Introduction
Abbreviated New Drug Applications (ANDAs) serve as a vital mechanism for the introduction of generic drugs into the market, offering a pathway that balances regulatory efficiency with public health needs. By enabling manufacturers to prove bioequivalence to established brand-name medications, ANDAs significantly contribute to the availability of affordable treatments.
As the landscape of generic drug approvals evolves, understanding the intricacies of the ANDA process becomes essential for stakeholders aiming to navigate regulatory challenges and capitalize on emerging opportunities.
This article delves into the key components of ANDA submissions, the role of regulatory agencies, and the implications of compliance, providing a comprehensive overview that underscores the importance of strategic planning and effective communication in achieving successful drug approvals.
Understanding Abbreviated New Drug Applications (ANDAs)
Abbreviated New Drug Applications (ANDAs) represent a crucial pathway for the approval of generic drugs by the FDA. These applications allow manufacturers to demonstrate that their products are bioequivalent to their branded counterparts, thus facilitating market entry without the necessity of conducting extensive clinical trials. The significance of ANDAs extends beyond regulatory compliance; they play a pivotal role in enhancing the availability of affordable medications, which is vital for public health.
Key subjects like Generic Drugs, Biopharmaceutics, and Product-Specific Guidances for Generic Drug Development are crucial for comprehending the intricacies involved in the Abbreviated New Drug Application process. According to recent projections from the Office of Generic Drugs (OGD), an estimated 784 ANDAs are expected to be submitted in FY 2024, reflecting a notable increase from the previous year's 733 applications. This increase in entries hints at a possible change in the market environment, even as the OGD expects to authorize 644 ANDAs for the fiscal year, indicating a reduction in consents compared to previous periods.
The case study titled 'Projections for FY2024' highlights these trends, emphasizing the implications of the projected data on the market. Furthermore, as Bob Pollock aptly states,
We will need to keep a close watch on CRLs over FY 2025 and hope that the downward trend seen in the second half of FY 2024 does not continue.
Understanding the FDA guidelines for ANDA submissions is critical, as these guidelines are designed to ensure compliance with safety and efficacy standards.
As the landscape of generic drug authorizations evolves, the impact of ANDAs on drug pricing and availability remains a critical concern for healthcare stakeholders.
Step-by-Step Guide to Submitting an ANDA
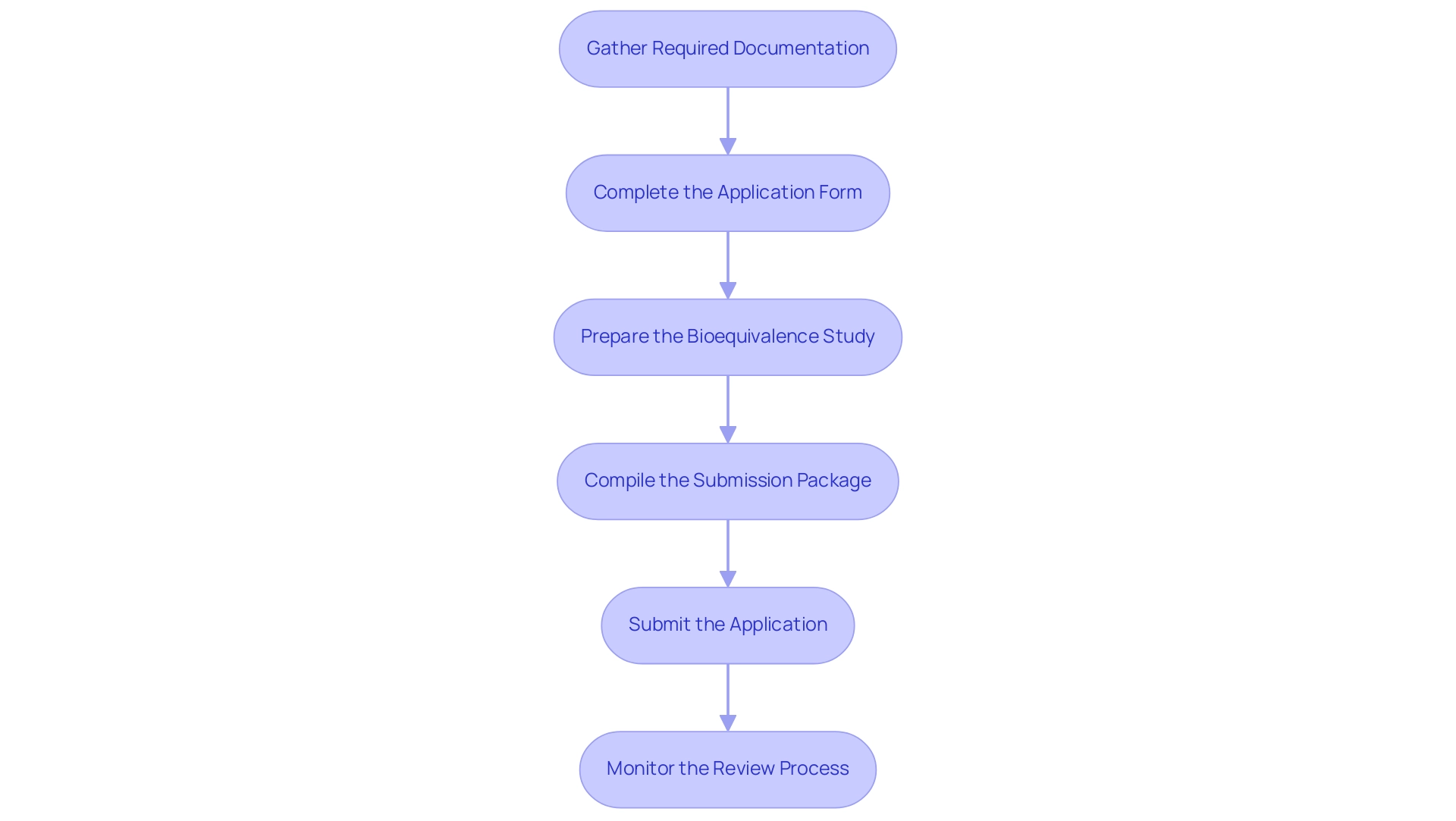
- Gather Required Documentation: Begin by compiling all necessary documents, which should include detailed information on product formulation, manufacturing methods, and stability data. Bioequivalence study results are particularly critical, as they serve to demonstrate the similarity of your product to the reference drug. A well-prepared documentation set lays the groundwork for a smoother approval process, an approach supported by experts like Ana Criado, our Director of Regulatory Affairs who possesses extensive experience in regulatory compliance and clinical trial management.
- Complete the application form: Accurately fill out the application form, ensuring that all required information about the drug—including its labeling and proposed manufacturing sites—is provided. Attention to detail is crucial, as incomplete or inaccurate entries can result in delays or rejections.
- Prepare the Bioequivalence Study: Conduct a comprehensive bioequivalence study in accordance with the latest FDA guidelines. The study design, methodology, and statistical analysis should be robust and meticulously documented. This step is essential, as bioequivalence is a cornerstone of the approval system.
- Compile the Submission Package: Organize your application materials to include all forms, studies, and supporting documents. Ensure that the package is well-structured and easy to navigate, facilitating a thorough review by FDA officials. As Bob Pollock emphasized, "We will need to keep a close watch on CRLs over FY 2025 and hope that the downward trend seen in the second half of FY 2024 does not continue," stressing the importance of monitoring feedback during the evaluation.
- Submit the application: Send your application electronically via the FDA’s electronic gateway. Be aware of any applicable user fees, as these are part of the process that must be completed to avoid further delays.
- Monitor the Review Process: After your ANDA submission, actively monitor its status on the FDA’s website. Be prepared to promptly respond to any inquiries or requests for additional information from the agency, as timely communication can significantly impact the review timeline. The latest data indicates that approximately 220 applicants submit around 275 pre-submissions and 198 certifications annually, underscoring the competitive nature of the certification landscape. Furthermore, as highlighted in the case study named 'Checklist for BLA Readiness,' recording and presenting proof of fulfilled commitments is essential to prevent unnecessary delays in the review. Consulting with experts like Ana Criado and Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics, can also help craft compelling briefing documents and prepare strategies to address potential FDA concerns. Our services also encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all of which are essential for navigating the intricate drug application process.
Navigating Challenges in the ANDA Approval Process
-
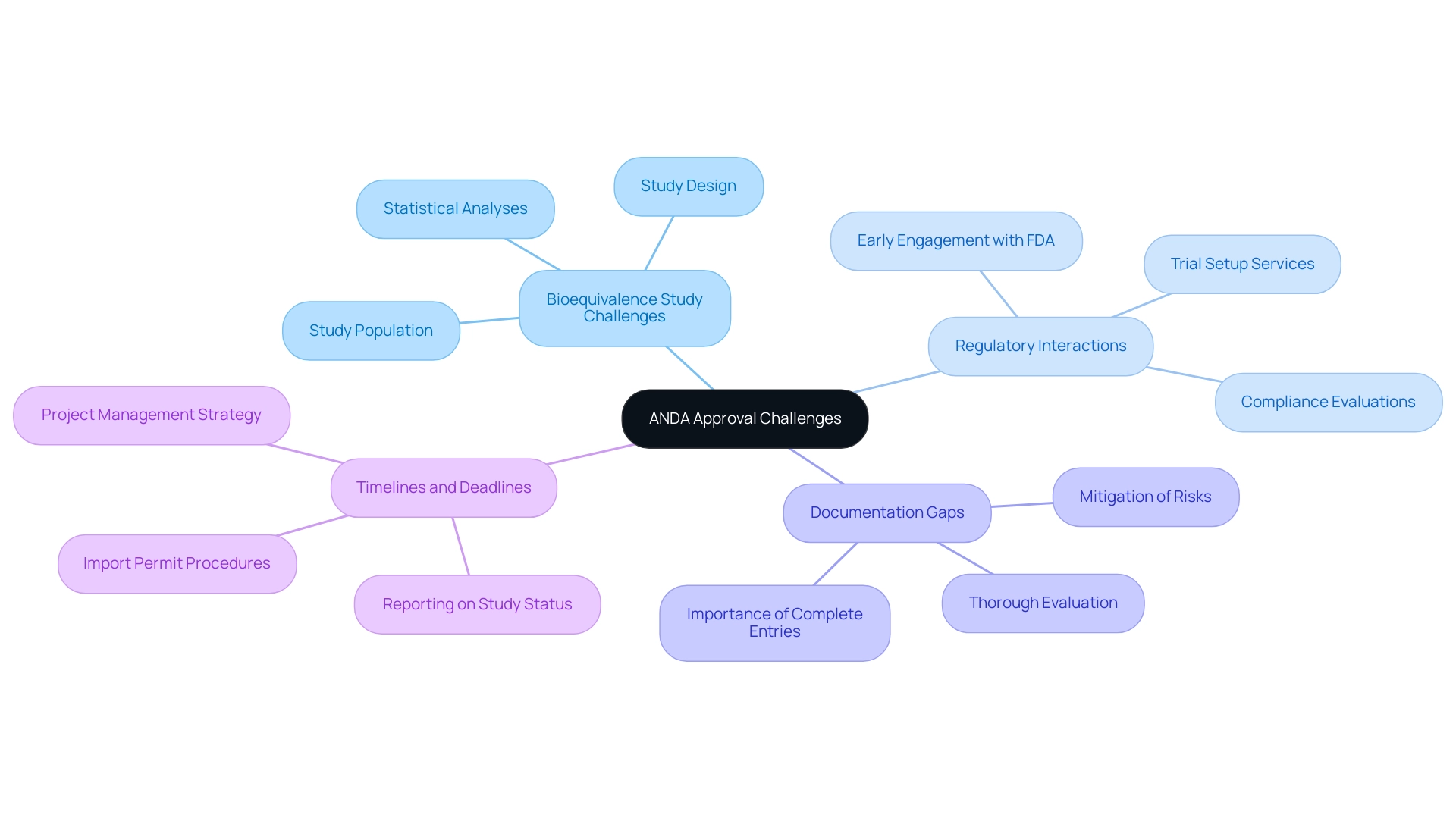
Bioequivalence Study Challenges: Conducting bioequivalence studies poses intricate challenges that require meticulous planning. It is crucial to ensure that the study population, design, and statistical analyses are meticulously aligned with FDA expectations to minimize the risk of delays. A thorough understanding of these requirements is essential for successful study execution. Our comprehensive clinical trial management services, which include feasibility studies and site selection, are designed to support this alignment. The rise in ANDA approvals from 102 in Q1 2018 to 262 in Q4 2018 highlights the significance of prompt filings and compliance with regulatory expectations.
Regulatory Interactions: Early engagement with the FDA is vital to clarify application requirements and address any uncertainties. This proactive communication, combined with our compliance evaluations and trial setup services, helps avoid potential misunderstandings that could obstruct the authorization stage later on. As noted by Joseph S. Ross, MD, MHS, the preparation for ANDA approval can be a lengthy endeavor, often taking manufacturers 6 to 12 months for regulatory review, making early dialogue even more critical. Furthermore, the successes of monoclonal antibodies in clinical settings, as highlighted by Reichert et al., exemplify effective strategies for navigating regulatory challenges.
-
Documentation Gaps: A thorough evaluation of all documentation before presenting it is crucial to prevent omitting vital information. Incomplete entries not only threaten the endorsement process but can also lead to substantial delays. Ensuring that all necessary documentation is intact is a foundational step for obtaining ANDA approval in any successful application. Our project management services include thorough documentation checks to mitigate these risks. The effect of priority review on drug acceptance rates illustrates the significance of regulatory incentives; drugs granted ANDA approval had a 67.9% acceptance rate on the first submission compared to only 40.3% for standard reviews, highlighting how prioritization can facilitate timely endorsements.
Timelines and Deadlines: Maintaining organization and adhering to established timelines is paramount. Delays at any stage can have a cascading effect, resulting in missed deadlines that can adversely impact overall project timelines and market entry strategies. It is essential to have a strong project management strategy established, directed by professionals like Katherine Ruiz, to navigate the intricacies of the ANDA approval process smoothly. Additionally, our services include overseeing the import permit procedures for investigational devices, ensuring compliance with national regulations. We also provide detailed reporting on study status, including tracking serious and non-serious adverse events, which is crucial for maintaining regulatory compliance and ensuring participant safety.
The Role of Regulatory Agencies in ANDA Approvals
The FDA plays a vital role in the ANDA approval validation procedure for Abbreviated New Drug Applications (ANDAs) by establishing thorough guidelines that oversee both the submission and evaluation stages. These guidelines are designed to ensure that all generic products meet rigorous safety and efficacy standards. Interacting with the FDA—especially via pre-submission meetings—can provide applicants with useful insights and assist in clarifying expectations, ultimately enabling a more seamless review.
Compliance with regulatory requirements is paramount; any deviations can lead to significant delays or outright denials of applications for ANDA approval. Comprehending the subtleties of the FDA's evaluation system, particularly concerning the analysis of bioequivalence, is vital for applicants maneuvering through the intricacies of submissions. The guidelines established by the FDA outline the standards for showing bioequivalence, a vital aspect of the ANDA approval process.
As Bob Pollock wisely queries, will we keep witnessing diminished filing rates, will applications for generic drugs continue to surpass new applications, and what effect will that have on the OGD, its personnel and responsiveness? This inquiry underscores the evolving landscape of abbreviated new drug applications and the importance of staying informed about the latest regulatory developments. With 2,023 ANDAs awaiting applicant action, the second lowest in FY 2023, comprehending the FDA's role is more essential than ever for successful navigation of the ANDA approval process in the regulatory landscape.
Furthermore, from 2009 to 2023, the number of approved ANDAs has shown various trends that reflect the changing dynamics of the generic drug market. Additionally, the case study titled 'Impact of Insufficient Generic Competition' highlights real-world implications of the FDA's measures to enhance the availability of generic drugs, illustrating the importance of compliance in ensuring patient access to essential medications.

Post-Approval Changes and Compliance for ANDAs
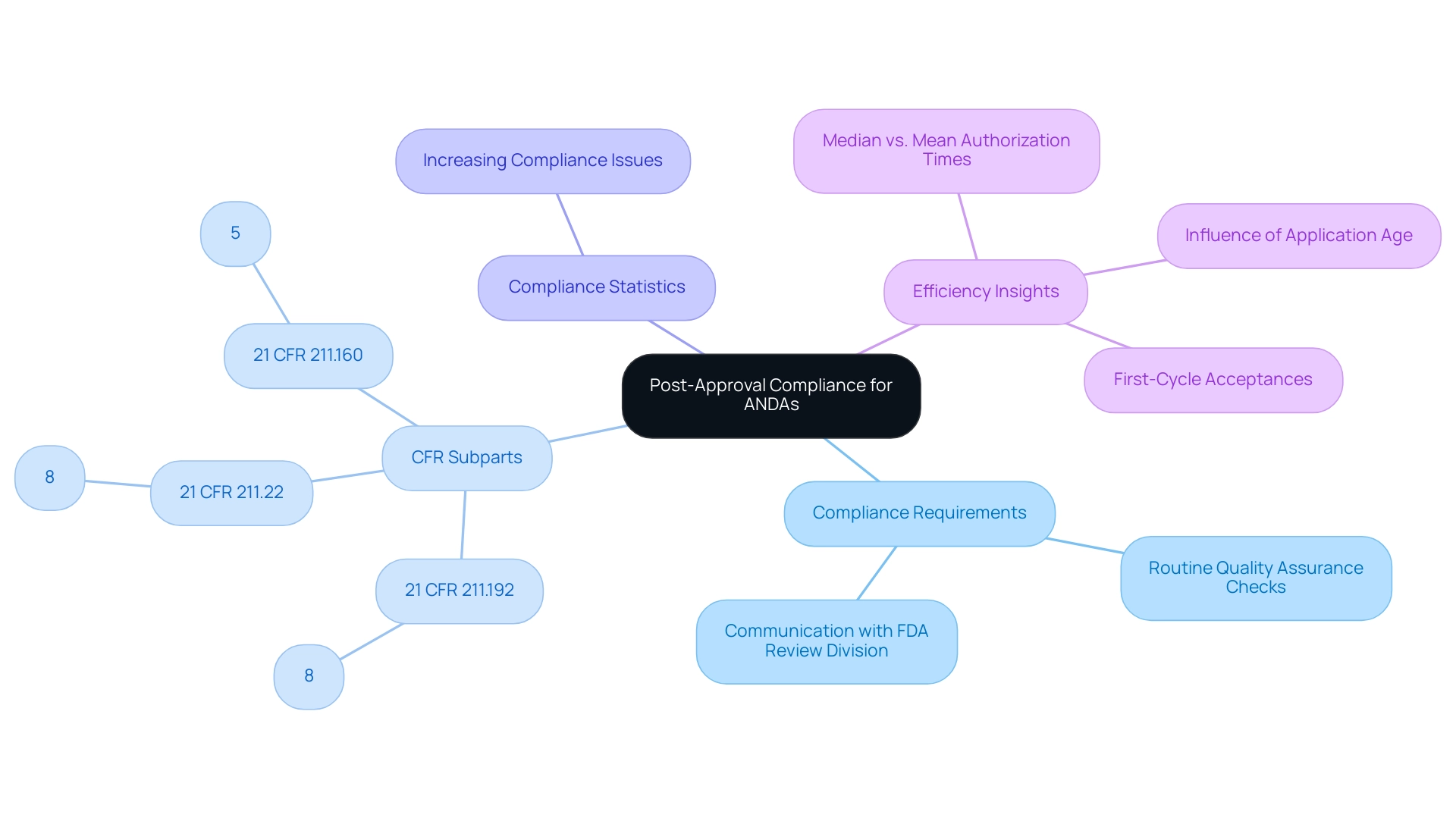
Post-approval, applicants must prioritize compliance with FDA regulations to mitigate risks associated with manufacturing changes, formulation adjustments, or labeling modifications. The FDA mandates that any significant changes be reported promptly; neglecting this requirement can lead to serious compliance issues, including product recalls. Recent statistics reveal that adherence to compliance standards is crucial, as cases of post-approval compliance issues have been increasingly documented in 2024.
Notably, the most commonly cited CFR subparts include:
- 21 CFR 211.192 (8%)
- 21 CFR 211.22 (8%)
- 21 CFR 211.160 (5%)
This highlights key areas of focus for compliance. To demonstrate the efficiency of the review procedure, the final report for FY 2023 indicated that the median authorization times for ANDAs were significantly lower than the mean authorization times, influenced by the age of applications and the increase in first-cycle acceptances. This indicates enhanced efficiency in the validation process, which is pertinent to the compliance challenges encountered by application holders.
A consulting company, Allucent, highlights their proficiency by declaring,
Allucent has experience with both ANDAs and 505(b)(2) application support and can assist in selecting the appropriate pathway for your drug development program.
This insight reflects the necessity of informed decision-making in the post-approval phase. Implementing routine quality assurance checks is essential to ensure that products consistently meet established specifications and regulatory requirements.
Furthermore, staying abreast of regulatory updates and maintaining open communication with the relevant FDA review division is vital for navigating post-approval challenges effectively. For further questions, the guideline recommends contacting the respective FDA review division. By doing so, holders can enhance their compliance strategies and adapt to any evolving regulatory landscape.
Building Effective Relationships with Regulatory Bodies
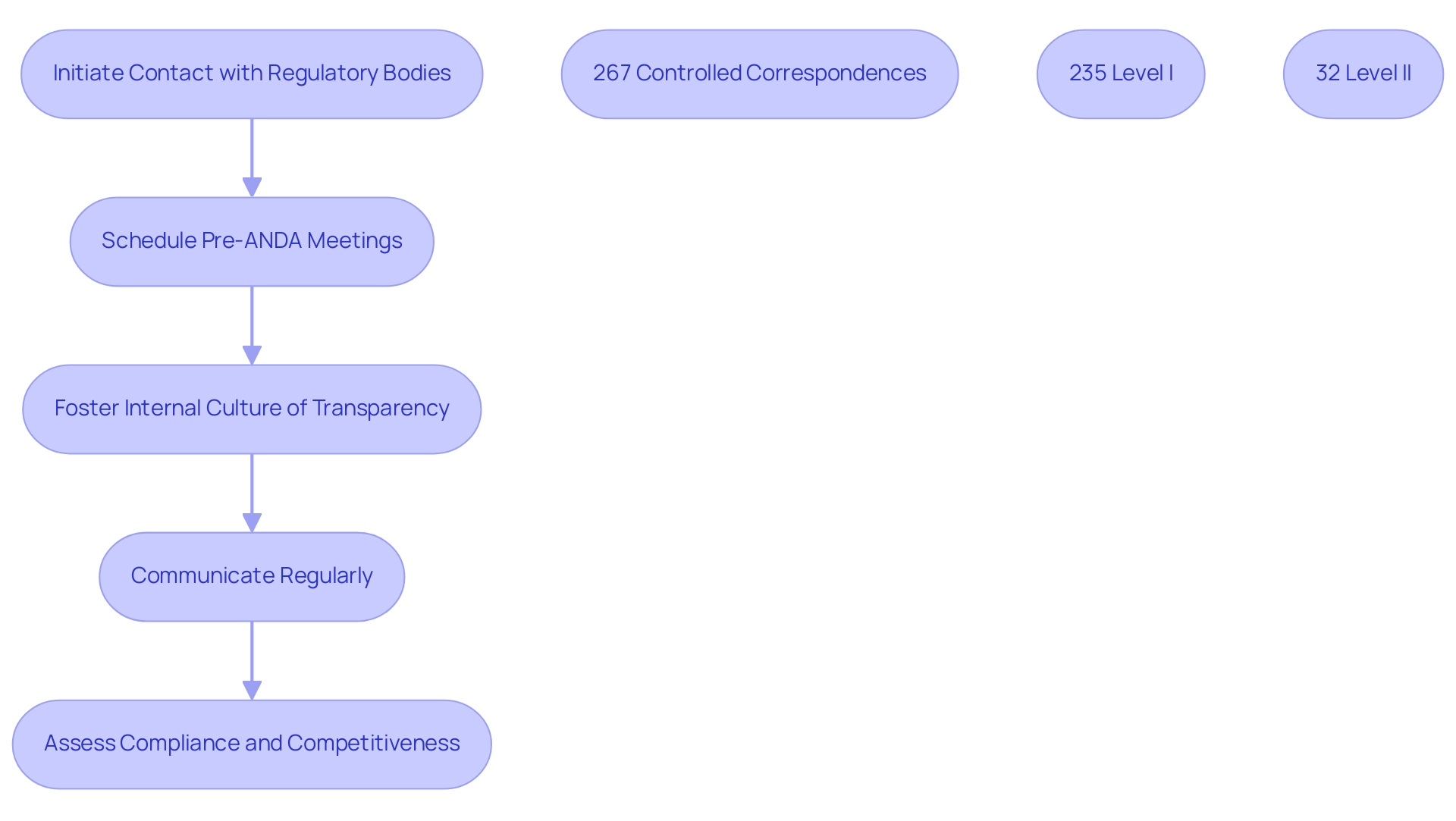
Building strong connections with regulatory agencies, like the FDA and INVIMA, is essential for successfully maneuvering through the ANDA approval process. Professionals like Ana Criado, Director of Regulatory Affairs and an expert in biomedical engineering and health economics, emphasize that regular communication with the FDA not only provides valuable insights into expectations and potential challenges but also enhances the overall approval experience. Ana's extensive experience at INVIMA, where she held various executive roles, provides her with a unique perspective on regulatory procedures.
Significantly, a statistic shows that there were 267 controlled correspondences, with 235 classified at Level I and 32 at Level II, illustrating the volume of interactions that can assist applicants in their entries. Scheduling pre-ANDA approval meetings is a strategic approach to discuss submission strategies and clarify any concerns, which can significantly enhance efficiency. Additionally, fostering a culture of transparency and collaboration within your organization can lead to more productive interactions with regulatory agencies.
This engagement not only assists in the endorsement process but also aids in grasping the evolving regulatory landscape, ensuring compliance and competitiveness. A case study titled 'Effects of GDUFA on Drug Costs' highlights that while additional generic authorizations can enhance competition and lower prices, the specific impact of GDUFA on drug pricing remains unclear due to various influencing factors. Nonetheless, by February 2018, approvals in 2017 resulted in $11.8 billion in savings for U.S. drug purchasers, demonstrating a positive financial impact.
As Bob Pollock aptly noted, 'Hopefully, now that the new report has taken its initial flight, it will be smooth sailing from here on out.' The FDA's commitment to enhancing its generic drug program, demonstrated by its hiring of over 1,500 personnel by fiscal 2016, further emphasizes the importance of establishing strong relationships with regulatory agencies. By prioritizing regular communication and collaboration, your organization can navigate the complexities of the regulatory environment more effectively, drawing on the expertise of professionals like Ana Criado, who also serves as a consultant for global companies in the medical field and leads Mahu Pharma, a company with licenses to cultivate cannabis for medicinal and scientific use.
Conclusion
The Abbreviated New Drug Application (ANDA) process is a critical component of the pharmaceutical landscape, facilitating the entry of generic drugs that enhance accessibility to affordable medications. By understanding the key steps involved in ANDA submissions—from gathering documentation and conducting bioequivalence studies to navigating regulatory interactions—manufacturers can position themselves for success in a competitive market. The importance of maintaining compliance with FDA guidelines and fostering effective relationships with regulatory agencies cannot be overstated, as these factors significantly influence the approval process and, ultimately, market entry.
As the number of ANDA submissions continues to rise, stakeholders must remain vigilant and proactive in their approaches to regulatory challenges. The evolving nature of the generic drug approval landscape underscores the necessity for strategic planning, meticulous documentation, and effective communication with regulatory bodies. By prioritizing these elements, manufacturers can not only streamline their submission processes but also contribute to a broader public health goal of increasing the availability of cost-effective treatment options.
In summary, the ANDA process is not merely a regulatory hurdle but a vital pathway that shapes the dynamics of drug pricing and availability in the healthcare system. As stakeholders navigate this complex terrain, the insights and strategies outlined in this article serve as a valuable guide for achieving successful drug approvals and fostering a more competitive marketplace that ultimately benefits patients and the healthcare community at large.
Frequently Asked Questions
What is an Abbreviated New Drug Application (ANDA)?
An ANDA is a submission to the FDA that allows manufacturers to demonstrate that their generic products are bioequivalent to their branded counterparts, facilitating market entry without extensive clinical trials.
Why are ANDAs significant?
ANDAs are crucial for enhancing the availability of affordable medications, which is vital for public health, and they ensure compliance with regulatory standards.
What key subjects should one understand regarding ANDAs?
Key subjects include Generic Drugs, Biopharmaceutics, and Product-Specific Guidances for Generic Drug Development.
How many ANDAs are projected to be submitted in FY 2024?
An estimated 784 ANDAs are expected to be submitted in FY 2024, an increase from 733 applications in the previous year.
What does the Office of Generic Drugs (OGD) expect regarding ANDA approvals in FY 2024?
The OGD expects to authorize 644 ANDAs for the fiscal year, indicating a reduction in approvals compared to previous periods.
What are the steps involved in preparing an ANDA submission?
The steps include gathering required documentation, completing the application form, preparing the bioequivalence study, compiling the submission package, submitting the application, and monitoring the review process.
What types of documentation are needed for an ANDA?
Required documentation includes product formulation details, manufacturing methods, stability data, and bioequivalence study results.
How should the bioequivalence study be conducted?
The bioequivalence study must be comprehensive and adhere to the latest FDA guidelines, with robust design, methodology, and statistical analysis.
How should the ANDA submission package be organized?
The submission package should be well-structured and easy to navigate, including all forms, studies, and supporting documents.
What should applicants do after submitting their ANDA?
Applicants should actively monitor the status of their application on the FDA’s website and be prepared to respond promptly to any inquiries or requests for additional information.
What additional services can assist in the ANDA submission process?
Services may include feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, which help navigate the intricate drug application process.




