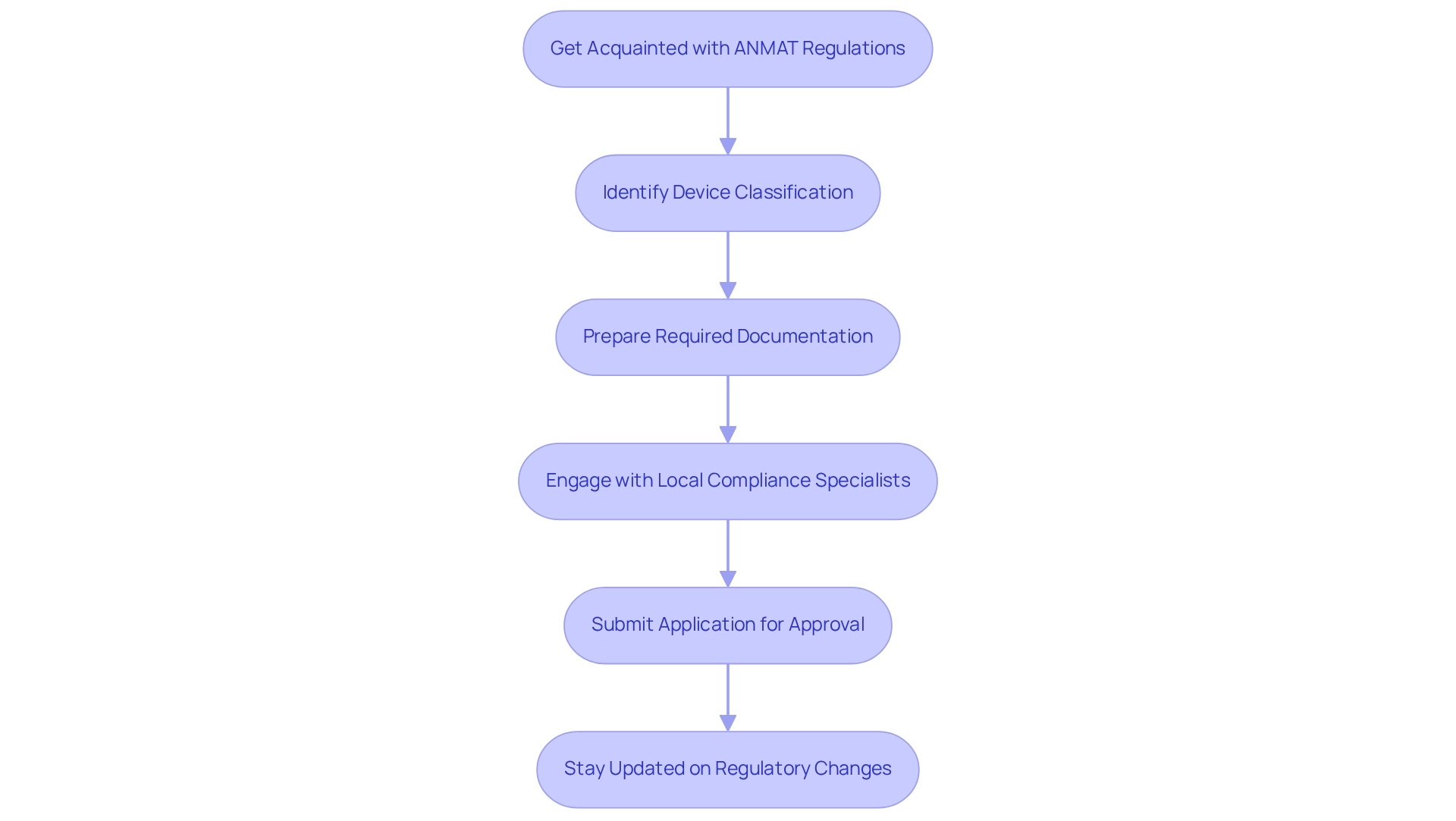
Get Acquainted with ANMAT Regulations: Examine the most recent regulations, especially Resolution 237/2024, which outlines the requirements for medical equipment studies. This resolution is essential as it broadens the range of oversight and adherence, ensuring that evaluations meet the highest standards.
-
Identify Device Classification: Classify your medical device as Class I, II, III, or IV. This classification will establish the approval pathway and specific requirements for your clinical studies, impacting the authorization process.
-
Prepare Required Documentation: Compile all necessary documentation, including clinical trial protocols, informed consent forms, and safety data. It is vital that these documents adhere to ANMAT guidelines to facilitate a smooth review process.
-
Engage with Local Compliance Specialists: Collaborate with local compliance specialists or legal advisors who focus on medical device regulations. Their insights can help ensure compliance and streamline the approval process, reducing potential delays. bioaccess® offers expertise in feasibility studies and site selection, which can be invaluable in this phase.
-
Submit Application for Approval: After preparing all documentation, submit your application to ANMAT for review. Be ready to address any queries or requests for additional information from the regulatory body, as this is a common part of the approval process.
-
Stay Updated on Regulatory Changes: Continuously monitor updates on regulations and guidelines from ANMAT. Staying informed about changes is crucial for maintaining compliance throughout the testing process.
Considering recent improvements in hospital facilities and digital health projects, Argentina is increasingly positioned to conduct clinical studies effectively. Ethical considerations, such as the significance of clinical study registration, further highlight the necessity for transparency and accountability in research involving human participants. As Luis G. Cuervo pointed out, "A campaign regarding the significance of clinical study registration, especially the ethical and moral justifications for registering, along with details about existing registries and accessible tools, could help reduce the knowledge barrier, inform sponsors of their responsibilities, and motivate investigators to act as enforcers."
This highlights the critical role of registration in enhancing the integrity of clinical research. Furthermore, adverse event reporting systems are crucial for pharmacovigilance, gathering information on safety risks linked to medical equipment. Recent research suggests that registration of experiments is becoming a requirement for publication in numerous journals, with 50% of responding journals acknowledging this necessity.
By following these guidelines and comprehending the changing compliance environment, researchers can effectively maneuver through the intricacies of medical device trial design in Argentina. Furthermore, bioaccess® is committed to ensuring information security and client trust, addressing any concerns through established grievance and data protection procedures.
Outline the Key Steps in Designing a Medical Device Trial
Designing a medical device trial involves several critical steps that ensure the study's success and compliance with regulatory standards:
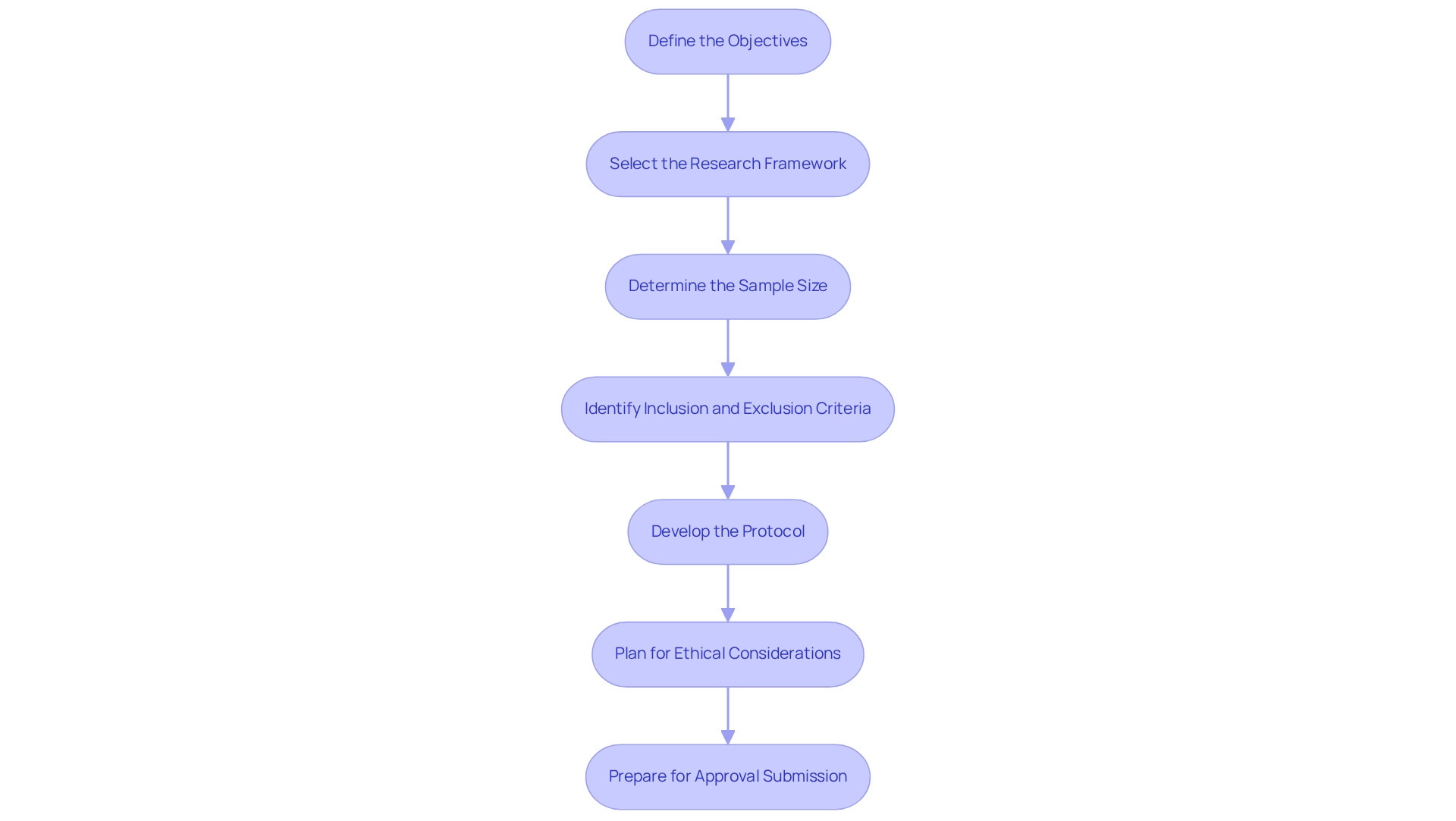
- Define the Objectives: Clearly outline both primary and secondary objectives. Comprehending what you intend to demonstrate or assess with your medical instrument is crucial for steering the study's course.
- Select the Research Framework: Choose an appropriate research framework, such as a randomized controlled experiment or observational analysis, tailored to your objectives and the specific characteristics of the device. bioaccess® specializes in various research types, including Early-Feasibility Research (EFS), First-In-Human Research (FIH), Pilot Research, Pivotal Research, and Post-Market Clinical Follow-Up Research (PMCF), ensuring a comprehensive approach to design.
- Determine the Sample Size: Calculate the necessary sample size to achieve statistically significant results. This calculation should consider the expected effect size and variability within the target population. bioaccess® utilizes its vast expertise to aid in this process, guaranteeing that studies are structured efficiently and effectively.
- Identify Inclusion and Exclusion Criteria: Establish clear criteria for participant selection to ensure that the study population aligns with the study's objectives, enhancing the validity of the results.
- Develop the Protocol: Create a comprehensive study protocol detailing the methodology, including recruitment strategies, data collection methods, and statistical analysis plans. This document acts as the blueprint for the experiment.
- Plan for Ethical Considerations: Integrate ethical considerations into the study design, ensuring informed consent and participant safety are prioritized throughout the research.
- Prepare for Approval Submission: Gather all essential documentation and get ready for submission to INVIMA, the Colombia National Food and Drug Surveillance Institute, for authorization before starting the study. This step is essential for compliance and successful execution of the process.
By following these steps, clinical research directors can effectively create studies that not only meet regulatory requirements but also contribute to the advancement of innovative medical devices through medical device trial design in Argentina. Insights from recent research, such as ReGelTec's Early Feasibility Analysis on HYDRAFIL™ for addressing chronic low back pain in Colombia, demonstrate the successful application of these principles in real-world scenarios. Furthermore, utilizing Bayesian methods in study design can further improve efficiency and results, as emphasized in the case study "Future Directions for Bayesian Approaches in Medical Devices."
This trend is highlighted by the endorsement of sixteen medical instruments by the FDA utilizing these approaches, stressing the significance of modifying testing designs to utilize contemporary methodologies for enhanced outcomes. Furthermore, decentralized research has demonstrated the capability to swiftly recruit, enroll, and complete clinical investigations with a diverse and satisfied participant pool, as noted by Ravindran et al.
Implement Effective Recruitment and Compliance Strategies
To ensure successful recruitment and compliance in your medical device trial, consider the following strategies:
-
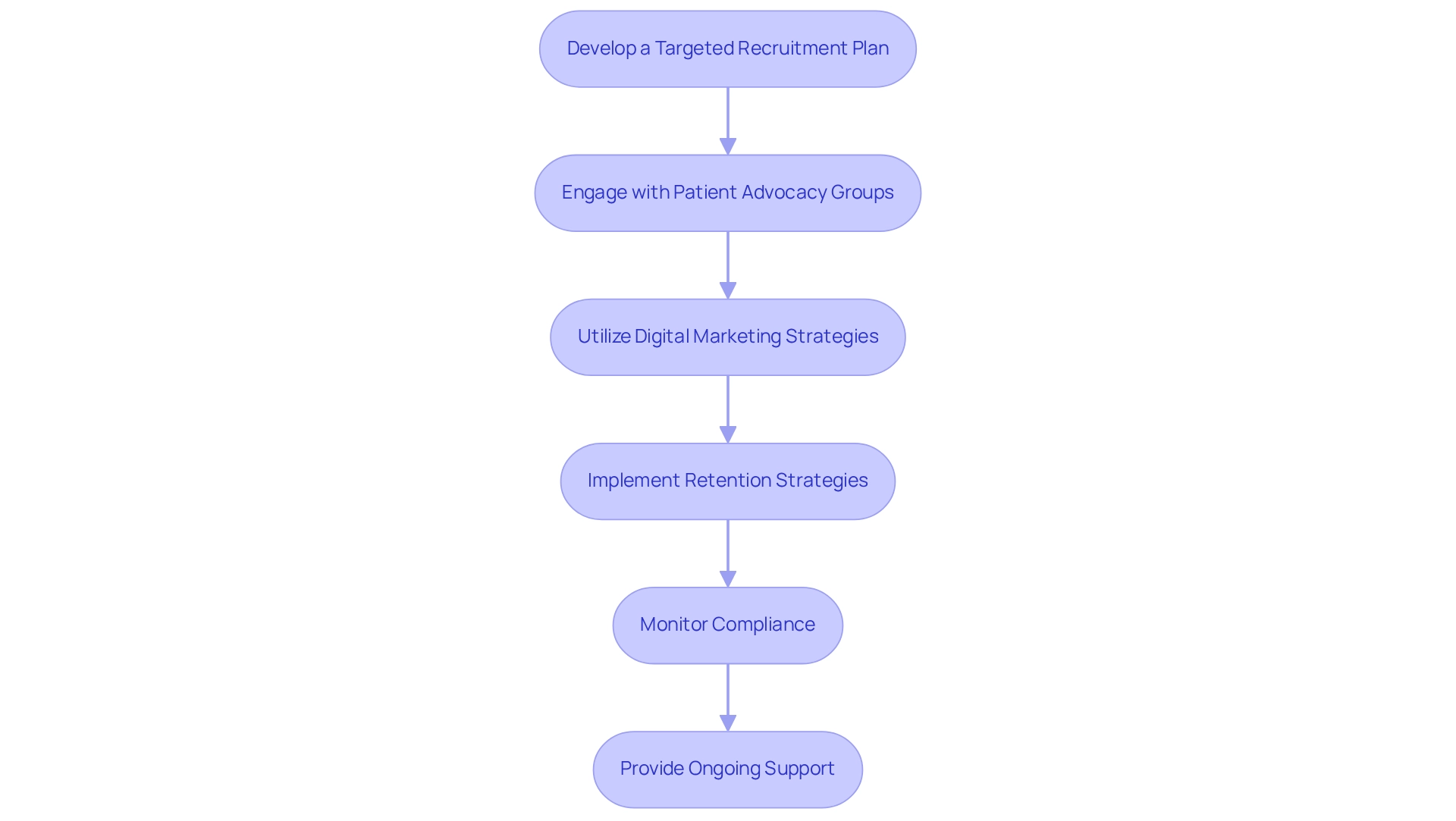
Develop a Targeted Recruitment Plan: Clearly define the demographics of your target population and customize your recruitment strategies to align with their needs. Engage local healthcare providers, community organizations, and online platforms to effectively reach potential participants in the medical device trial design in Argentina.
-
Engaging with patient advocacy groups can significantly enhance recruitment efforts for medical device trial design in Argentina. These organizations can assist in increasing awareness about the medical device trial design in Argentina and its potential advantages, cultivating trust and interest among possible participants.
-
Utilize digital marketing strategies, including social media campaigns, to broaden your outreach in the context of medical device trial design in Argentina. This method can draw in participants who may not be informed about the study, indicating a transition towards greater online involvement in recruitment tactics relevant to medical device trial design in Argentina. According to a 2019 study, traditional recruitment tactics were more commonly used among sponsor companies and CROs, indicating a need for adaptation in current practices. Ken Getz from the Tufts Center for the Study of Drug Development noted that comparisons from 2012 to 2023 show increased usage of websites and social media, highlighting the trend towards online recruitment post-pandemic.
-
Implement Retention Strategies: Retaining participants throughout the study is crucial. Develop strategies within the medical device trial design in Argentina that include regular communication, updates on trial progress, and prompt responses to participant concerns, ensuring they feel valued and informed. The case study titled 'Team Effort in Recruitment and Retention' emphasizes that medical device trial design in Argentina involves collaborative efforts requiring the involvement of various team members to achieve common goals.
-
Monitor Compliance: Establish a robust compliance monitoring system in the context of medical device trial design in Argentina to ensure adherence to regulatory requirements and ethical standards. This should include regular audits and thorough training for all personnel engaged in the experiment. Utilizing the knowledge of bioaccess® in medical device trial design in Argentina can improve compliance and simplify processes, guaranteeing that your research adheres to all required regulations.
-
Provide Ongoing Support: Offer continuous assistance to participants throughout the study. Ensure they have access to healthcare professionals who can address any questions or concerns related to the medical device trial design in Argentina, enhancing their overall experience and commitment to the study. Emphasizing patient experience has been demonstrated to enhance the chances of successful recruitment and retention in clinical studies, highlighting the significance of participant involvement throughout the study process. Furthermore, bioaccess®'s dedication to data protection and client trust guarantees that participant information is managed with the highest regard, further boosting their confidence in the study.
Evaluate Outcomes and Ensure Regulatory Compliance
To successfully assess results and ensure compliance with regulations in medical equipment trials, it is imperative to follow these steps:
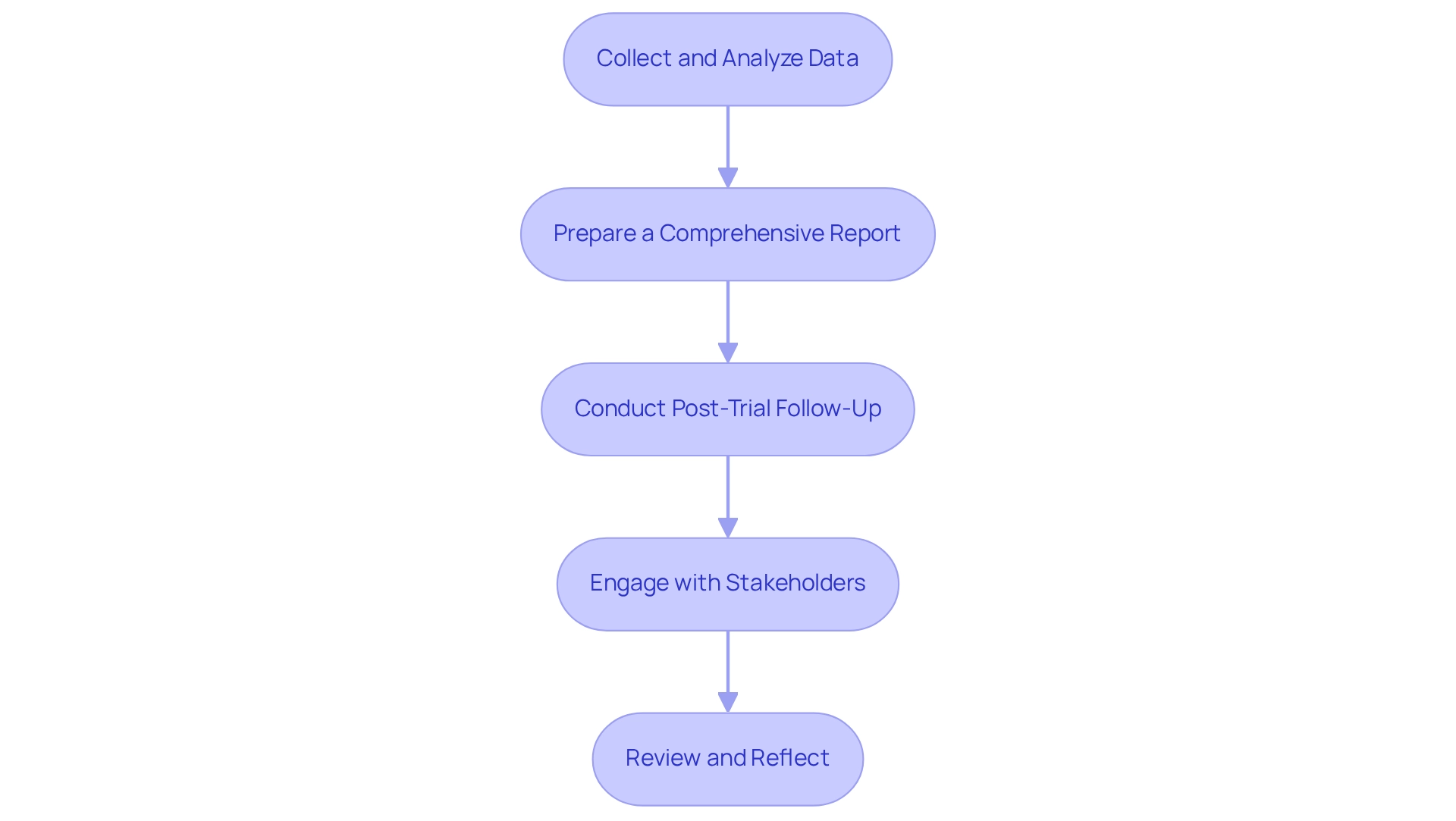
- Collect and Analyze Data: Systematically gather data in accordance with your protocol. Employ robust statistical techniques to analyze the data, which is essential for drawing valid conclusions regarding the device's safety and efficacy. The FDA recommends simulating various scenarios to estimate type I and type II error rates, which can enhance the reliability of your findings. Additionally, there is a pressing need for improved compliance and validation methods in biostatistical analysis for drug approvals, underscoring the importance of rigorous data analysis.
- Prepare a Comprehensive Report: Compile all findings into a detailed report that encompasses methodology, results, and interpretations. This document is essential for compliance submissions and will serve as a reference for future research endeavors. Ensure that your findings related to the medical device trial design in Argentina are meticulously prepared and submitted to ANMAT, adhering to their compliance guidelines. Include all necessary documentation to facilitate a smooth review process.
- Conduct Post-Trial Follow-Up: Develop a post-trial follow-up strategy to monitor long-term outcomes and any potential adverse effects associated with the device. This step is essential for ongoing safety evaluations and adherence to regulations. Engage with Stakeholders: Actively communicate your findings with stakeholders, including sponsors, regulatory bodies, and the medical community. Sharing insights encourages teamwork and can improve the design of future research. It is also essential to understand statistical methods to effectively evaluate study datasets and report findings.
- Review and Reflect: After the examination concludes, conduct a thorough review of the entire process. Identify lessons learned and areas for enhancement to refine methodologies for future experiments. Combining decision analysis with sequential clinical evaluations, as shown in case examples, can greatly enhance the effectiveness assessment of therapeutic interventions in small groups, resulting in more informed clinical choices. As Georgios D Panos observed, although the procedure may seem intimidating, the appropriate information can clarify significant elements of statistical analysis.
At bioaccess®, we focus on extensive clinical study management services, including feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting. Our expertise spans Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), ensuring that your clinical trials are conducted efficiently and effectively in the Latin American market.
Conclusion
Navigating the landscape of medical device trials in Argentina necessitates a comprehensive understanding of the regulatory framework established by ANMAT, strategic planning, and an unwavering commitment to ethical practices. Familiarizing oneself with ANMAT regulations is paramount, as these guidelines dictate the essential steps for successful trial execution. From the appropriate classification of devices to the preparation of meticulous documentation and the engagement of local regulatory experts, each step is critical in ensuring compliance and streamlining the approval process.
Furthermore, the design of a medical device trial involves defining clear objectives, selecting suitable study designs, and establishing robust recruitment strategies. Engaging with patient advocacy groups and leveraging digital marketing are vital tactics for effective participant recruitment and retention, which are crucial for the success of any clinical trial. Continuous monitoring of compliance, alongside ongoing support for participants, further enhances the overall trial experience.
Ultimately, the successful evaluation of trial outcomes relies on rigorous data collection and analysis, comprehensive reporting, and proactive stakeholder engagement. By adhering to these principles and remaining vigilant about regulatory updates, researchers can adeptly navigate the complexities of medical device trials in Argentina. As the demand for innovative medical solutions continues to expand, the commitment to ethical standards and participant safety will be paramount in advancing the field of medical research. The journey may be challenging; however, with the right approach and resources, stakeholders can pave the way for groundbreaking advancements in healthcare.
Frequently Asked Questions
What is the importance of understanding ANMAT regulations for medical device trial design in Argentina?
A thorough comprehension of ANMAT regulations, particularly Resolution 237/2024, is crucial as it outlines the requirements for medical equipment studies, ensuring that evaluations meet high standards of oversight and adherence.
How are medical devices classified in Argentina?
Medical devices are classified into four categories: Class I, II, III, or IV. This classification determines the approval pathway and specific requirements for clinical studies, impacting the authorization process.
What documentation is required for medical device trials?
Required documentation includes clinical trial protocols, informed consent forms, and safety data. These documents must adhere to ANMAT guidelines to facilitate a smooth review process.
Why is it beneficial to engage with local compliance specialists?
Collaborating with local compliance specialists or legal advisors can ensure compliance with regulations and streamline the approval process, reducing potential delays.
What steps should be taken after preparing the required documentation?
After preparing the necessary documentation, submit your application to ANMAT for review and be prepared to address any queries or requests for additional information from the regulatory body.
How can one stay updated on regulatory changes in Argentina?
It is important to continuously monitor updates on regulations and guidelines from ANMAT to maintain compliance throughout the testing process.
What ethical considerations are associated with clinical studies in Argentina?
Ethical considerations include the significance of clinical study registration, which promotes transparency and accountability in research involving human participants.
What role does clinical study registration play in research integrity?
Registration enhances the integrity of clinical research and is increasingly becoming a requirement for publication in many journals, as it informs sponsors of their responsibilities and motivates investigators.
What is the significance of adverse event reporting systems?
Adverse event reporting systems are crucial for pharmacovigilance, as they gather information on safety risks linked to medical equipment.
How does bioaccess® support researchers in medical device trial design?
Bioaccess® offers expertise in feasibility studies and site selection, ensures information security, and addresses client concerns through established grievance and data protection procedures.




