Overview
The ANMAT approval process for clinical trials represents a structured procedure that necessitates the submission of comprehensive documentation, technical and administrative evaluations, and the assurance of ongoing compliance and monitoring throughout the study. This process is pivotal in the realm of clinical research, as meticulous documentation and strict adherence to regulatory standards are crucial.
Effective navigation of the ANMAT approval process not only enhances the likelihood of securing approval but also significantly contributes to the successful conduct of clinical trials in Argentina. Understanding these nuances is essential for stakeholders aiming to thrive in this competitive landscape.
Introduction
In the intricate realm of clinical trials, grasping regulatory frameworks is crucial for achieving success. In Argentina, the National Administration of Drugs, Foods, and Medical Devices (ANMAT) assumes a pivotal role in safeguarding the safety and efficacy of medical products. Organizations aiming to conduct clinical research in this region must adeptly navigate ANMAT's stringent guidelines and processes, which not only protect participant rights but also maintain data integrity.
As interest in clinical studies in Argentina escalates, particularly with projections indicating increased research activity by 2025, a thorough understanding of ANMAT's functions becomes indispensable. This article explores the complexities of ANMAT's regulatory landscape, providing insights into the documentation, evaluation, and compliance processes that are essential for executing successful clinical trials in Argentina.
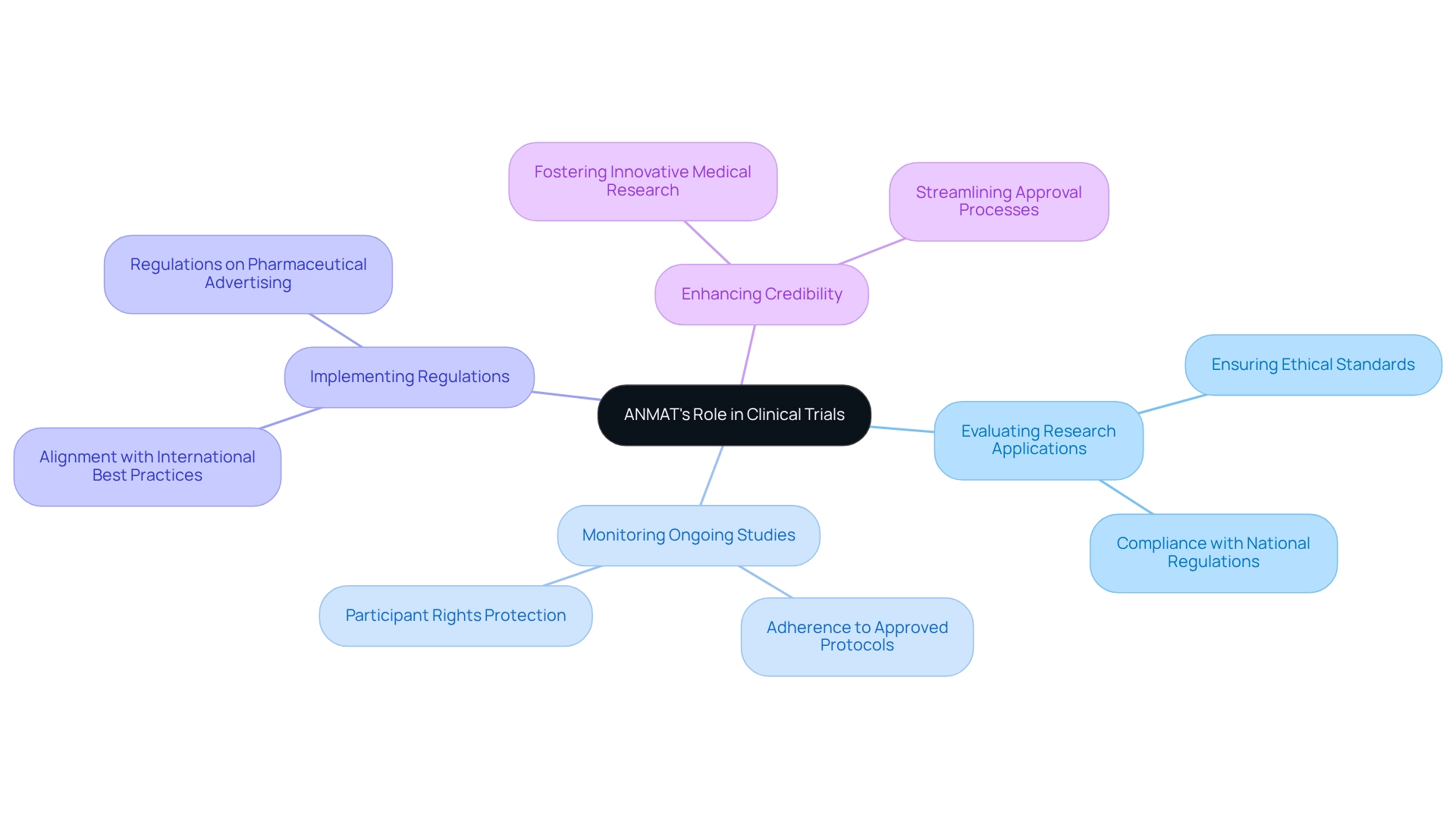
Understand ANMAT and Its Role in Clinical Trials
The National Administration of Drugs, Foods, and Medical Devices (ANMAT) serves as Argentina's regulatory authority, overseeing the safety and efficacy of medical products, including those utilized in research studies. For organizations aiming to conduct medical research in Argentina, understanding the agency's role is crucial. ANMAT ensures compliance with both national and international regulations, protecting participant rights and upholding data integrity.
Familiarity with the agency's guidelines and processes lays a solid foundation for effectively navigating the approval procedure.
Key functions of ANMAT include:
- Evaluating research applications to ensure they meet ethical standards.
- Monitoring ongoing studies to guarantee adherence to approved protocols.
- Implementing regulations that align with international best practices, thereby enhancing the credibility of medical research conducted in Argentina.
In 2025, research studies in Argentina are anticipated to expand across various phases, including early feasibility assessments, pivotal studies, and post-market medical follow-up studies. This trend reflects a growing interest in the region's research potential. Dr. Wanda Dobrzanski has expressed a willingness to discuss future research needs in Argentina and Latin America, highlighting the regulatory body's pivotal role in fostering these advancements. Furthermore, recent updates to health regulations are expected to streamline the approval process, ultimately improving success rates for research studies in the country.
The agency's rigorous oversight, which includes regulations on pharmaceutical advertising, ensures that ethical standards are maintained, equipping healthcare professionals with accurate information about products. This regulatory framework not only safeguards public health but also cultivates an environment conducive to innovative medical research. Notably, innovative treatments like pegzilarginase have the potential to benefit patients with arginase 1 deficiency, underscoring ANMAT's regulatory role in advancing such therapies.
At bioaccess®, we leverage over 20 years of expertise in Medtech to navigate the complexities of the ANMAT approval process. We offer tailored solutions that encompass regulatory approval, research site activation, subject recruitment, and data management, enhancing our clients' prospects for success. Our commitment to data protection and transparency ensures that client concerns are addressed promptly, fostering trust and compliance throughout the research journey.
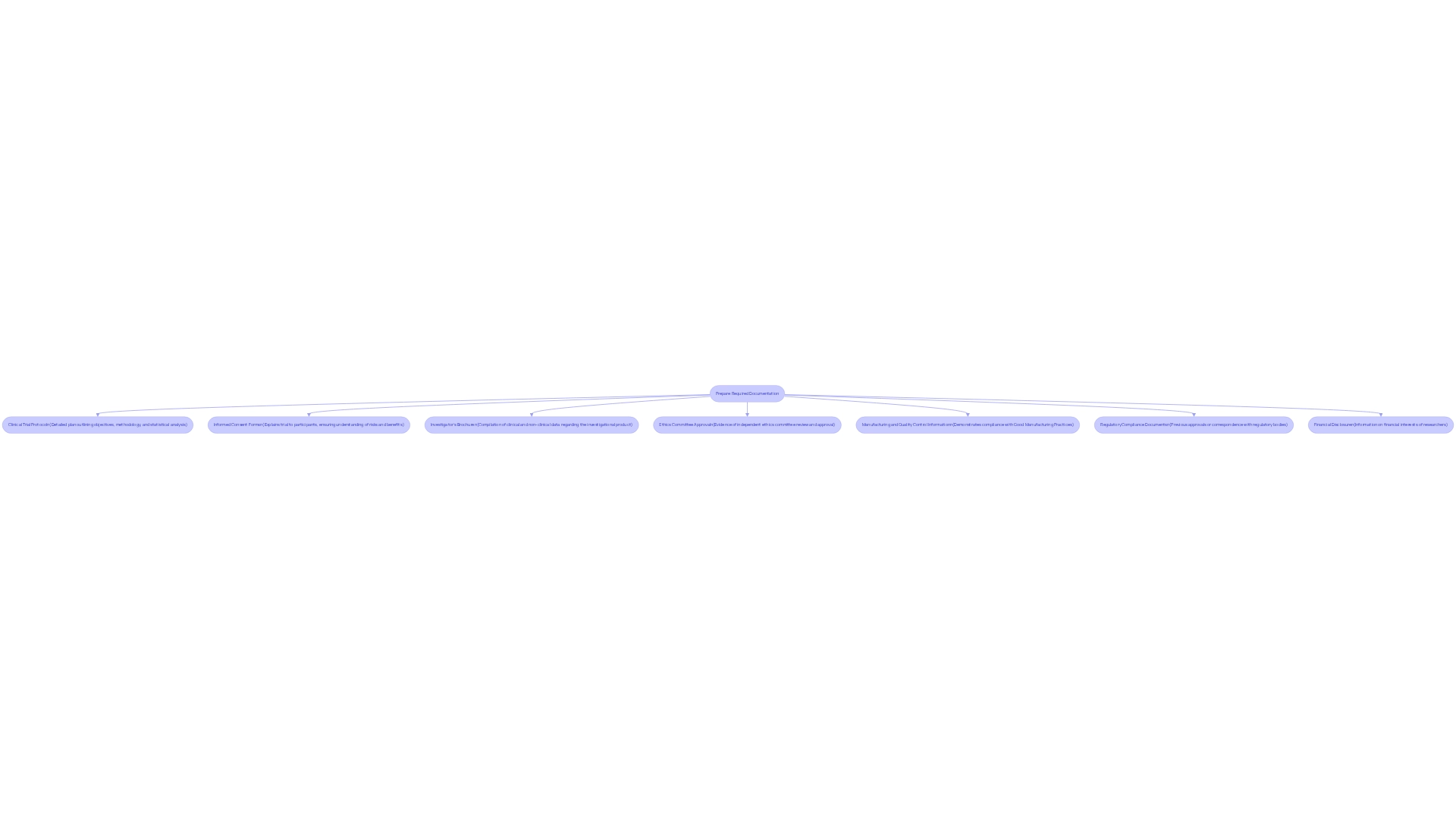
Prepare Required Documentation for Submission
To successfully submit a clinical study application to ANMAT, it is essential to prepare a comprehensive set of documents for the ANMAT approval process for clinical trials. bioaccess®, as a leading contract research organization in Latin America, offers expertise in managing this process effectively. The following essential documentation is required:
- Clinical Trial Protocol: A detailed plan that outlines the study's objectives, methodology, and statistical analysis.
- Informed Consent Forms: Documents that clearly explain the trial to participants, ensuring they understand the associated risks and benefits.
- Investigator's Brochure: A compilation of both clinical and non-clinical data regarding the investigational product.
- Ethics Committee Approval: Evidence that an independent ethics committee has reviewed and approved the study protocol.
- Manufacturing and Quality Control Information: Documentation demonstrating compliance with Good Manufacturing Practices (GMP).
- Regulatory Compliance Documents: Any previous approvals or correspondence with other regulatory bodies.
- Financial Disclosure: Information concerning any financial interests of the researchers involved in the study.
It is crucial to ensure that all documents are translated into Spanish and arranged in accordance with the organization's guidelines. This attention to detail can significantly reduce the likelihood of common documentation errors, which statistics indicate can occur in up to 20% of submissions, based on a sample size of 3,400 fields. Furthermore, a case study named "Limitations of Traditional Data Quality Assessments" emphasized that conventional audits may overlook all sources of error, stressing the necessity for a comprehensive approach to precisely assess data quality in research studies.
By following these guidelines and utilizing bioaccess's extensive clinical trial management services, you can enable a smoother review process and possibly reduce the typical duration required for the ANMAT approval process for clinical trials. As Roberto Leiva noted, "We gratefully acknowledge the importance of meticulous documentation preparation in ensuring successful submissions.
Navigate the Evaluation Process by ANMAT
Upon submission of your application, the agency initiates a structured evaluation process, which includes several key steps that are critical for the success of clinical trials.
The ANMAT approval process for clinical trials begins with an initial review, where a preliminary assessment is conducted to verify that all necessary documents have been submitted. Following this, the technical evaluation involves a comprehensive review that examines both the scientific and ethical dimensions of the trial, typically lasting up to 60 business days. During this phase, it is essential to ensure that your study complies with country requirements, including the review and feedback on study documents. Experts, such as those at bioaccess, can facilitate this process, ensuring that your application meets all necessary criteria.
After the technical evaluation, an additional 10 business days are dedicated to the administrative review within the ANMAT approval process for clinical trials. The agency will then communicate the results of the evaluation, which may consist of approval, requests for further information, or a rejection.
To ensure a seamless evaluation process, maintaining open lines of communication with the organization is crucial. Be prepared to address any inquiries or requests for clarification promptly, as this can significantly influence the efficiency of your application review. Leveraging the expertise of a team with over 20 years in Medtech, such as bioaccess, can enhance your chances of navigating this process successfully.
Manage Post-Approval Compliance and Monitoring
Efficient oversight of adherence and observation following the ANMAT approval process for clinical trials is vital for the success of clinical studies. Key responsibilities include:
- Adherence to Protocol: Conduct the study strictly in accordance with the approved protocol, ensuring that any modifications align with regulatory standards.
- Regular Reporting: Provide periodic updates to ANMAT, detailing study progress, adverse events, and any protocol deviations. This clarity is essential, as research indicates that 92.5% of published articles thoroughly document withdrawals due to adverse events. Furthermore, the case study 'Future Directions for Clinical Trials Reporting' underscores the necessity for improved adherence to the ANMAT approval process for clinical trials, emphasizing that thorough reporting can enhance the overall integrity of clinical studies.
- Participant Safety Monitoring: Continuously monitor participant safety, with immediate reporting of any serious adverse events to ANMAT. This proactive approach is crucial, particularly given that concordance for withdrawals due to adverse events is notably low, with only 44.1% of reports aligning with the ANMAT approval process for clinical trials. This highlights the importance of accurate reporting and monitoring to maintain participant safety and regulatory compliance.
- Final Study Report: Upon completion of the study, submit a detailed report summarizing findings, methodologies, and any encountered issues. This report should reflect compliance with established frameworks, such as the CONSORT Harms statement, to enhance clarity and accountability.
Incorporating extensive study management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, is essential for effective compliance management. Bioaccess provides these services to ensure that studies comply with regulatory standards and uphold participant safety. As Warman O'Brien aptly states, "Embracing rigorous post-marketing surveillance enhances market competitiveness and supports trust and credibility among healthcare providers and patients."
By prioritizing rigorous compliance and maintaining open communication with ANMAT throughout the ANMAT approval process for clinical trials, you not only uphold the integrity of your clinical trial but also significantly contribute to the advancement of medical knowledge and participant safety.
Conclusion
Navigating the regulatory landscape of clinical trials in Argentina is essential for success, particularly given the pivotal role played by ANMAT, the National Administration of Drugs, Foods, and Medical Devices. A thorough understanding of ANMAT's comprehensive guidelines and processes is crucial for organizations to effectively manage the complexities of clinical research, safeguard participant rights, and maintain data integrity. As the demand for clinical studies in Argentina is projected to rise by 2025, a firm grasp of ANMAT's functions becomes increasingly important.
Key elements such as the preparation of essential documentation, the structured evaluation process, and the significance of post-approval compliance and monitoring have been highlighted. Each step in the clinical trial journey necessitates meticulous attention to detail, from the initial application submission to the final study report, ensuring adherence to ethical standards and regulatory requirements. The insights provided emphasize that success in clinical trials is not solely dependent on innovative treatments; it also relies on the rigorous processes that underpin their development.
In conclusion, fostering a collaborative relationship with ANMAT and leveraging expert resources can significantly enhance the likelihood of successful clinical trials in Argentina. By prioritizing regulatory compliance and participant safety, organizations contribute not only to the advancement of medical research but also to the overall integrity of the healthcare landscape. As the clinical research environment evolves, embracing these practices will be key to unlocking the full potential of innovative therapies for patients in need.
Frequently Asked Questions
What is the role of ANMAT in Argentina?
ANMAT, or the National Administration of Drugs, Foods, and Medical Devices, is Argentina's regulatory authority responsible for overseeing the safety and efficacy of medical products, including those used in research studies.
Why is it important for organizations to understand ANMAT's role?
Understanding ANMAT's role is crucial for organizations conducting medical research in Argentina, as it ensures compliance with national and international regulations, protects participant rights, and upholds data integrity.
What are the key functions of ANMAT?
Key functions of ANMAT include evaluating research applications for ethical standards, monitoring ongoing studies for adherence to approved protocols, and implementing regulations that align with international best practices.
What changes are anticipated in research studies in Argentina by 2025?
By 2025, research studies in Argentina are expected to expand across various phases, including early feasibility assessments, pivotal studies, and post-market medical follow-up studies, reflecting a growing interest in the region's research potential.
How does ANMAT contribute to the credibility of medical research?
ANMAT enhances the credibility of medical research by implementing regulations that align with international best practices and maintaining rigorous oversight, including regulations on pharmaceutical advertising.
What recent updates to health regulations are expected to impact research studies in Argentina?
Recent updates to health regulations are anticipated to streamline the approval process for research studies, ultimately improving success rates in the country.
How does bioaccess® assist with the ANMAT approval process?
bioaccess® leverages over 20 years of expertise in Medtech to navigate the complexities of the ANMAT approval process, offering tailored solutions that include regulatory approval, research site activation, subject recruitment, and data management.
What commitment does bioaccess® make regarding data protection and transparency?
bioaccess® is committed to data protection and transparency, ensuring that client concerns are addressed promptly and fostering trust and compliance throughout the research journey.




