Overview
This article delineates the essential steps for effectively conducting multi-site trials in Ecuador, underscoring the critical need to comprehend the regulatory landscape, implement effective recruitment strategies, and establish robust data management protocols. It articulates vital practices, including:
- The acquisition of ethics approval
- The utilization of local networks for recruitment
- The deployment of centralized data management systems
These elements are indispensable for enhancing trial efficiency and fostering participant engagement.
Introduction
In the realm of clinical research, multi-site trials are revolutionizing the way studies are conducted, offering a dynamic approach that transcends geographical boundaries. This innovative strategy not only amplifies participant diversity but also accelerates the recruitment process, ultimately leading to more reliable and generalizable findings.
As the landscape of clinical trials evolves, understanding the intricacies of executing these studies becomes paramount. From navigating regulatory frameworks in countries like Ecuador to implementing effective recruitment strategies and establishing robust data management protocols, the success of multi-site trials hinges on a comprehensive understanding of these key components.
This article delves into the essentials of multi-site trials, shedding light on the benefits, challenges, and best practices that can empower researchers to harness the full potential of this approach in today's fast-paced research environment.
Understand the Basics of Multi-Site Trials
Multi-site experiments are essential in medical research, facilitating the execution of a single investigation across various locations. This approach not only broadens the participant pool but also enhances the generalizability of findings, ultimately expediting the enrollment process. Key elements to consider include:
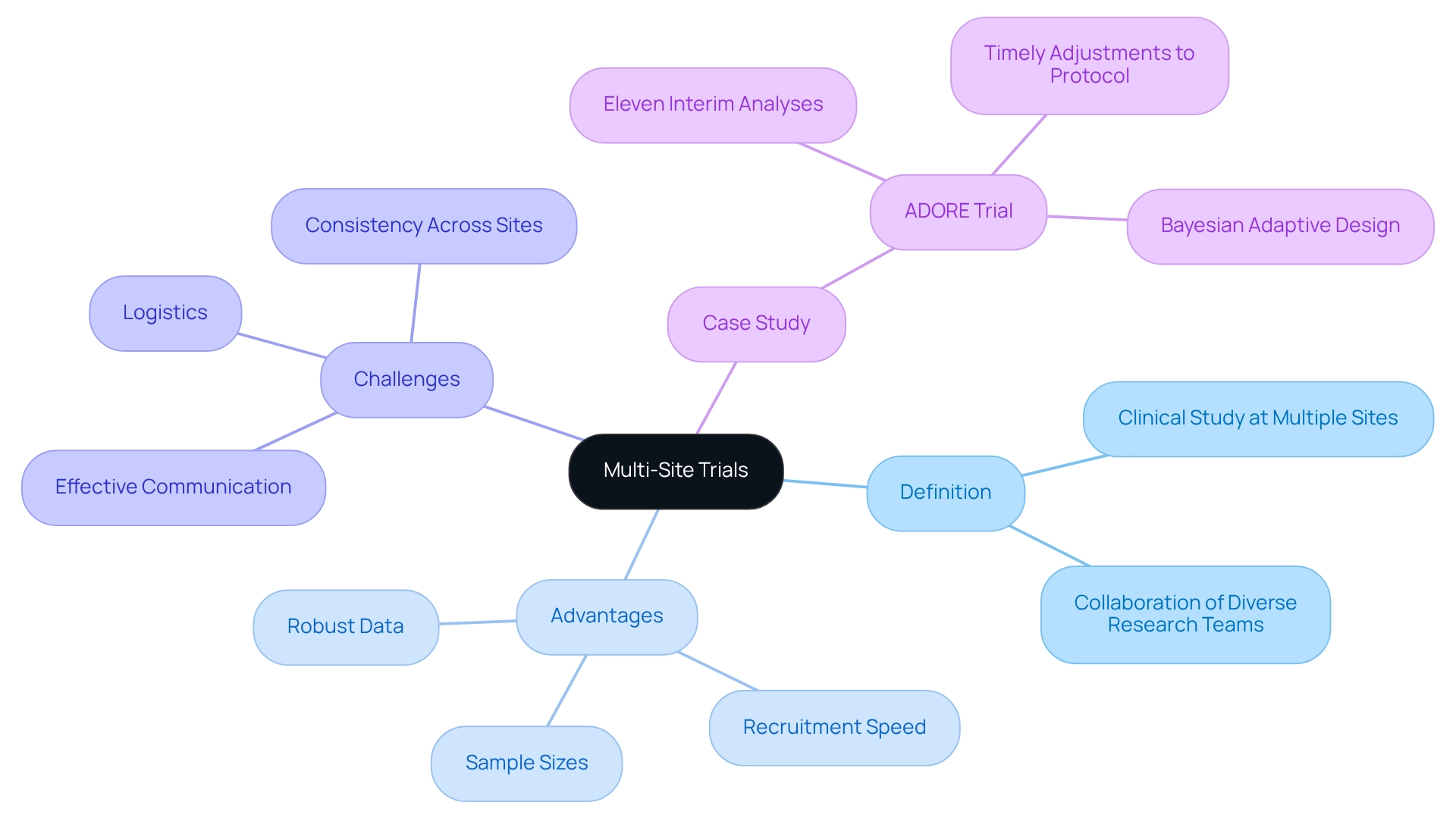
- Definition: A multi-site trial is a clinical study carried out at multiple sites, often involving diverse research teams collaborating towards a unified objective.
- Advantages: These studies significantly boost recruitment speed, increase sample sizes, and yield more robust data, thereby enhancing the reliability of results. Statistics reveal that the number of centers in systematic reviews varies from 1 to 1,161, with a median of 24, underscoring the growing importance of multi-site research in the clinical landscape. Furthermore, comprehensive clinical study management services, such as those offered by bioaccess™, include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting, all of which are vital for successful execution.
- Challenges: Despite the clear advantages, managing logistics, ensuring consistency across sites, and maintaining effective communication present significant challenges. As Dinesh Pal Mudaranthakam notes, "We would suggest the adoption of similar tools for any research center that often serves as a main location in multisite studies." Addressing these complexities, including differing regulatory requirements and varying site capabilities, is crucial for the success of the experiment.
- Case Study: The ADORE study illustrates the practical application of interim analyses in multi-site investigations, having conducted eleven interim analyses to adjust treatment randomization based on a Bayesian Adaptive Design. These analyses facilitated timely modifications to the study protocol, ensuring that the experiment remained aligned with its objectives and participant safety.
Moreover, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading hub for medical research in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances the local research landscape but also fosters job creation and economic growth in the region.
Understanding these fundamentals equips you to navigate the complexities of conducting successful multi-site trials in Ecuador, where opportunities for diverse participant involvement are abundant. Current trends in 2025 indicate an increasing reliance on multi-location studies to enhance data quality and recruitment efficiency, making it imperative for research directors to stay informed and adaptable.
Navigate Regulatory Requirements in Ecuador
Ecuador's research study regulations play a crucial role in upholding ethical standards and ensuring participant safety. To effectively navigate these requirements, consider the following key steps:
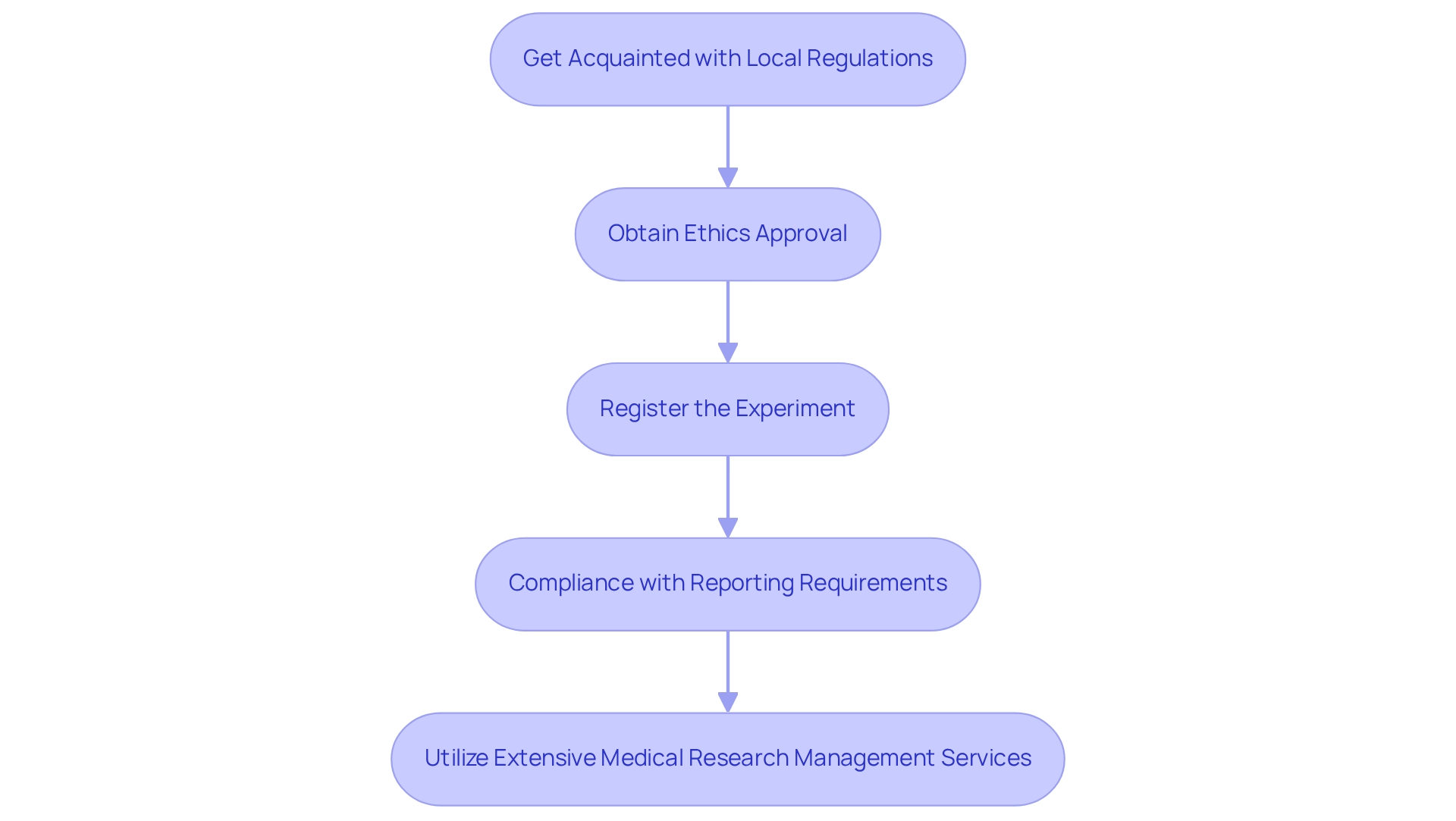
- Get Acquainted with Local Regulations: Examine the Ministerial Agreement (MA) 0075-2017 and its amendments, which establish the legal framework for conducting clinical studies in Ecuador. Staying informed about updates to this agreement is essential for compliance.
- Obtain Ethics Approval: Prior to commencing a study, secure approval from an Ethics Committee or Institutional Review Board (IRB). This process necessitates the submission of comprehensive documentation detailing the study's design, objectives, and participant recruitment strategies.
- Register the Experiment: Ensure your experiment is registered with the Agencia Nacional de Regulación, Control y Vigilancia Sanitaria (ARCSA). Notably, only 24 out of 75 recognized studies were found in the ARCSA national registry, underscoring the necessity of proper registration and the urgency of adherence. Compliance with Reporting Requirements: Regularly submit progress reports and document any adverse events to maintain compliance with regulatory standards. Non-compliance with these regulations can result in significant delays or even the termination of your trial.
- Utilize Extensive Medical Research Management Services: Collaborate with bioaccess®, a leading Contract Research Organization (CRO) dedicated to supporting medical device studies in Latin America. Their services encompass feasibility studies, site selection, compliance reviews, testing setup, import permits, project management, and reporting, ensuring that your study adheres to all necessary legal and ethical standards. Stay Informed: Given the evolving nature of regulations, it is crucial to remain aware of new guidelines or amendments that may impact your study.
The pressing need for increased investment in research, particularly those addressing prevalent health issues such as chronic non-communicable diseases, highlights the importance of adhering to these regulations. The significant gap in clinical research development related to these health issues reinforces the urgency for compliance and investment. By following these steps and collaborating with local stakeholders, you can effectively navigate the regulatory environment and enhance the impact of your study.
Implement Effective Recruitment Strategies
Recruiting participants for multi-site trials in Ecuador necessitates a strategic and multifaceted approach to ensure effective engagement at each location. Here are essential strategies to enhance recruitment efforts:
-
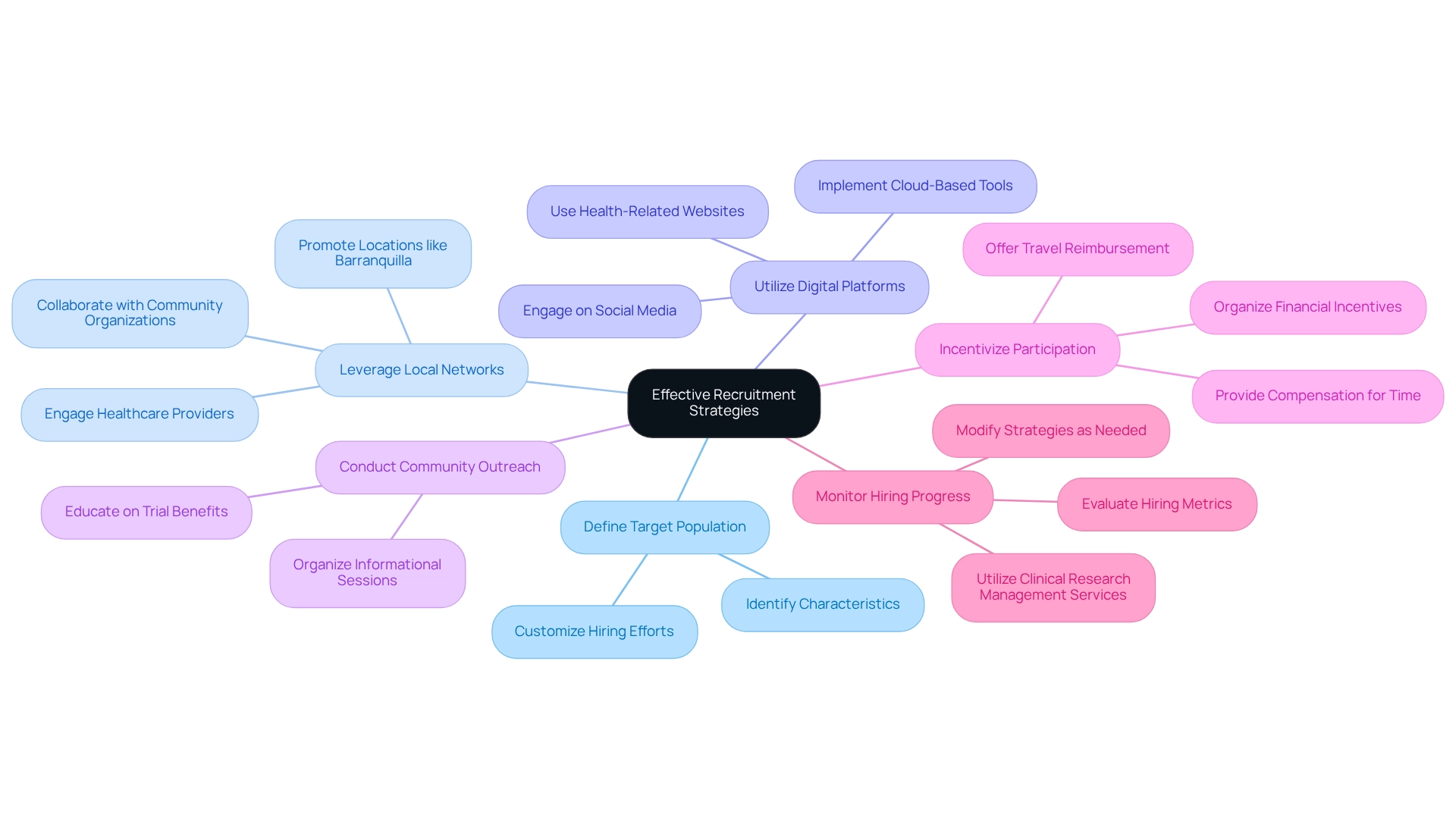
Define Your Target Population: Clearly delineate the characteristics of your ideal participants based on the trial's objectives and specific inclusion/exclusion criteria. This accuracy aids in customizing hiring efforts effectively.
-
Leverage Local Networks: Tap into existing relationships with healthcare providers and community organizations. These connections can encourage word-of-mouth referrals and strengthen trust within the community, significantly improving hiring efforts. The partnership between bioaccess™ and Caribbean Health Group illustrates how local collaborations can enhance hiring efforts, particularly in areas such as Barranquilla, which is being promoted as a premier location for clinical studies in Latin America. The support from Colombia's Minister of Health further underscores the importance of this initiative.
-
Utilize Digital Platforms: Engage potential participants through social media, online forums, and health-related websites. Digital outreach can broaden your audience and attract diverse participants, particularly younger demographics who are more active online. Tools like RingCentral's cloud-based platform can enable seamless engagement with patients and hiring firms, enhancing your outreach efforts.
-
Conduct Community Outreach: Organize informational sessions in local communities to educate potential participants about the trial's purpose and benefits. This direct engagement fosters a sense of community involvement and can alleviate concerns about participation.
-
Incentivize Participation: Offering incentives, such as travel reimbursement or compensation for time, can significantly enhance enrollment rates. A case study titled 'Impact of Financial Incentives on Hiring' demonstrated that financial compensation was a crucial factor in attracting participants, contributing to the overall success of hiring efforts. bioaccess™ can aid in organizing these incentives efficiently to maximize enrollment potential.
-
Monitor Hiring Progress: Regularly evaluate hiring metrics at each location to identify challenges and modify strategies accordingly. This ongoing assessment enables prompt actions to enhance hiring processes. The research group gained important insights and abilities throughout the selection process, which can guide upcoming medical experiments and enhance participant engagement strategies. The extensive clinical research management services offered by bioaccess™, including feasibility assessments and project oversight, further bolster these efforts.
By implementing these strategies, you can significantly enhance participant enrollment for multi-site trials in Ecuador, ensuring that your trial is adequately powered to meet its objectives. The effectiveness of these methods is emphasized by recent findings, which suggest that systematic hiring strategies can improve participation rates and overall results. As M.H., a Principal Investigator, noted, "By employing these systematic approaches, future studies can more accurately assess and refine recruitment strategies to enhance participation rates and study outcomes.
Establish Robust Data Management and Communication Protocols
Effective data management and communication protocols are essential for the success of multi-site trials in Ecuador. To establish these protocols, consider the following key steps:
-
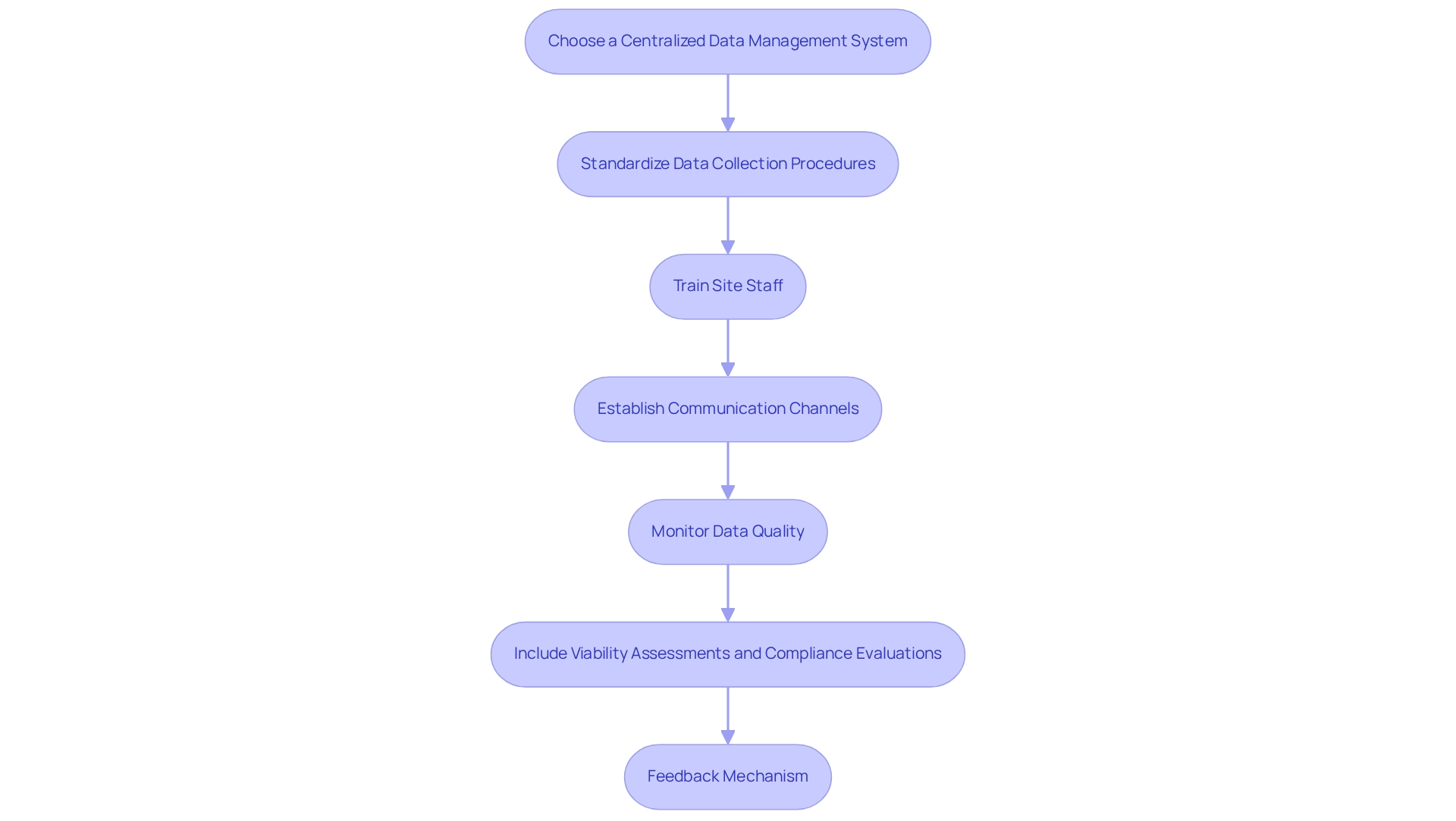
Choose a Centralized Data Management System: Implement a cloud-based platform that facilitates real-time data entry and monitoring across all sites, ensuring consistency and accessibility of data. Notably, North America represented 45.9% of the worldwide Clinical Data Management Systems (CDMS) market in 2022, underscoring the critical role of strong data management systems in trials.
-
Standardize Data Collection Procedures: Develop and distribute standardized forms and templates for data collection to minimize variability and reduce errors, which can significantly impact study outcomes. As Maryam Y. Garza emphasizes, reporting research results without specifying the error rate and its measurement limits the ability to interpret findings, highlighting the necessity for accurate data management.
-
Train Site Staff: Provide comprehensive training for all site personnel on data management practices and the use of the data management system, ensuring compliance and accuracy in data handling. Bioaccess provides extensive training as part of its thorough research management services, ensuring that all team members are well-equipped to handle data effectively.
-
Establish Communication Channels: Set up regular meetings and communication channels, such as email and messaging apps, to foster ongoing dialogue among site teams and promptly address any issues that arise. Bioaccess emphasizes the importance of clear communication in its project management approach, facilitating seamless collaboration across multiple sites.
-
Monitor Data Quality: Conduct regular audits and checks to ensure data integrity and adherence to research protocols, which is essential for maintaining the reliability of experiment results. The safeguarding of clinical data is regulated by stringent rules, including adherence to import permits and the regulatory framework, as demonstrated in the case analysis on clinical data protection and anonymization. Bioaccess's project management services include rigorous monitoring to uphold these standards.
-
Include Viability Assessments and Compliance Evaluations: Integrate viability assessments and compliance evaluations into the data management process to ensure that all aspects of the experiment setup are thoroughly evaluated and documented. This comprehensive approach enhances the overall effectiveness of the management process.
-
Feedback Mechanism: Create a system for collecting feedback from site staff to continuously refine data management practices and address challenges as they emerge. This feedback loop is crucial for improving processes and ensuring that all stakeholders are aligned with the objectives of the multi-site trials in Ecuador. By implementing these robust protocols, including critical elements such as feasibility studies and compliance reviews, you can enhance the efficiency and effectiveness of multi-site trials in Ecuador, ensuring high-quality data and seamless collaboration among all stakeholders.
Conclusion
Multi-site trials represent a transformative approach in clinical research, enabling studies to expand beyond geographical constraints while enhancing participant diversity and recruitment efficiency. This article highlights the essential elements of executing successful multi-site trials, including an understanding of their definition, benefits, and challenges. By leveraging comprehensive clinical trial management services, such as those offered by bioaccess™, researchers can navigate the complexities involved, from regulatory compliance in Ecuador to effective recruitment strategies.
Navigating the regulatory landscape is critical for the success of these trials. Familiarizing themselves with local regulations, obtaining necessary approvals, and ensuring proper trial registration allow researchers to mitigate risks and enhance their studies' credibility. Additionally, implementing effective recruitment strategies—such as leveraging local networks and utilizing digital platforms—is vital to attracting a diverse participant pool that aligns with the trial's objectives.
Moreover, establishing robust data management and communication protocols is essential for maintaining data integrity and facilitating collaboration among multiple sites. By adopting a centralized data management system and standardizing data collection procedures, researchers can ensure consistency and accuracy across all locations. Continuous training, regular audits, and an effective feedback mechanism further contribute to the overall success of multi-site trials.
Ultimately, multi-site trials are not just a trend but a necessary evolution in clinical research that can lead to more reliable and generalizable findings. By understanding and implementing best practices in the areas discussed, researchers are better equipped to harness the full potential of multi-site trials, driving advancements in healthcare and benefiting diverse populations worldwide.
Frequently Asked Questions
What is a multi-site experiment in medical research?
A multi-site experiment is a clinical study conducted at multiple locations, often involving various research teams working together towards a common goal.
What are the advantages of conducting multi-site trials?
Multi-site trials enhance recruitment speed, increase sample sizes, and provide more robust data, leading to more reliable results. They also allow for a broader participant pool and improved generalizability of findings.
What challenges are associated with multi-site experiments?
Challenges include managing logistics, ensuring consistency across different sites, maintaining effective communication, and addressing varying regulatory requirements and site capabilities.
Can you provide an example of a multi-site study?
The ADORE study is an example, which conducted eleven interim analyses to adjust treatment randomization based on a Bayesian Adaptive Design, allowing timely modifications to the study protocol for participant safety.
How do organizations like bioaccess™ support multi-site trials?
Bioaccess™ offers comprehensive clinical study management services, including feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting, which are crucial for successful execution of multi-site studies.
What is the significance of the partnership between bioaccess™ and Caribbean Health Group?
This partnership aims to position Barranquilla as a leading hub for medical research in Latin America, enhancing the local research landscape, fostering job creation, and promoting economic growth in the region.
What trends are expected in multi-site studies by 2025?
Current trends indicate an increasing reliance on multi-location studies to improve data quality and recruitment efficiency, making it essential for research directors to remain informed and adaptable.




